Abstract
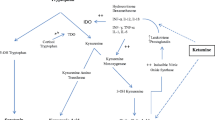
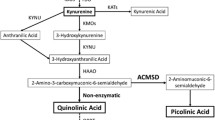
Glutamate is the major excitatory neurotransmitter in the central nervous system, and it is linked with the amino acid glutamine through a metabolic relationship of enzymatic compound interconversion and transportation, also known as the glutamate-glutamine cycle.
A growing body of evidence suggests involvement of the glutamatergic neurotransmitter system in suicidal behaviours. The initial evidence comes from the pathophysiology of neuropsychiatric disorders, as disruptions in glutamate neurotransmission have been found underlying pathology in multiple suicide-related psychiatric conditions such as major depressive disorder, schizophrenia, post-traumatic stress disorder, and bipolar disorder.
Existing data from experimental animal models and human in vivo studies also demonstrate that glutamate plays a key role in suicide-related personality traits including aggression and impulsive aggression.
Further studies on glutamate system dysfunction underlying suicidal behaviours have focused on the different steps of the glutamate-glutamine cycle: an inflammation-mediated reduction of glutamine synthetase activity has been found in depressed suicide attempters, phosphate-activated glutaminase genes are reduced in suicide completers, and gene expression abnormalities in NMDA receptors have also been discovered in suicide victims.
Evidence of a role of the glutamate-glutamine cycle in suicidal behaviours unveils new targets for anti-suicide interventions. Lithium’s mechanism to reduce the risk of suicide in people with mood disorders may be related to its ability to increase glutamine synthetase, whereas novel NMDA antagonists such as ketamine [or its S(+) enantiomer esketamine] have already demonstrated positive results in reducing suicidal ideation.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Ballard ED, Ionescu DF, Vande Voort JL, Niciu MJ, Richards EM, Luckenbaugh DA et al (2014) Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. https://doi.org/10.1016/j.jpsychires.2014.07.027
Bartoli F, Riboldi I, Crocamo C, Di Brita C, Clerici M, Carrà G (2017) Ketamine as a rapid-acting agent for suicidal ideation: a meta-analysis. Neurosci Biobehav Rev https://doi.org/10.1016/j.neubiorev.2017.03.010
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. https://doi.org/10.1016/S0006-3223(99)00230-9
Bernstein H-G, Tausch A, Wagner R, Steiner J, Seeleke P, Walter M et al (2013) Disruption of glutamate-glutamine-GABA cycle significantly impacts on suicidal behaviour: survey of the literature and own findings on glutamine synthetase. CNS Neurol Disord Drug Targets. https://doi.org/10.2174/18715273113129990091
Bhat RV, Budd Haeberlein SL, Avila J (2004) Glycogen synthase kinase 3: a drug target for CNS therapies. J Neurochem. https://doi.org/10.1111/j.1471-4159.2004.02422.x
Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P et al (2018) Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatr. https://doi.org/10.1176/appi.ajp.2018.17060720
Choudhury PR, Lahiri S, Rajamma U (2012) Glutamate mediated signaling in the pathophysiology of autism spectrum disorders. Pharmacol Biochem Behav. https://doi.org/10.1016/j.pbb.2011.06.023
Cipriani A, Hawton K, Stockton S, Geddes JR (2013) Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. https://doi.org/10.1136/bmj.f3646
Coccaro EF, Lee R, Vezina P (2013) Cerebrospinal fluid glutamate concentration correlates with impulsive aggression in human subjects. J Psychiatr Res. https://doi.org/10.1016/j.jpsychires.2013.05.001
Courtet P, Giner L, Seneque M, Guillaume S, Olie E, Ducasse D (2016) Neuroinflammation in suicide: toward a comprehensive model. World J Biol Psychiatry. https://doi.org/10.3109/15622975.2015.1054879
Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC et al (2018) Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiat. https://doi.org/10.1001/jamapsychiatry.2017.3739
Dean B, Gibbons AS, Boer S, Uezato A, Meador-Woodruff J, Scarr E, McCullumsmith RE (2016) Changes in cortical N-methyl-d-aspartate receptors and post-synaptic density protein 95 in schizophrenia, mood disorders and suicide. Aust N Z J Psychiatry. https://doi.org/10.1177/0004867415586601
DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA et al (2010a) Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. https://doi.org/10.4088/JCP.09m05327blu
Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S et al (2010b) A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. https://doi.org/10.1001/archgenpsychiatry.2010.90
Emnett CM, Eisenman LN, Taylor AM, Izumi Y, Zorumski CF, Mennerick S (2013) Indistinguishable synaptic pharmacodynamics of the N-methyl-D-aspartate receptor channel blockers memantine and ketamine. Mol Pharmacol. https://doi.org/10.1124/mol.113.089334
Ende G, Cackowski S, van Eijk J, Sack M, Demirakca T, Kleindienst N et al (2016) Impulsivity and aggression in female BPD and ADHD patients: association with ACC glutamate and GABA concentrations. Neuropsychopharmacology. https://doi.org/10.1038/npp.2015.153
Fan W, Yang H, Sun Y, Zhang J, Li G, Zheng Y, Liu Y (2017) Ketamine rapidly relieves acute suicidal ideation in cancer patients: a randomized controlled clinical trial. Oncotarget. https://doi.org/10.18632/oncotarget.13743
Fiori LM, Turecki G (2012) Broadening our horizons: gene expression profiling to help better understand the neurobiology of suicide and depression. Neurobiol Dis. https://doi.org/10.1016/j.nbd.2010.11.004
Freed WJ, Dillon-Carter O, Kleinman JE (1993) Properties of [3H]AMPA binding in postmortem human brain from psychotic subjects and controls: increases in caudate nucleus associated with suicide. Exp Neurol. https://doi.org/10.1006/exnr.1993.1070
Fudalej S, Klimkiewicz A, Mach A, Jakubczyk A, Fudalej M, Wasilewska K et al (2017) An association between genetic variation in the glutamatergic system and suicide attempts in alcohol-dependent individuals. Am J Addict. https://doi.org/10.1111/ajad.12571
Gleeson M (2008) Dosing and efficacy of glutamine supplementation in human exercise and sport training. J Nutr. https://doi.org/10.1093/jn/138.10.2045S
Grunebaum MF, Ellis SP, Keilp JG, Moitra VK, Cooper TB, Marver JE et al (2017) Ketamine versus midazolam in bipolar depression with suicidal thoughts: a pilot midazolam-controlled randomized clinical trial. Bipolar Disord. https://doi.org/10.1111/bdi.12487
Grunebaum MF, Galfalvy HC, Choo TH, Keilp JG, Moitra VK, Parris MS et al (2018) Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatr. https://doi.org/10.1176/appi.ajp.2017.17060647
Hayashi MK (2018) Structure-function relationship of transporters in the glutamate–glutamine cycle of the central nervous system. Int J Mol Sci. https://doi.org/10.3390/ijms19041177
Holemans S, De Paermentier F, Horton RW, Crompton MR, Katona CLE, Maloteaux JM (1993) NMDA glutamatergic receptors, labelled with [3H]MK-801, in brain samples from drug-free depressed suicides. Brain Res. https://doi.org/10.1016/0006-8993(93)90202-X
Hu S, Martella A, Anderson WR, Chao CC (1994) Role of cytokines in lipopolysaccharide-induced functional and structural abnormalities of astrocytes. Glia. https://doi.org/10.1002/glia.440100309
Hu S, Sheng WS, Ehrlich LC, Peterson PK, Chao CC (2000) Cytokine effects on glutamate uptake by human astrocytes. Neuroimmunomodulation. https://doi.org/10.1159/000026433
Huang TL, O’Banion MK (2002) Interleukin-1β and tumor necrosis factor-α suppress dexamethasone induction of glutamine synthetase in primary mouse astrocytes. J Neurochem. https://doi.org/10.1046/j.1471-4159.1998.71041436.x
Ionescu DF, Swee MB, Pavone KJ, Taylor N, Akeju O, Baer L et al (2016) Rapid and sustained reductions in current suicidal ideation following repeated doses of intravenous ketamine: secondary analysis of an open-label study. J Clin Psychiatry. https://doi.org/10.4088/JCP.15m10056
Jiménez E, Arias B, Mitjans M, Goikolea JM, Roda E, Sáiz PA et al (2013) Genetic variability at IMPA2, INPP1 and GSK3β increases the risk of suicidal behavior in bipolar patients. Eur Neuropsychopharmacol. https://doi.org/10.1016/j.euroneuro.2013.01.007
Kalkman HO (2011) Circumstantial evidence for a role of glutamine-synthetase in suicide. Med Hypotheses. https://doi.org/10.1016/j.mehy.2011.03.005
Kim S, Choi KH, Baykiz AF, Gershenfeld HK (2007) Suicide candidate genes associated with bipolar disorder and schizophrenia: an exploratory gene expression profiling analysis of post-mortem prefrontal cortex. BMC Genomics. https://doi.org/10.1186/1471-2164-8-413
Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, Ffrench-Mullen J, Turecki G (2009) Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatry. https://doi.org/10.1038/sj.mp.4002110
Kudoh A, Takahira Y, Katagai H, Takazawa T (2002) Small-dose ketamine improves the postoperative state of depressed patients. Anesth Analg. https://doi.org/10.1097/00000539-200207000-00020
Laje G, Paddock S, Manji H, Rush AJ, Wilson AF, Charney D, McMahon FJ (2007) Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatr. https://doi.org/10.1176/appi.ajp.2007.06122018
Larkin GL, Beautrais AL (2011) A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. https://doi.org/10.1017/S1461145711000629
Lascelles K, Marzano L, Brand F, Trueman H, McShane R, Hawton K (2019) Effects of ketamine treatment on suicidal ideation: a qualitative study of patients’ accounts following treatment for depression in a UK ketamine clinic. BMJ Open. https://doi.org/10.1136/bmjopen-2019-029108
Masugi-Tokita M, Flor PJ, Kawata M (2016) Metabotropic glutamate receptor subtype 7 in the bed nucleus of the stria terminalis is essential for intermale aggression. Neuropsychopharmacology. https://doi.org/10.1038/npp.2015.198
Molero P, Ramos-Quiroga JA, Martin-Santos R, Calvo-Sánchez E, Gutiérrez-Rojas L, Meana JJ (2018) Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. https://doi.org/10.1007/s40263-018-0519-3
Murphy TM, Ryan M, Foster T, Kelly C, McClelland R, O’Grady J et al (2011) Risk and protective genetic variants in suicidal behaviour: association with SLC1A2, SLC1A3, 5-HTR1B &NTRK2 polymorphisms. Behav Brain Funct. https://doi.org/10.1186/1744-9081-7-22
Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM et al (2013) Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatr. https://doi.org/10.1176/appi.ajp.2013.13030392
Murrough JW, Soleimani L, Dewilde KE, Collins KA, Lapidus KA, Iacoviello BM et al (2015) Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol Med. https://doi.org/10.1017/S0033291715001506
Noga JT, Wang H (2002) Further postmortem autoradiographic studies of AMPA receptor binding in schizophrenia. Synapse. https://doi.org/10.1002/syn.10106
Nowak G, Ordway GA, Paul IA (1995) Alterations in the N-methyl-d-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. https://doi.org/10.1016/0006-8993(95)00057-W
Nudmamud-Thanoi S, Reynolds GP (2004) The NR1 subunit of the glutamate/NMDA receptor in the superior temporal cortex in schizophrenia and affective disorders. Neurosci Lett. https://doi.org/10.1016/j.neulet.2004.09.035
O’Donovan SM, Sullivan CR, McCullumsmith RE (2017) The role of glutamate transporters in the pathophysiology of neuropsychiatric disorders. NPJ Schizophr. https://doi.org/10.1038/s41537-017-0037-1
Parkin GM, Udawela M, Gibbons A, Dean B (2018) Glutamate transporters, EAAT1 and EAAT2, are potentially important in the pathophysiology and treatment of schizophrenia and affective disorders. World J Psychiatry. https://doi.org/10.5498/wjp.v8.i2.51
Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P et al (2019) Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatr. https://doi.org/10.1176/appi.ajp.2019.19020172
Price RB, Nock MK, Charney DS, Mathew SJ (2009) Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. https://doi.org/10.1016/j.biopsych.2009.04.029
Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ et al (2014) Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety. https://doi.org/10.1002/da.22253
Ragguett RM, Rong C, Kratiuk K, McIntyre RS (2019) Rapastinel – an investigational NMDA-R modulator for major depressive disorder: evidence to date. Expert Opin Investig Drugs. https://doi.org/10.1080/13543784.2019.1559295
Sanacora G, Smith MA, Pathak S, Su HL, Boeijinga PH, McCarthy DJ, Quirk MC (2014) Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol Psychiatry. https://doi.org/10.1038/mp.2013.130
Sanacora G, Johnson MR, Khan A, Atkinson SD, Riesenberg RR, Schronen JP et al (2017) Adjunctive lanicemine (AZD6765) in patients with major depressive disorder and history of inadequate response to antidepressants: a randomized, placebo-controlled study. Neuropsychopharmacology. https://doi.org/10.1038/npp.2016.224
Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V et al (2009) Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One. https://doi.org/10.1371/journal.pone.0006585
Singh JB, Fedgchin M, Daly E, Xi L, Melman C, de Bruecker G et al (2016) Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. https://doi.org/10.1016/j.biopsych.2015.10.018
Sokolowski M, Ben-Efraim YJ, Wasserman J, Wasserman D (2013) Glutamatergic GRIN2B and polyaminergic ODC1 genes in suicide attempts: associations and gene-environment interactions with childhood/adolescent physical assault. Mol Psychiatry. https://doi.org/10.1038/mp.2012.112
Sowa-Kućma M, Szewczyk B, Sadlik K, Piekoszewski W, Trela F, Opoka W et al (2013) Zinc, magnesium and NMDA receptor alterations in the hippocampus of suicide victims. J Affect Disord. https://doi.org/10.1016/j.jad.2013.08.009
Takahashi A, Lee RX, Iwasato T, Itohara S, Arima H, Bettler B et al (2015) Glutamate input in the dorsal raphe nucleus as a determinant of escalated aggression in male mice. J Neurosci. https://doi.org/10.1523/JNEUROSCI.2450-14.2015
Thakurta RG, Das R, Bhattacharya AK, Saha D, Sen S, Singh OP et al (2012) Rapid response with ketamine on suicidal cognition in resistant depression. Indian J Psychol Med. https://doi.org/10.4103/0253-7176.101793
Vande Voort JL, Morgan RJ, Kung S, Rasmussen KG, Rico J, Palmer BA et al (2016) Continuation phase intravenous ketamine in adults with treatment-resistant depression. J Affective Disord. https://doi.org/10.1016/j.jad.2016.09.008
Wickens MM, Bangasser DA, Briand LA (2018) Sex differences in psychiatric disease: a focus on the glutamate system. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2018.00197
Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A et al (2018) The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatr. https://doi.org/10.1176/appi.ajp.2017.17040472
Willard SS, Koochekpour S (2013) Glutamate, glutamate receptors, and downstream signaling pathways. Int J Biol Sci. https://doi.org/10.7150/ijbs.6426
Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA et al (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. https://doi.org/10.1001/archpsyc.63.8.856
Zhan Y, Zhang B, Zhou Y, Zheng W, Liu W, Wang C et al (2019) A preliminary study of anti-suicidal efficacy of repeated ketamine infusions in depression with suicidal ideation. J Affective Disord. https://doi.org/10.1016/j.jad.2019.03.071
Zhao J, Verwer RWH, Gao SF, Qi XR, Lucassen PJ, Kessels HW, Swaab DF (2018) Prefrontal alterations in GABAergic and glutamatergic gene expression in relation to depression and suicide. J Psychiatr Res. https://doi.org/10.1016/j.jpsychires.2018.04.020
Zhou Y, Danbolt NC (2013) GABA and glutamate transporters in brain. Front Endocrinol. https://doi.org/10.3389/fendo.2013.00165
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jimenez-Trevino, L., Gonzalez-Blanco, L., Alvarez-Vazquez, C., Rodriguez-Revuelta, J., Saiz Martinez, P.A. (2020). Glutamine and New Pharmacological Targets to Treat Suicidal Ideation. In: Baca-Garcia, E. (eds) Behavioral Neurobiology of Suicide and Self Harm. Current Topics in Behavioral Neurosciences, vol 46. Springer, Cham. https://doi.org/10.1007/7854_2020_168
Download citation
DOI: https://doi.org/10.1007/7854_2020_168
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-57573-1
Online ISBN: 978-3-030-57574-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)