Abstract
Aim
In several commonly used regimens, chemotherapy doses are split across different days of the cycle. We aimed to determine the feasibility of growth factor support with once-per-cycle pegfilgrastim in this setting.
Methods
This phase II study in breast cancer patients assessed the utility of a single 6 mg subcutaneous dose of pegfilgrastim administered on day 9 of an intravenous (IV) “split” CMF (cyclophosphamide 600 mg/m2, methotrexate 40 mg/m2 and 5-fluorouracil 600 mg/m2) chemotherapy regimen administered on days 1 and 8 and repeated every 28 days for 6 cycles.
Results
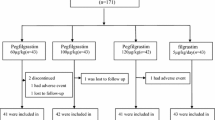
Fifty-eight patients were enrolled, with 49 completing the study. For the primary endpoint, 48 patients (83%) received ≥85% of the relative dose intensity (RDI) of chemotherapy over all 6 cycles (95% confidence interval [CI], 71–91%). Across all chemotherapy cycles, 41 patients (71%) received all scheduled cycles on time and most patients (n=49, 84%) received ≥85% of the planned dose of all chemotherapy agents in all cycles. In total, 295/319 cycles (92%) were delivered on schedule and ≥85% of the planned dose of all chemotherapy agents were administered in 309/319 cycles (97%). Febrile neutropenia was reported in only 2 patients (3%). There were no grade 4 adverse events related to pegfilgrastim.
Discussion
Day 9 pegfilgrastim administration was well tolerated and provided effective protection against neutropenia in patients receiving IV CMF on days 1 and 8, allowing chemotherapy to be delivered on time and at the scheduled dose in most patients.
Similar content being viewed by others
References
Budman DR, Berry DA, Cirrincione CT et al (1998) Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst 90:1205–1211
Leonard RC, Miles D, Thomas R, Nussey F (2003) Impact of neutropenia on delivering planned adjuvant chemotherapy: UK audit of primary breast cancer patients. Br J Cancer 89:2062–2068
Bonadonna G, Valagussa P, Moliterni A et al (1995) Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer. The results of 20 years of follow-up. N Engl J Med 332:901–906
Mackey JR, Cantin J, Chang J et al (2001) Role of hematopoietic growth factors in the prevention of neutropenic complications for breast cancer patients treated with docetaxel, doxorubicin, and cyclophosphamide (TAC): results of a randomized double-blind phase III trial -BCIRG 004. Proc Am Soc Clin Oncol 20:10a (Abstract 38)
Pettengell R, Gurney H, Radford JA et al (1992) Granulocyte colony-stimulating factor to prevent dose-limiting neutropenia in non-Hodgkin’s lymphoma: a randomized controlled trial. Blood 80:1430–1436
Trillet-Lenoir V, Green J, Manegold C et al (1993) Recombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapy. Eur J Cancer 29A:319–324
Meropol NJ, Miller LL, Korn EL et al (1992) Severe myelosuppression resulting from concurrent administration of granulocyte colony-stimulating factor and cytotoxic chemotherapy. J Natl Cancer Inst 84:1201–1203
Rowinsky EK, Grochow LB, Sartorius SE et al (1996) Phase I and pharmacologic study of high doses of the topoisomerase I inhibitor topotecan with granulocyte colony-stimulating factor in patients with solid tumors. J Clin Oncol 14:1224–1235
Ackland SP, Anton A, Breitbach GP for the HEPI 013 study group (2001) Dose-intensive epirubicinbased chemotherapy is superior to an intensive intravenous cyclophosphamide, methotrexate, and fl uorouracil regimen in metastatic breast cancer: a randomized multinational study. J Clin Oncol 19:943–953
Link BK, Budd GT, Scott S for the Oncology Practice Pattern Study Working Group (2001) Delivering adjuvant chemotherapy to women with early-stage breast carcinoma: current patterns of care. Cancer 92:1354–1367
Zamboni WC (2003) Pharmacokinetics of pegfilgrastim. Pharmacotherapy 23:9S–14S
Green MD, Koelbl H, Baselga J for the International Pegfilgrastim 749 Study Group (2003) A randomized, double-blind, multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 14:29–35
Holmes FA, O’shaughnessy JA, Vukelja S et al (2002) Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol 20:727–731
Siena S, Piccart MJ, Holmes FA et al (2003) A combined analysis of two pivotal randomized trials of a single dose of pegfilgrastim per chemotherapy cycle and daily filgrastim in patients with stage II–IV breast cancer. Onc Rep 10:715–724
Wolf T, Densmore JL (2004) Pegfilgrastim use during chemotherapy: current and future applications. Curr Hematol Rep 3:419–423
Aapro MS, Cameron DA, Pettengell R et al (2006) EORTC guidelines for the use of granulocytecolony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer 42:2433–2453
Riedel R, Garst J, Dunphy F et al (2003) Dosedense carboplatin/vinorelbine with pegfilgrastim support for the treatment of thoracic malignancies. Proc Am Soc Clin Oncol 22:703 (Abstract 2826)
Fortner BV, Schwartzberg LS, Ashley J et al (2003) A single pegfilgrastim dose per cycle maintains neutrophil counts after docetaxel (D) and gemcitabine (Gem) chemotherapy for advanced non-small cell lung cancer. Proc Am Soc Clin Oncol 22:696 (Abstract 2799)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mattioli, R., Gridelli, C., Castellanos, J. et al. Use of pegfilgrastim support on day 9 to maintain relative dose intensity of chemotherapy in breast cancer patients receiving a day 1 and 8 CMF regimen. Clin Transl Oncol 11, 842–848 (2009). https://doi.org/10.1007/s12094-009-0453-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-009-0453-4