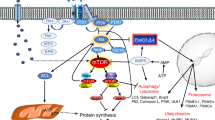
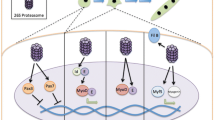
Abstract
Skeletal muscle has a remarkable ability to rapidly adjust to changes in physiological requirements. This includes hypertrophic muscle growth and the atrophic loss of muscle mass, both of which occur in response to hormonal, endocrine and mechanical stimuli. In ageing muscle, sarcopenia (the loss of muscle fibres) can aggravate hormonally and mechanically induced atrophy. Hypertrophy and atrophy are associated with changes in sarcomeric protein composition and metabolic enzymes. The coordinated changes of transcriptional and splice mechanisms, protein turnover and cell fate integrates signalling pathways from hormone and cytokine receptors, as well as the sarcomere itself. This involves a number of proteins that shuttle between sarcomeric and nonsarcomeric localisations and thus convey signals from the contractile machinery to the nucleus. The M-band is emerging as a hub mainly for protein-kinase regulated ubiquitin signalling and protein turnover, whereas the I-band and Z-disk contain stretch-sensitive pathways involving transcriptional modifiers. Disruptions of these pathways can cause hereditary myopathies.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Sartorelli VG, Caretti G. Mechanisms underlying the transcriptional regulation of skeletal myogenesis. Curr Opin Genet Dev 2005; 15(5):528–535.
Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem 2006; 75:18–37
Schiaffino S, Sandri M Murgia M. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology (Bethesda) 2007; 22:269–278.
Potthoff MJ, Wu H, Arnold MA et al. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J Clin Invest 2007; 117(9):2459–2467.
Li S, Czubryt MPJ, Anally Mc et al. Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc Natl Acad Sci USA 2005; 102(4):1082–1087.
Parlakian A, Tuil D, Hamard G et al. Targeted Inactivation of serum response factor in the developing heart results in myocardial defects and ebryonic lethality. Mol Cell Biol 2004; 24(12):5281–5289.
Molkentin JD. Dichotomy of Ca2+ in the heart: Contraction versus intracellular signaling. J Clin Invest 2006; 116(3):623–626.
Tskhovrebova, Trinick LJ. Titin: Properties and family relationships. Nat Rev Mol Cell Biol 2003; 4(9):679–689.
Goldstein MA, Michael LH, Schroeter JP et al. Structural states in the Z band of skeletal muscle correlate with states of active and passive tension. J Gen Physiol 1988; 92(1):113–119.
Yoshikawa Y, Yasuike T, Yagi A et al. Transverse elasticity of myofibrils of rabbit skeletal muscle studied by atomic force microscopy. Biochem Biophys Res Commun 1999; 256(1):13–19.
Arber S, Halder G, Caroni P. Muscle LIM protein, a novel essential regulator of myogenesis, promotes myogenic differentiation. Cell 1994; 79(2):221–231.
Kong Y, Flick MJ, Kudla AJ et al. Muscle LIM protein promotes myogenesis by enhancing the activity of MyoD. Mol Cell Biol 1997; 17(8):4750–4760.
Flick MJ, Konieczny SF. The muscle regulatory and structural protein MLP is a cytoskeletal binding partner of betaI-spectrin. J Cell Sci 2000; 113(Pt 9):1553–1564.
Ecarnot-Laubriet A, De Luca K, Vandroux D et al. Downregulation and nuclear relocation of MLP during the progression of right ventricular hypertrophy induced by chronic pressure overload. J Mol Cell Cardiol 2000; 32(12):2385–2395.
Boateng SY, Belin RJ, Geenen DL et al. Cardiac dysfunction and heart failure are associated with abnormalities in the subcellular distribution and amounts of oligomeric muscle LIM protein. Am J Physiol Heart Circ Physiol 2007; 292(1):H259–269.
Pomiès P, Louis HA, Beckerle MC. CRP1, a LIM domain protein implicated in muscle differentiation, interacts with α-actinin. J Cell Biol 1997; 139:157–168.
Louis HA, Pino JD, Schmeichel KL et al. Comparison of three members of the cysteine-rich protein family reveals functional conservation and divergent patterns of gene expression. J Biol Chem 1997; 272(43):27484–27891.
Knoll R, Hoshijima M, Hoffman HM et al. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell 2002; 111(7):943–955.
Chang AN, Potter JD. Sarcomeric protein mutations in dilated cardiomyopathy. Heart Fail Rev 2005; 10(3):225–235.
Guglieri M, Magri F, Comi GP. Molecular etiopathogenesis of limb girdle muscular and congenital muscular dystrophies: Boundaries and contiguities. Clinica Chimica Acta 2005; 361(1–2):54–79.
Heineke J, Ruetten H, Willenbockel C et al. Attenuation of cardiac remodeling after myocardial infarction by muscle LIM protein-calcineurin signaling at the sarcomeric Z-disc. Proc Natl Acad Sci USA 2005; 102(5):1655–1660.
da Costa N, Edgar J, Ooi PT et al. Calcineurin differentially regulates fast myosin heavy chain genes in oxidative muscle fibre type conversion. Cell Tissue Res 2007; 329(3):515–527.
Willmann R, Kusch J, Sultan KR et al. Muscle LIM protein is upregulated in fast skeletal muscle during transition toward slower phenotypes. Am J Physiol Cell Physiol 2001; 280(2):C273–279.
Schneider AG, Sultan KR, Pette D. Muscle LIM protein: Expressed in slow muscle and induced in fast muscle by enhanced contractile activity. Am J Physiol 1999; 276(4 Pt 1):C900–906.
Barash IA, Mathew L, Lahey M et al. Muscle LIM protein plays both structural and functional roles in skeletal muscle. Am J Physiol Cell Physiol 2005; 289(5):C1312–1320.
Valle G, Faulkner G, De Antoni A et al. Telethonin, a novel sarcomeric protein of heart and skeletal muscle. FEBS Lett 1997; 415:163–168.
Gregorio CC, Trombitas K, Centner T et al. The NH2 terminus of titin spans the Z-disc: Its interaction with a novel 19-kD ligand (T-cap) is required for sarcomeric integrity. J Cell Biol 1998; 143(4):1013–1027.
Mues A, Van der Ven PFM, Young P et al. Two immunoglobulin-like domains of the Z-disk portion of titin interact in a conformation-dependent way with telethonin. FEBS Let 1998; 428:111–114.
Zou P, Pinotsis N, Lange S et al. Palindromic assembly of the giant muscle protein titin in the sarcomeric Z-disk. Nature 2006; 439:229–233.
Moreira ES, Wiltshire TJ, Faulkner G et al. Limb-girdle muscular dystrophy type 2G is caused by mutations in the gene encoding the sarcomeric protein telethonin. Nat Genet 2000; 24(2):163–166.
Hayashi T, Arimura T, Itoh-Satoh M et al. Tcap gene mutations in hypertrophic cardiomyopathy and dilated cardiomyopathy. J Am Coll Cardiol 2004; 44(11):2192–2201.
Schröder R, Iakovenko A, Reimann J et al. Early and selective downregulation of telethonin in neurogenic atrophy. J Muscle Res Cell Motil 2001; 22:259–264.
Wang J, Shaner N, Mittal B et al. Dynamics of Z-band based proteins in developing skeletal muscle cells. Cell Motil Cytoskeleton 2005; 61(1):34–48.
Lee EH, Gao M, Pinotsis N et al. Mechanical strength of the titin Z1Z2-telethonin complex. Structure 2006; 14(3):497–509.
Frey, NEN, Olson. Calsarcin-3, a novel skeletal muscle-specific member of the calsarcin family, interacts with multiple Z-disc proteins. J Biol Chem 2002; 12:13998–14004.
Faulkner G, Lanfranchi G, Valle G. Telethonin and other new proteins of the Z-disc of skeletal muscle. IUBMB Life 2001; 51(5):275–282.
Furukawa T, Ono Y, Tsuchiya H et al. Specific interaction of the potassium channel beta-subunit minK with the sarcomeric protein T-cap suggests a T-tubule-Myofibril linking system. J Mol Biol 2001; 313(4):775–784.
Nicholas G, Thomas M, Langley B et al. Titin-cap associates with and regulates secretion of Myostatin. J Cell Physiol 2002; 193(1):120–131.
Solomon AM, Bouloux PM. Modifying muscle mass—the endocrine perspective. J Endocrinol 2006; 191(2):349–360.
Haworth RS, Cuello F, Herron TJ et al. Protein Kinase D is a novel mediator of cardiac troponin i phosphorylation and regulates myofilament function. Circ Res 2004; 95(11):1091–1099.
Mayans O, Van der Ven P, Wilm M et al. Structural basis of the activation of the titin kinase domain during myofibrillogenesis. Nature 1998; 395:863–869.
Takada F, Vander DL, Woude Tong HQ et al. Myozenin: An alpha-actinin-and gamma-filamin-binding protein of skeletal muscle Z lines. Proc Natl Acad Sci USA 2001; 98(4):1595–600.
Frey N, Richardson JA, Olson EN. Calsarcins, a novel family of sarcomeric calcineurin-binding proteins. Proc Natl Acad Sci USA 2000; 97(26):14632–14637.
Faulkner G, Pallavicini A, Comelli A et al. FATZ, a filamin-, actinin-and telethonin-binding protein of the Z-disc of skeletal muscle. J Biol Chem 2000; 275(52):41234–41242.
Gontier Y, Taivainen A, Fontao L et al. The Z-disc proteins myotilin and FATZ-1 interact with each other and are connected to the sarcolemma via muscle-specific filamins. J Cell Sci 2005; 118(16):3739–3749.
Schiaffino, Serrano SA. Calcineurin signaling and neural control of skeletal muscle fiber type and size. Trends Pharmacol Sci 2002, 23(12):569–575.
Frey N, Barrientos T, Shelton JM et al. Mice lacking calsarcin-1 are sensitized to calcineurin signaling and show accelerated cardiomyopathy in response to pathological biomechanical stress. Nat Med 2004; 10(12):1336–1343.
Osio A, Tan L, Chen SN et al. Myozenin 2 is a novel gene for human hypertrophic cardiomyopathy. Circ Res 2007; 100(6):766–768.
Zhou Q, Ruiz-Lozano P, Martone ME et al. Cypher, a striated muscle-restricted PDZ and LIM domain-containing protein, binds to alpha-actinin-2 and protein kinase C. J Biol Chem 1999; 274(28):19807–19813.
Faulkner G, Pallavicini A, Formentin E et al. ZASP: A new Z-band alternatively spliced PDZ-motif protein. J Cell Biol 1999; 146(2):465–475.
Zhou Q, Chu PH, Huang C et al. Ablation of Cypher, a PDZ-LIM domain Z-line protein, causes a severe form of congenital myopathy. J Cell Biol 2001; 155(4):605–612.
Vatta M, Mohapatra B, Jimenez S et al. Mutations in Cypher/ZASP in patients with dilated cardiomyopathy and left ventricular noncompaction. J Am Coll Cardiol 2003; 42(11):2014–2027.
Arimura T, Hayashi T, Terada H et al. A Cypher/ZASP mutation associated with dilated cardiomyopathy alters the binding affinity to protein kinase C. J Biol Chem 2004; 279(8):6746–6752.
Griggs R, Vihola A, Hackman P et al. Zaspopathy in a large classic late-onset distal myopathy family. Brain 2007; 130:1477–1484.
Horowits R, Kempner ES, Bisher ME et al. A physiological role for titin and nebulin in skeletal muscle. Nature 1986; 323:160–164.
Horowits R, Podolsky RJ. The positional stability of thick filaments in activated skeletal muscle depends on sarcomere length: Evidence for the role of titin filaments. J Cell Biol 1987; 105:2217–2223.
Chu W, Burns DK, Swerlick RA et al. Identification and characterization of a novel cytokine-inducible nuclear protein from human endothelial cells. J Biol Chem 1995; 270(17):10236–10245.
Zou Y, Evans S, Chen J et al. CARP, a cardiac ankyrin repeat protein, is downstream in the Nkx2-5 homeobox gene pathway. Development 1997; 124(4):793–804.
Jeyaseelan R, Poizat C, Baker RK et al. A novel cardiac-restricted target for doxorubicin. CARP, a nuclear modulator of gene expression in cardiac progenitor cells and cardiomyocytes. J Biol Chem 1997; 272(36):22800–22808.
Baumeister A, Arber S, Caroni P. Accumulation of muscle ankyrin repeat protein transcript reveals local activation of primary myotube endcompartments during muscle morphogenesis. J Cell Biol 1997; 139(5):1231–1242.
Kemp TJ, Sadusky TJ, Saltisi F et al. Identification of Ankrd2, a novel skeletal muscle gene coding for a stretch-responsive ankyrin-repeat protein. Genomics 2000; 66(3):229–241.
Barash IA, Mathew L, Ryan AF et al. Rapid muscle-specific gene expression changes after a single bout of eccentric contractions in the mouse. Am J Physiol Cell Physiol 2004; 286(2):C355–364.
Hentzen ER, Lahey M, Peters D et al. Stress-dependent and-independent expression of the myogenic regulatory factors and the MARP genes after eccentric contractions in rats. J Physiol 2006; 570 (Pt 1):157–167.
Pallavicini A, Kojic S, Bean C et al. Characterization of human skeletal muscle Ankrd2. Biochem Biophys Res Commun 2001; 285(2):378–386.
Ishiguro N, Baba T, Ishida T et al. Carp, a cardiac ankyrin-repeated protein and its new homologue, Arpp, are differentially expressed in heart, skeletal muscle and rhabdomyosarcomas. Am J Pathol 2002; 160(5):1767–1778.
Tsukamoto Y, Senda T, Nakano T et al. Arpp, a new homolog of carp, is preferentially expressed in type 1 skeletal muscle fibers and is markedly induced by denervation. Lab Invest 2002; 82(5):645–655.
Kojic S, Medeot E, Guccione E et al. The Ankrd2 protein, a link between the sarcomere and the nucleus in skeletal muscle. J Mol Biol 2004; 339(2):313–325.
Bang ML, Mudry RE, McElhinny AS et al. Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J Cell Biol 2001; 153(2):413–427.
Miller MK, Bang ML, Witt CC et al. The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules. J Mol Biol 2003; 333(5):951–964.
Zolk O, Frohme M, Maurer A et al. Cardiac ankyrin repeat protein, a negative regulator of cardiac gene expression, is augmented in human heart failure. Biochem Biophys Res Commun 2002; 293(5):1377–1382.
Barash I, Bang M-L, Mathew L et al. Structural and regulatory roles of the muscle ankyrin repeat protein family in skeletal muscle. Am J Physiol Cell Physiol 2007; 293:C218–227.
Johannessen M, Moller S, Hansen T et al. The multifunctional roles of the four-and-a-half-LIM only protein FHL2. Cell Mol Life Sci 2006; 63(3):268–284.
Lange S, Ehler E, Gautel M. From A to Z and back? Multicompartment proteins in the sarcomere. Trends Cell Biol 2006; 16(1):11–18.
Scholl FA, McLoughlin P, Ehler E et al. DRAL is a p53-responsive gene whose four and a half LIM domain protein product induces apoptosis. J Cell Biol 2000; 151(3):495–506.
McLoughlin P, Ehler E, Carlile G et al. The LIM-only protein DRAL/FHL2 interacts with and is a corepressor for the promyelocytic leukemia zinc finger protein. J Biol Chem 2002; 277:37045–37053.
Muller JM, Metzger E, Greschik H et al. The transcriptional coactivator FHL2 transmits Rho signals from the cell membrane into the nucleus. EMBO J 2002; 21(4):736–48.
Hsu CL, Chen YL, Yeh S et al. The use of phage display technique for the isolation of androgen receptor interacting peptides with (F/W)XXL(F/W) and FXXLY new signature motifs. J Biol Chem 2003; 278(26):23691–23698.
Samson T, Smyth N, Janetzky S et al. The LIM-only proteins FHL2 and FHL3 interact with alpha-and beta-subunits of the muscle alpha7beta1 integrin receptor. J Biol Chem 2004; 279(27):28641–28652.
Martin B, Schneider R, Janetzky S et al. The LIM-only protein FHL2 interacts with ta-catenin and promotes differentiation of mouse myoblasts. J Cell Biol 2002; 159:113–122.
McGrath MJ, Mitchell CA, Coghill ID et al. Skeletal muscle LIM protein 1 (SLIM1/FHL1) induces alpha 5 beta 1-integrin-dependent myocyte elongation. Am J Physiol Cell Physiol 2003; 285(6): C1513–1526.
Wei Y, Renard CA, Labalette C et al. Identification of the LIM protein FHL2 as a coactivator of beta-catenin. J Biol Chem 2003; 278(7):5188–94.
Labalette C, Renard CA, Neuveut C et al. Interaction and functional cooperation between the LIM protein FHL2, CBP/p300 and ta-catenin. Mol Cell Biol 2004; 24(24):10689–10702.
Roth J-F, Shikama N, Henzen C et al. Differential role of p300 and CBP acetyltransferase during myogenesis: p300 acts upstream of MyoD and Myf5. EMBO J 2003; 22(19):5186–5196.
Miyagishi M, Fujii R, Hatta M et al. Regulation of Lef-mediated transcription and p53-dependent pathway by associating beta-catenin with CBP/p300. J Biol Chem 2000; 275(45):35170–35175.
Lee SW, Kim EJ, Um SJ. FHL2 mediates p53-induced transcriptional activation through a direct association with HIPK2. Biochem Biophys Res Commun 2006; 339(4):1056–1062.
Stilo R, Leonardi A, Formisano L et al. TUCAN/CARDINAL and DRAL participate in a common pathway for modulation of NF-[kappa]B activation. FEBS Letters 2002; 521(1–3):165–169.
Purcell NH, Tang G, Yu C et al. Activation of NF-kappa B is required for hypertrophic growth of primary rat neonatal ventricular cardiomyocytes. Proc Natl Acad Sci USA 2001; 98(12):6668–6673.
Kramer, HFLJ Goodyear. Exercise, MAPK and NF-[kappa]B signaling in skeletal muscle. J Appl Physiol 2007; 103(1):388–395.
Purcell NH, Darwis D, Bueno OF et al. Extracellular signal-regulated kinase 2 interacts with and is negatively regulated by the LIM-only protein FHL2 in cardiomyocytes. Mol Cell Biol 2004; 24(3):1081–1095.
Sun J, Yan G, Ren A et al. FHL2/SLIM3 decreases cardiomyocyte survival by inhibitory interaction with sphingosine kinase-1. Circ Res 2006; 99(5):468–476.
Nagata Y, Partridge TA, Matsuda R et al. Entry of muscle satellite cells into the cell cycle requires sphingolipid signaling. J Cell Biol 2006; 174(2):245–253.
Lange S, Auerbach D, McLoughlin P et al. Subcellular targeting of metabolic enzymes to titin in heart muscle may be mediated by DRAL/FHL-2. J Cell Sci 2002; 115(Pt 24):4925–4936.
Obermann WMJ, Gautel M, Steiner F et al. The structure of the sarcomeric M band: Localization of defined domains of myomesin, M-protein and the 250 kD carboxy-terminal region of titin by immunoelectron microscopy. J Cell Biol 1996; 134:1441–1453.
Labeit S, Gautel M, Lakey A et al. Towards a molecular understanding of titin. EMBO J 1992; 11(5):1711–1716.
Agarkova I, Perriard JC. The M-band: An elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol 2005; 15(9):477–485.
Grater F, Shen J, Jiang H et al. Mechanically induced titin kinase activation studied by force-probe molecular dynamics simulations. Biophys J 2005; 88(2):790–804.
Lange S, Xiang F, Yakovenko A et al. The kinase domain of titin controls muscle gene expression and protein turnover. Science 2005; 308:1599–1603.
Spencer JA, Eliazer S, Ilaria RL Jr et al. Regulation of microtubule dynamics and myogenic differentiation by MURF, a striated muscle RING-finger protein. J Cell Biol 2000; 150(4):771–784.
McElhinny MS, Kakinuma K, Sorimachi H et al. Muscle-specific RING finger-1 interacts with titin to regulate sarcomeric M-line and thick filament structure and may have nuclear functions via its interaction with glucocorticoid modulatory element binding protein-1. J Cell Biol 2002; 157(1):125–136.
Sudo T, Maruyama M, Osada H. p62 functions as a p38 MAP kinase regulator. Biochem Biophys Res Commun 2000; 269(2):521–525.
Diaz-Meco, MTJ Moscat. MEK5, a new target of the atypical protein kinase C isoforms in mitogenic signaling. Mol Cell Biol 2001; 21(4):1218–1227.
Sanz L, Sanchez P, Lallena MJ et al. The interaction of p62 with RIP links the atypical PKCs to NF-kappaB activation. EMBO J 1999; 18(11):3044–3053.
Puls A, Schmidt S, Grawe F et al. Interaction of protein kinase C zeta with ZIP, a novel protein kinase C-binding protein. Proc Natl Acad Sci USA 1997; 94(12):6191–6196.
Park I, Chung J, Walsh CT et al. Phosphotyrosine-independent binding of a 62-kDa protein to the src homology 2 (SH2) domain of p56lck and its regulation by phosphorylation of Ser-59 in the lck unique N-terminal region. Proc Natl Acad Sci USA 1995; 92(26):12338–12342.
Joung I, Strominger JL, Shin J. Molecular cloning of a phosphotyrosine-independent ligand of the p56lck SH2 domain. Proc Natl Acad Sci USA 1996; 93(12):5991–5995.
Cariou B, Perdereau D, Cailliau K et al. The adapter protein ZIP binds Grb14 and regulates its inhibitory action on insulin signaling by recruiting protein kinase c{zeta}. Mol Cell Biol 2002; 22(20):6959–6970.
Holt LJ, Siddle K. Grb10 and Grb14: Enigmatic regulators of insulin action—And more? Biochem J 2005; 388(Pt 2):393–406.
Lehti TM, Silvennoinen M, Kivela R et al. Effects of streptozotocin-induced diabetes and physical training on gene expression of titin-based stretch sensing complexes in mouse striated muscle Am J Physiol Endocrinol Metab 2006; 292(2):E533–542.
Sanz L, Diaz-Meco MT, Nakano H et al. The atypical PKC-interacting protein p62 channels NF-kappaB activation by the IL-1-TRAF6 pathway. EMBO J 2000; 19(7):1576–1586.
Wooten MW, Seibenhener ML, Mamidipudi V et al. The atypical protein kinase C-interacting protein p62 Is a scaffold for NF-kB activation by nerve growth factor. J Biol Chem 2001; 276(11):7709–7712.
Zetser A, Gredinger E, Bengal E. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J Biol Chem 1999; 274(8):5193–5200.
Puri PL, Wu Z, Zhang P et al. Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells. Genes Dev 2000; 14(5):574–584.
Nicol RL, Frey N, Pearson G et al. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J 2001; 20(11):2757–2767.
Dinev D, Jordan BW, Neufeld B et al. Extracellular signal regulated kinase 5 (ERK5) is required for the differentiation of muscle cells. EMBO Rep 2001; 2(9):829–834.
Barsyte-Lovejoy D, Galanis A, Clancy A et al. ERK5 is targeted to myocyte enhancer factor 2A (MEF2A) through a MAPK docking motif. Biochem J 2004; 381(Pt 3):693–699.
Wang X, Merritt AJ, Seyfried J et al. Targeted deletion of mek5 causes early embryonic death and defects in the extracellular signal-regulated kinase 5/myocyte enhancer factor 2 cell survival pathway. Mol Cell Biol 2005; 25(1):336–345.
Marcus SL, Winrow CJ, Capone JP et al. A p56(lck) ligand serves as a coactivator of an orphan nuclear hormone receptor. J Biol Chem 1996; 271(44):27197–27200.
Sack MN, Disch DL, Rockman HA et al. A role for Sp and nuclear receptor transcription factors in a cardiac hypertrophic growth program. Proc Natl Acad Sci USA 1997; 94(12):6438–6443.
Myers SA, Wang SC, Muscat GE. The chicken ovalbumin upstream promoter-transcription factors modulate genes and pathways involved in skeletal muscle cell metabolism. J Biol Chem 2006; 281(34):24149–24160.
Sun L, Chen ZJ. The novel functions of ubiquitination in signaling. Curr Opin Cell Biol 2004; 16(2):119–126.
Haglund K, Dikic I. Ubiquitylation and cell signaling. EMBO J 2005; 24(19):3353–3359.
Seibenhener ML, Geetha T, Wooten MW. Sequestosome 1/p62—More than just a scaffold. FEBS Lett 2007; 581(2):175–179.
Pankiv S, Clausen TH, Lamark T et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 2007; 282(33):24131–24145.
Whitehouse C, Chambers J, Howe K et al. NBR1 interacts with fasciculation and elongation protein zeta-1 (FEZ1) and calcium and integrin binding protein (CIB) and shows developmentally restricted expression in the neural tube. Eur J Biochem 2002; 269(2):538–545.
Centner T, Yano J, Kimura E et al. Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J Mol Biol 2001; 306(4):717–726.
Pizon V, Iakovenko A, Van der Ven PFM et al. Transient association of titin and myosin with microtubules in nascent myofibrils directed by the MURF2 RING-finger protein. J Cell Sci 2002; 115(23):4469–4482.
Bodine SC, Latres E, Baumhueter S et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001; 294(5547):1704–1708.
Fielitz J, Kim MS, Shelton JM et al. Myosin accumulation and striated muscle myopathy result from the loss of muscle RING finger 1 and 3. J Clin Invest 2007; 117(9):2486–2495.
Jackson PK, Eldridge AG, Freed E et al. The lore of the RINGs: Substrate recognition and catalysis by ubiquitin ligases. Trends Cell Biol 2000; 10(10):429–439.
Gill G. SUMO and ubiquitin in the nucleus: Different functions, similar mechanisms? Genes Dev 2004; 18(17):2046–59.
Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol 2003; 5(2):87–90.
Dai KS, Liew CC. A novel human striated muscle RING zinc finger protein, SMRZ, interacts with SMT3b via its RING domain. J Biol Chem 2001; 276(26):23992–23999.
Salmena P, Pandolfi LP. Changing venues for tumour suppression: Balancing destruction and localization by monoubiquitylation. Nat Rev Cancer 2007; 7(6):409–413.
von Mikecz A. The nuclear ubiquitin-proteasome system. J Cell Sci 2006; 119(10):1977–1984.
Arya R, Kedar V, Hwang JR et al. Muscle ring finger protein-1 inhibits PKC<epsilon> activation and prevents cardiomyocyte hypertrophy. J Cell Biol 2004; 167(6):1147–1159.
Witt SH, Granzier H, Witt CC et al. MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: Towards understanding MURF-dependent muscle ubiquitination. J Mol Biol 2005; 350(4):713–722.
Agarkova I, Schoenauer R, Ehler E et al. The molecular composition of the sarcomeric M-band correlates with muscle fiber type. Eur J Cell Biol 2004; 83(5):193–204.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2008 Landes Bioscience and Springer Science+Business Media
About this chapter
Cite this chapter
Gautel, M. (2008). The Sarcomere and the Nucleus: Functional Links to Hypertrophy, Atrophy and Sarcopenia. In: Laing, N.G. (eds) The Sarcomere and Skeletal Muscle Disease. Advances in Experimental Medicine and Biology, vol 642. Springer, New York, NY. https://doi.org/10.1007/978-0-387-84847-1_13
Download citation
DOI: https://doi.org/10.1007/978-0-387-84847-1_13
Publisher Name: Springer, New York, NY
Print ISBN: 978-0-387-84846-4
Online ISBN: 978-0-387-84847-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)