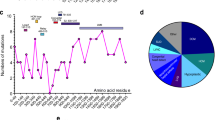
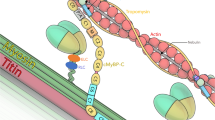
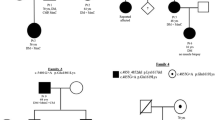
Abstract
Hereditary myosin myopathies are a newly emerged group of diseases caused by mutations in skeletal muscle myosin heavy chain (MyHC) genes. The phenotypes of these diseases are varied, ranging from prenatal nonprogressive arthrogrypotic syndromes to adult-onset progressive muscle weakness. They are caused by mutations in skeletal muscle myosin heavy chain (MyHC) genes. Mutations have been reported in two of three MyHC isoforms expressed in adult limb skeletal muscle: type I (slow/β-cardiac MyHC; MYH7) and type IIa (MYH2). Most of the mutations described in MYH7 are associated with hypertrophic/dilated cardiomyopathy, with no skeletal muscle involvement. However, some mutations are associated with two distinct skeletal myopathies, namely Laing distal myopathy and myosin storage myopathy. Although initially thought not to have associated cardiac involvement, recent reports have indicated co-existent cardiac and skeletal muscle disease can occur in both. A myopathy associated with a specific mutation in MYH2 is associated with congenital joint contractures and external ophthalmoplegia. Mutations in embryonic MyHC (MYH3) and perinatal MyHC (MYH8) are associated with distal arthrogryposis syndromes with no or minor muscle weakness. This may be expected in myosin isoforms expressed predominantly during muscle development. Clinical findings, muscle morphology and molecular genetics in hereditary myosin myopathies are summarized in this chapter.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Oldfors A. Hereditary myosin myopathies. Neuromuscul Disord 2007; 17:355–367.
Kjellgren D, Thornell LE, Andersen J et al. Myosin heavy chain isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci 2003; 44:1419–25.
Ching YH, Ghosh TK, Cross SJ et al. Mutation in myosin heavy chain 6 causes atrial septal defect. Nat Genet 2005; 37:423–8.
Zhu L, Vranckx R, Khau Van Kien P et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet 2006; 38:343–9.
Poetter K, Jiang H, Hassanzadeh S et al. Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle. Nat Genet 1996; 13:63–69.
Darin N, Kyllerman M, Wahlstrom J et al. Autosomal dominant myopathy with congenital joint contractures, ophthalmoplegia and rimmed vacuoles. Ann Neurol 1998; 44:242–248.
Martinsson T, Darin N, Kyllerman M et al. Dominant hereditary inclusion-body myopathy gene (IBM3) maps to chromosome region 17p13.1. Am J Hum Genet 1999; 64:1420–1426.
Martinsson T, Oldfors A, Darin N et al. Autosomal dominant myopathy: Missense mutation (Glu-706 to Lys) in the myosin heavy chain IIa gene. Proc Natl Acad Sci USA 2000; 97:14614–14619.
Sunnerhagen KS, Darin N, Tasjharghi H. The effects of endurance training in persons with a hereditary myosin myopathy. Acta Neurol Scand 2004; 110:80–86.
Tajsharghi H, Stibrant Sunnerhagen K, Darin N et al. Induced shift in myosin heavy chain expression in myosin myopathy by endurance training. J Neurol 2004; 251:179–183.
Tajsharghi H, Thornell LE, Darin N et al. Myosin heavy chain IIa gene mutation E706K is pathogenic and its expression increases with age. Neurology 2002; 58:780–786
Jansson M, Darin N, Kyllerman M et al. Multiple mitochondrial DNA deletions in hereditary inclusion body myopathy. Acta Neuropathol (Berl) 2000; 100:23–28.
Li M, Lionikas A, Yu F et al. Muscle cell and motor protein function in patients with a IIa myosin missense mutation (Glu-706 to Lys). Neuromuscul Disord 2006; 16:782–791.
Zeng W, Conibear PB, Dickens JL et al. Dynamics of actomyosin interactions in relation to the cross-bridge cycle. Phil Trans R Soc B 2004; 359:1843–1855.
Tajsharghi H, Pilon M, Oldfors A. A Caenorhabditis elegans model of the myosin heavy chain IIa E706K mutation. Ann Neurol 2005; 58:442–448.
Tajsharghi H, Darin N, Rekabdar E et al. Mutations and sequence variation in the human myosin heavy chain IIa gene (MYH2). Eur J Hum Genet 2005; 13:617–622.
Kimber E, Tajsharghi H, Kroksmark AK et al. A mutation in the fast skeletal muscle troponin I gene causes myopathy and distal arthrogryposis. Neurology 2006; 67:597–601.
Sung SS, Brassington AM, Grannatt K et al. Mutations in genes encoding fast-twitch contractile proteins cause distal arthrogryposis syndromes. Am J Hum Genet 2003; 72:681–690.
Sung SS, Brassington AM, Krakowiak PA et al. Mutations in TNNT3 cause multiple congenital contractures: A second locus for distal arthrogryposis type 2B. Am J Hum Genet 2003; 73:212–214.
Tajsharghi H, Kimber E, Holmgren D et al. Distal arthrogryposis and muscle weakness associated with a β-tropomyosin mutation. Neurology 2007; 68:772–775.
Toydemir RM, Chen H, Proud VK et al. Trismus-pseudo camptodactyly syndrome is caused by recurrent mutation of MYH8. Am J Med Genet A 2006; 140:2387–2393.
Toydemir RM, Rutherford A, Whitby FG et al. Mutations in embryonic myosin heavy chain (MYH3) cause Freeman-Sheldon syndrome and Sheldon-Hall syndrome. Nat Genet 2006; 38:561–565.
Veugelers M, Bressan M, McDermott DA et al. Mutation of perinatal myosin heavy chain associated with a Carney complex variant. N Engl J Med 2004; 351:460–469.
Bamshad M, Jorde LB, Carey JC. A revised and extended classification of the distal arthrogryposes. Am J Med Genet 1996; 65:277–281.
Hall JG. Arthrogryposis multiplex congenita: Etiology, genetics, classification, diagnostic approach and general aspects. J Pediatr Orthop B 1997; 6:159–166.
Stevenson DA, Carey JC, Palumbos J et al. Clinical characteristics and natural history of Freeman-Sheldon syndrome. Pediatrics 2006; 117:754–762.
Laing NG, Laing BA, Meredith C et al. Autosomal dominant distal myopathy: Linkage to chromosome 14. Am J Hum Genet 1995; 56:422–427.
Lamont PJ, Udd B, Mastaglia FL et al. Laing early onset distal myopathy: Slow myosin defect with variable abnormalities on muscle biopsy. J Neurol Neurosurg Psychiatry 2006; 77:208–215.
Voit T, Kutz P, Leube B et al. Autosomal dominant distal myopathy: Further evidence of a chromosome 14 locus. Neuro muscul Disord 2001; 11:11–19.
Hedera P, Petty EM, Bui MR et al. The second kindred with autosomal dominant distal myopathy linked to chromosome 14q: Genetic and clinical analysis. Arch Neurol 2003; 60:1321–1325.
Caforio AL, Rossi B, Risaliti R et al. Type 1 fiber abnormalities in skeletal muscle of patients with hypertrophic and dilated cardiomyopathy: Evidence of subclinical myogenic myopathy. J Am Coll Cardiol 1989; 14:1464–1473.
Fananapazir L, Dalakas MC, Cyran F et al. Missense mutations in the beta-myosin heavy-chain gene cause central core disease in hypertrophic cardiomyopathy. Proc Natl Acad Sci USA 1993; 90:3993–3997.
Darin N, Tajsharghi H, Oldfors A. New skeletal myopathy and cardiomyopathy associated with a missense mutation in MYH7. Neurology 2007; (68)23:2041–2042.
Overeem S, Schelhaas HJ, Blijham PJ et al. Symptomatic distal myopathy with cardiomyopathy due to a MYH7 mutation. Neuromuscul Disord 2007; 17:490–493.
Meredith C, Herrmann R, Parry C et al. Mutations in the slow skeletal muscle fiber myosin myosin chain gene (MYH7) cause Laing early-onset distal myopathy (MPD1). Am J Hum Genet 2004; 75:703–708.
Cancilla PA, Kalayanaraman K, Verity MA et al. Familial myopathy with probable lysis of myofibrils in type I fibers. Neurology 1971; 21:579–585.
Sahgal V, Sahgal S. A new congenital myopathy: A morphological, cytochemical and histochemical study. Acta Neuropathol (Berl) 1977; 37:225–230.
Engel A, Banker BQ. Ultrastructural changes in diseased muscle. In: Engel A, Banker BQ, eds. Myology Basic and Clincal. New York: McGraw-Hill Company, 1986; 909–1044.
Ceuterick C, Martin JJ, Martens C. Hyaline bodies in skeletal muscle of a patient with a mild chronic nonprogressive congenital myopathy. Clin Neuropathol 1993; 12:79–83.
Barohn RJ, Brumback RA, Mendell JR. Hyaline body myopathy Neuromuscul Disord 1994; 4:257–262.
Masuzugawa S, Kuzuhara S, Narita Y et al. Autosomal dominant hyaline body myopathy presenting as scapuloperoneal syndrome: Clinical features and muscle pathology. Neurology 1997; 48:253–257.
Bohlega S, Lach B, Meyer BF et al. Autosomal dominant hyaline body myopathy: Clinical variability and pathologic findings. Neurology 2003; 61:1519–1523.
Önengüt S, Ugur SA, Karasoy H et al. Identification of a locus for an autosomal recessive hyaline body myopathy at chromosome 3p22.2–p21.32. Neuromuscul Disord 2004; 14:4–9.
Tajsharghi H, Thornell LE, Lindberg C et al. Myosin storage myopathy associated with a heterozygous missense mutation in MYH7. Ann Neurol 2003; 54:494–500.
Laing NG, Ceuterick-de Groote C, Dye De et al. Myosin storage myopathy: Slow skeletal myosin (MYH7) mutation in two isolated cases. Neurology 2005; 64:527–529.
Dye DE, Azzarelli B, Goebel HH, Laing NG. Novel slow-skeletal myosin (MYH7) mutation in the original myosin storage myopathy kindred. Neuromuscul Disord 2006; 16:357–360.
Tajsharghi H, Oldfors A, Macleod DP et al. Homozygous mutation in MYH7 in myosin storage myopathy and cardiomyopathy. Neurology 2007; 68:962.
Shingde MV, Spring PJ, Maxwell A et al. Myosin storage (hyaline body) myopathy: A case report. Neuromuscul Disord 2006; 16:882–886.
Pegoraro E, Gavassini BF, Borsato C et al. MYH7 gene mutation in myosin storage myopathy and scapulo-peroneal myopathy. Neuromuscul Disord 2007; 17:321–329.
Sohn RL, Vikstrom KL, Strauss M et al. A 29 residue region of the sarcomeric myosin rod is necessary for filament formation. J Mol Biol 1997; 266:317–330.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2008 Landes Bioscience and Springer Science+Business Media
About this chapter
Cite this chapter
Oldfors, A., Lamont, P.J. (2008). Thick Filament Diseases. In: Laing, N.G. (eds) The Sarcomere and Skeletal Muscle Disease. Advances in Experimental Medicine and Biology, vol 642. Springer, New York, NY. https://doi.org/10.1007/978-0-387-84847-1_7
Download citation
DOI: https://doi.org/10.1007/978-0-387-84847-1_7
Publisher Name: Springer, New York, NY
Print ISBN: 978-0-387-84846-4
Online ISBN: 978-0-387-84847-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)