Abstract
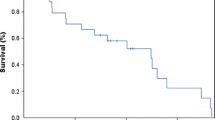
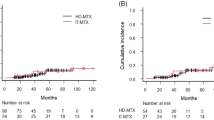
Systemic high-dose methotrexate (HD-MTX) is the most effective chemotherapeutic agent in the treatment of primary central nervous system lymphoma (PCNSL). Leptomeningeal involvement is common and intrathecal methotrexate (IT-MTX) is frequently used in combination with HD-MTX, but its benefits are not established. Using a case-controlled retrospective study, matching patients treated with HD-MTX with or without IT-MTX, we found no difference in survival, disease control, or neurotoxicity.
Similar content being viewed by others
References
Abrey LE, Yahalom J, DeAngelis LM: Treatment for primary CNS lymphoma: The next step. J Clin Oncol 18: 3144–3150, 2000
Hiraga S, Arita N, Ohnishi T, Kohmura E, Yamamoto K, Oku Y, Taki T, Sato M, Aozasa K, Yoshimine T: Rapid infusion of high-dose methotrexate resulting in enhanced penetration into cerebrospinal fluid and intensified tumor response in primary central nervous system lymphomas. J Neurosurg 91: 221–230, 1999
Guha-Thakurta N, Damek D, Pollack C, Hochberg FH: Intravenous methotrexate as initial treatment for primary central nervous system lymphoma: Response to therapy and quality of life of patients. J Neuro-Oncol 43: 259–268, 1999
McAllister LD, Doolittle ND, Guastadisegni PE, Kraemer DF, Lacy CA, Crossen JR, Neuwelt EA: Cognitive outcomes and long-term follow-up results after enhanced chemotherapy delivery for primary central nervous system lymphoma. Neurosurgery 46: 51–60, 2000
Chamberlain MC, Kormanik PA, Barba D: Complications associated with intraventricular chemotherapy in patients with leptomeningeal metastases. J Neurosurg 87: 694–699, 1997
Grossman SA, Finkelstein DM, Ruckdeschel JC, Trump DL, Moynihan T, Ettinger DS: Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. Eastern Cooperative Oncology Group. J Clin Oncol 11: 561–569, 1993
Balmaceda C, Gaynor JJ, Sun M, Gluck JT, DeAngelis LM: Leptomeningeal tumor in primary central nervous system lymphoma: Recognition, significance, and implications. Ann Neurol 38: 202–209, 1995
Evans WE, Hutson PR, Stewart CF, Cairnes DA, Bowman WP, Rivera G, Crom WR: Methotrexate cerebrospinal fluid and serum concentrations after intermediatedose methotrexate infusion. Clin Pharmacol Ther 33: 301–307, 1983
Vassal G, Valteau D, Bonnay M, Patte C, Aubier F, Lemerle J: Cerebrospinal fluid and plasma methotrexate levels following high-dose regimen given as a 3-hour intravenous infusion in children with non Hodgkin's lymphoma. Pediatr Hematol Oncol 7: 71–77, 1990
Mahoney DH, Shuster JJ, Nitschke R, Lauer SJ, Steuber CP, Winick N, Camitta B: Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: An association with intermediate-dose intravenous methotrexate and intrathecal triple therapy-a Pediatric Oncology Group study. J Clin Oncol 16: 1712–1722, 1998
Recht L, Straus DJ, Cirrincione C, Thaler HT, Posner JB: Central nervous system metastases from non-Hodgkin's lymphoma: Treatment and prophylaxis. Am J Med 84: 425–435, 1988
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Khan, R.B., Shi, W., Thaler, H.T. et al. Is Intrathecal Methotrexate Necessary in the Treatment of Primary CNS Lymphoma?. J Neurooncol 58, 175–178 (2002). https://doi.org/10.1023/A:1016077907952
Issue Date:
DOI: https://doi.org/10.1023/A:1016077907952