Abstract
Introduction
In adult patients, most inguinal hernias are treated by implanting a prosthetic mesh. To prevent mesh dislocation and thus recurrence, different types of fixation have been proposed. In contrast to penetrating fixation known to cause acute chronic pain, adhesive fixation is becoming increasingly popular as it reduces markedly the risk of injury and chronic pain. Apart from the biological sealants (e.g., fibrin glue), surgical adhesives include a group of synthetic glues and genetically engineered protein glues. For example, cyanoacrylate is used in various medical and veterinary indications due to its fast action, excellent bonding strength and low price.
Objective
The main objective of this paper was to communicate positive results obtained using n-butyl-cyanoacrylate glue to fix prosthetic meshes in over 1,300 TAPP repairs of primary and recurrent inguinal hernias. The secondary objective was to highlight the rationale (e.g., safety) for using non-fibrin based glue in this type of procedure.
Method
We present the in vitro and in vivo data necessary for the approval of n-butyl cyanoacrylate Histoacryl® glue. We use an equivalent glue, Glubran-2®, to fix prosthetic meshes in 1,336 laparoscopic TAPP repairs.
Results
Standardized tests to detect sensitization, irritation, genotoxicity or systemic toxicity demonstrated the safety and biocompatibility of Histoacryl®, which met all requirements, including those of ISO 10993. Histological long-term studies in rabbits yielded results comparable to routine suture fixations, with full integration of the mesh into the abdominal wall. The clinical results showed the following advantages: fast application of the glue, reduced postoperative pain, 0.0% infection rate, continuously low recurrence rate and shorter hospital stay. No adverse effects and no complaints were recorded.
Conclusion
The experimental and clinical data demonstrate the safe use and the excellent cost-benefit ratio of n-butyl cyanoacrylate compared with other techniques of mesh fixation.
Similar content being viewed by others
Introduction
Scientific background
Cyanoacrylate is the generic name of a group of fast-acting adhesives such as ethyl-2-cyanoacrylate, n-butyl cyanoacrylate, 2-octyl cyanoacrylate sold under various trade names like Histoacryl®, Indermil®, Dermabond® or Glubran®. Cyanoacrylate is an acrylic resin that polymerizes exothermically in the presence of water, especially with hydroxide ions, joining the bonded surfaces in 5–6 s and reaching the final stage in 60 s. It bonds body tissue excellently and shows bacteriostatic effects. The film of glue is eliminated by hydrolytic breakdown, a process whose duration varies according to tissue type and quantity of glue applied. Due to these properties, it has been used in sutureless surgery since the early 1970s. n-Butyl cyanoacrylate, known as Enbucrilate, is a butylester of 2-cyano-2-propenoic acid.
In medical and veterinary applications, n-butyl- and octyl-cyanoacrylate are used commonly and in general do not cause pain. Butyl esters provide stronger but more rigid bonds, octyl esters weaker but more flexible bonds, with much slower degradation due to the longer side chain. Since the 1970s, butyl cyanoacrylate has been used as a medical adhesive, or as hemostatic or embolic agent to repair lacerations, to close the skin in pediatric surgery, to obliterate the processus vaginalis in congenital inguinal hernias, to control hemostasis of parenchymal organs, to perform aortic and thoracic surgery, to treat arteriovenous malformations, or bleeding gastric varices, and to carry out interventional radiology and vascular neuroradiology [1–4].
Clinical background
Preamble
The recurrence rate of preperitoneal mesh hernia repair would approach zero if a correctly implanted mesh remained in place.
Since the introduction of prosthetic meshes in the 1960s, a major concern has been to prevent dislocation of the mesh before it is incorporated and stabilized in its original position.
Renee Stoppa—one of the pioneers of modern herniology—has demonstrated in his GPRVS (giant prosthetic reinforcement of the visceral sac) that a large prosthetic mesh placed in the preperitoneal space reduced the risk of recurrence even without excessive fixation [5]. Intra-abdominal pressure helps to keep the mesh in place until it is definitively fixed by scar tissue.
In the early 1990s, laparo-endoscopic surgeons started imitating the successful Stoppa procedure in the form of TEP and TAPP. However, they found the extent of dissection of the preperitoneal space recommended by Stoppa to be too large, so they reduced the dissection and used smaller meshes but stronger fixation. Nevertheless, they could not prevent mesh dislocation being one of the most frequent causes of recurrence. Various tacking devices, staplers and suturing techniques were developed to overcome this problem. During the same period, two new anatomic areas of the groin were delineated: the triangle of doom and the triangle of pain. The vulnerable structures in these anatomic areas made penetrating fixation hazardous due to the risk of vascular injury or chronic pain. Yet the non-fixed lateral lower mesh quadrant was obviously prone to dislocation. Thus, the surgeons began using bigger (or wider) meshes, without cutting them, and fixing them with adhesives [6–10].
In 2001, Katkhouda et al. [11] proposed to use fibrin sealants to fix the mesh. Several experienced surgeons or specialized centers went even further, they used bigger meshes and did not fix them at all [12–19]. Some investigators observed a marked increase in recurrence [20], but others noted only a slight increase, especially since the introduction of the light meshes [21].
After a decade attempting to cope with recurrence, the present decade must address chronic pain. Although the pain scores after laparoscopic repairs are lower than after open repair [22, 23], the penetrating fixation used may cause acute or chronic pain and increases both direct and indirect costs. In their randomized study, Koch et al. [24] conclude that elimination of tack fixation reduces significantly the need for postoperative narcotic analgesia, hospital stay and postoperative urinary retention.
Lovisetto [25] reports lower incidence of postoperative neuralgia and earlier resumption of physical and social activities when comparing fibrin glue with staple fixation in TAPP repair.
Since 2001, to prevent both recurrence and injury causing chronic pain, one of the authors (J.F.K.) started routinely using a glue to fix meshes. In contrast to most cyanoacrylate glues used for external applications like skin wound closure, the glue used, “Glubran-2®”, a n-butyl-cyanoacrylate, is CE-certified for both external and internal use.
The following experimental and clinical evidence has been provided for the safe use of n-butyl cyanoacrylate (nBCA) in this context.
Materials and methods
Experimental approach
Biocompatibility testing
Any implant that remains in the body for more than 30 days must be subjected to tests described in the ISO 10993 Standard [26] for approval as a medical device. These tests include assessment of cytotoxicity, sensitization, irritation, systemic toxicity, genotoxicity and implantation.
For the assessment of cytotoxicity, L929 mouse fibroblasts were incubated for 48 h at 37°C with an extract of Histoacryl® in 1XMEM (single strength minimum essential medium supplemented with 5% serum and 2% antibiotics). Changes in cell morphology were examined microscopically. The original extraction medium served as a control, a sample of tin-stabilized polyvinylchloride as a positive control, and a sample of high density polyethylene as a negative control.
The sensitization test was carried out using extracts of Histoacryl® with 0.9% sodium chloride and with sesame oil [27]. Each extract was injected intradermally and patched occlusively to ten guinea pigs to induce sensitization. A second induction followed 7 days later. After a recovery period of 14 days, a challenge patch of the extract was applied. The dermal reaction was recorded 24 and 48 h after patch removal and compared to the dermal reaction induced by the original extraction medium using the same procedure.
Histoacryl® extracts with 0.9% sodium chloride and with sesame oil were injected into rabbits to test for irritation and delayed-type hypersensitivity. The skin reaction was examined immediately after injection and after 24, 48 and 72 h for erythema or oedema compared to the injection site of the original sodium chloride solution or sesame oil.
Systemic toxicity was evaluated by intravenous and intraperitoneal injection of a 0.9% sodium chloride or sesame oil extract of Histoacryl® to five mice. As control, five mice were treated with the original extraction medium. All animals were observed for signs of systemic toxicity immediately after injection and at 4, 24, 48 und 72 h post-injection.
Genotoxicity was assessed by the Ames test (a bacterial reverse mutation test) performed according to the OECD guidelines: Test No. 471 [28]. In summary, the ability of Histoacryl® extracts in a 95% ethanol solution or a 0.9% sodium chloride solution to induce reverse mutations was evaluated either in the presence or absence of mammalian microsomal enzymes at the histidine locus of five strains of Salomella typhimurium, or the tryptophan locus of Escherichia coli. Molten top agar was supplemented with histidine-biotin or tryptophan for S. typhimurium or E. coli strains, respectively. This addition allowed the bacteria on the plate to undergo several divisions to produce a faint background lawn, which was examined under a darkfield colony counter.
The original extraction medium was used as negative control, and sodium azide, methyl methanesulfonate, 2-aminoanthracene, benzo[a]pyrene and 2-nitrofluorene and acridine mutagen ICR-191 as positive control.
Animal study
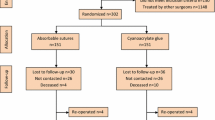
The study was carried out on 48 female SPFalbino rabbits [Chbb:HM(SPF)-Kleinrusse], weighing 2,100–3,300 g, from Charles River Deutschland (Kisslegg, Germany), according to the requirements of the OECD “Good laboratory practice” guidelines (GLP). The animals were allowed 5 days to acclimate, and were kept under standard laboratory conditions (filtered air, temperature 20°C ± 3°C, relative humidity 30–70%, 12 h light, 12 h dark, food and water ad libitum).
Surgical procedure
Surgery was carried out under aseptic conditions. Anesthesia was induced by intramuscular injection of 40 mg/kg Ketamine and 6 mg/kg Xylazine. After shaving and disinfecting the skin, a midline incision was made, and the subcutis dissected to expose the linea alba. A 35 mm × 35 mm light-weight hernia mesh (Optilene LP, large pores of 1 mm, weight 36 g/m2, thin and flexible) was placed centred on the fascia of the abdominal wall muscles. The mesh was fixed with 10 μl Histoacryl or with an absorbable suture material in each corner (MonoPlus USP 3-0, single stitch). After 3, 10, 30, 90, 180 and 360 days, the adhesion of the mesh to the fascia was evaluated qualitatively, and the implantation site examined macroscopically and histologically. For each time point, the abdominal wall of four rabbits, together with the mesh implant was taken from the abdominal wall as a whole and fixed immediately in 4% formaldehyde. The samples were examined macroscopically, and relevant parts embedded in paraffin. Sections were cut on a microtome and fixed on a microscope slide. The paraffin was removed by Roti-Histol®, and the samples rehydrated by isopropanol, successively in ethanol 96, 80, 70, 50% and distilled water.
The samples were stained with hematoxylin and eosin (H&E), embedded in xylol-carbol and covered by a glass coverslip.
Clinical approach
Between December 2001 and January 2010, we treated 1,467 inguinofemoral hernias using the TAPP technique. Initially, we used Vypro-2® mesh (329) and TiMesh® (28).
Since 2004, we have routinely used Ultrapro® mesh (1,110) for its convenient properties.
We fixed 1,336 of these 11 × 13 cm–10 × 15 cm meshes with only a few drops of nBCA in all four quadrants. As the glue was temporarily unavailable, we tacked 93 meshes with EMS alone (Endopath®, Endoscopic Multifeed Stapler, Ethicon Endo-Surgery).
In 38 primary inguinal hernias after retropubic prostatectomy or recurrent hernias after preperitoneal mesh repair, we thought it was too risky to use only a light-weight mesh and glue fixation, according to our new concept. To dissect the preperitoneal space was more difficult, and the fibroblastic reaction may be delayed in the scar, thus we expected higher risk of mesh dislocation. We used a heavy-weight mesh with higher flexural rigidity (Prolene®) in this group and fixed it with EMS in addition to glue.
In all cases, 1 cc nBCA was sufficient to fix both the unilateral and bilateral mesh. The remaining glue was often used to adapt the peritoneal flaps in order to facilitate final peritoneal closure with a USP 3-0 polydioxanon running suture.
Technique
Once the preperitoneal space is dissected, and all hernia sacs and preperitoneal fat prolapses are reduced, the mesh is secured to the abdominal wall. The glue application catheter (Cavafix®, Braun, Melsungen, Germany) is introduced through the skin incision of T2 (pararectal right) (Figs. 1, 2). It must be kept dry as long as possible to prevent the nBCA from polymerizing in contact with the humid tissue. An insulin syringe is used to facilitate dropwise distribution of the glue (Fig. 3). Two 5 mm graspers allow control of glue application (Fig. 4). An experienced assistant is able to apply over 20 “drops” from a 1 cc glue content. The glue is ejected from the applicator by expelling the air. The drops of glue are placed on the mesh, which is pressed gently against the underlying tissue, above the symphysis pubis, above the pubic arch medial of femoral vein, over the triangle of doom and triangle of pain, at the level of the superior iliac spine and medial and lateral of the inferior epigastric vessels. The peritoneal edges are then sutured, the CO2 is evacuated, and any trocar incision bigger than 5 mm is closed in layers. Finally, the wounds are infiltrated with long-acting local anesthetic.
During the procedure, the tip of the catheter may gradually clog up, causing spitting or complete obstruction. However, catheter patency can be ensured by simple measures. If one understands the behavior of the glue and the underlying tissue, fixation becomes a clear procedure: the glued spots should be as small as possible, as tissue in-growth in these areas is limited until the glue degrades.
Results
Experimental approach
To prove the safety and biocompatibility of nBCA, several genotoxicity, sensitization, irritation and systemic toxicity tests and an implantation study were carried out on Histoacryl® over 4 or 12 weeks according to ISO 10993 requirements for internal use. The Histoacryl® extracts were non-mutagenic and non-irritating with no evidence of systemic toxicity, and passed all the tests prescribed by ISO 10993. Additionally, the implanted material showed good local tolerance with only slight neo-vascularisation and degradation, and no cell or tissue degeneration.
Furthermore, a long-term implantation study with fixed meshes was conducted in rabbits over a period of 360 days. All animals survived until the end of the study. All skin incisions showed a normal healing process. At each time point, four animals were investigated in the Histoacryl® group and four in the MonoPlus® group. Macroscopic and microscopic analysis showed similar results for both groups, thus only results for Histoacryl® are presented here in detail. All meshes were found in the implantation site, only one in the Histoacryl® group was dislocated by a few centimeters. All meshes adhered to the abdominal wall at every time point. This shows the effectiveness of the fixation method. The histological analysis at different time points yielded the following results:
3 and 10 days postoperatively
The meshes were attached to the muscles without visible tissue reaction. After 10 days, they were covered with yellowish tissue by up to 5–50%. No difference could be seen between implants fixed by glue or suture.
As expected at this stage of wound healing, histological images revealed a slight-to-moderate inflammation. Diffuse infiltration of mixed inflammatory cells (granulocytes, lymphocytes and macrophages) and a multifocal vacuolar cavernous fibrinous fibroblastic tissue reaction were identified, including a slight to medium grade leucocytostasis. After 10 days, multinuclear giant cells emerged sporadically as well as slight-to-moderate formation of directed vascularized fibroangioblastic connective tissue.
30 days postoperatively
In all animals, the mesh was attached to the fascia and completely integrated into the connective tissue. Histology showed slight-to-moderate diffuse infiltration of inflammatory cells (lymphocytes and macrophages, few granulocytes). Basophilic or eosinophilic material was enclosed, reflecting a slight-to-moderate tissue reaction and formation. Partial multinuclear giant cells were observed. No difference was found, either quantitatively or qualitatively, between suture and Histoacryl®. No signs of decomposition or resorption of the glue were detectable (Fig. 5).
180 days postoperatively
All meshes had adhered superficially to the muscle tissue. In the area of the mesh, a very slight formation of fibrous and collagenous connective tissue with low nucleated cell counts and a slight infiltration of inflammatory cells (mostly lymphocytes and few giant cells) were detected. Tissue adhesive was not visible. The changes were macroscopically and histologically equivalent with both suture and Histoacryl®.
360 days post-operatively
Few variable alterations and a slight formation of white opaque tissue with complete attachment to the sample were observed. All samples adhered superficially to the muscle tissue. The surface of the meshes was covered extensively or completely with white opaque tissue.
Histology showed a slight formation of non-cellular fibrous connective tissue and slight infiltration of inflammatory cells, including mononuclear cells (lymphocytes, plasma cells, macrophages) and focal accumulation of multinuclear giant cells. The glue was detected as sporadic basophilic material in phagocytosis. No difference was found, either quantitatively or qualitatively, between suture and Histoacryl® (Fig. 6, Table 1).
Histoacryl® fixed meshes after 360 days: mesh residue (up arrows), slight formation of fibrous connective tissue consisting of collagen fibers and few cells (triangles), giant cells (up open arrow), isolated chunky slightly basophilic amorphous particles under phagocytosis (probably residue of glue, square)
Clinical approach
Between December 2001 and January 2010, 1,467 TAPP repairs were performed. In 1,336 cases, the meshes were fixed with Glubran-2 only, and 131 meshes either stapled with EMS or stapled in combination with glue.
In both unilateral and bilateral repairs, 1 cc nBCA glue or less was sufficient to fix the mesh in all cases. During our learning curve, we grew confident and used less glue drops on the mesh and used the rest to adapt the peritoneal flaps before final closure with a USP 3-0 polydioxanon running suture.
Over 98.5% of the patients were discharged on the following day. The remaining patients stayed longer either for social indications or prostatic pathology, but never for reasons related to the procedure.
Two to three days after patient discharge, a first telephonic feedback was obtained from 100% of patients. Ten days after surgery, 99% of the patients had been examined by the operating surgeon; 5–6 weeks postoperatively, 97% of all the patients had had either a clinical control by the operating surgeon or had given a detailed personal telephonic feedback in the absence of any symptoms or complaints.
Postoperative pain
The typical minor discomfort at the operation site in the first postoperative days has nearly disappeared since we started to use glue fixation.
Years before abandoning stapled mesh fixation, we started to close the peritoneal incision by running suture instead of stapling it. Then, the only temporary complaint concerned the T1 and T2 trocar wounds (10–12 mm). The use of a smaller T2 trocar (3 instead of 12 mm) caused another major improvement in postoperative pain perception.
In the postoperative follow-up, we recorded VAS (0–10) scores to improve pain management in all patients. But our VAS records are not complete (especially for the historical stapled control group) and pain reduction was due to different factors. Thus, we do not use these as evidence.
Handling
The most valuable property of cyanoacrylate is that it polymerizes within seconds to form a strong tissue bond. In contrast, fibrin-based glues take much more time (ca. 60 s) to set, and provide often only a weak bond that must be reinforced by spraying over the surface of the mesh. The viscosity of the glue is only slightly greater than that of water. Therefore, it must be distributed cautiously, using a grasper.
Complications
During the 98 months of using this technique, we did not observe, either intraoperatively or postoperatively, any adverse reaction that could be directly or even indirectly related to its use. The infection rate of both mesh and trocar wounds was 0.0%.
Recurrence
In a long-term follow up of a large patient collective, it is difficult to determine exactly the true recurrence rate. We are aware of eight patients with ten recurrences (0.7%). Seven patients with eight hernias were explored laparoscopically at our institution and repaired by TAPP. There were three patients in the stapled group (n = 131) with three true recurrences (2.3%) and four patients in the glue group (n = 1,336) with five recurrences (0.37%). The five recurrences in the glue group (inguinal swelling) were two true herniations due to mesh displacement and three preperitoneal fat-prolapses/missed funicular lipomas under the correctly lying mesh.
Cost
Significant cost reduction is another positive aspect of using glue. The price of 1 cc glue and the application set is 2.5 times lower than that of a tacking device.
Patient satisfaction
Patient outcomes in the first 18 months convinced us to stop using staple fixation in the daily routine and reserve it, in combination with glue, for difficult recurrences, reoperations after mesh implantation in the preperitoneal space or after radical prostatectomy.
Accidental spills
Especially when the application catheter becomes obstructed by the polymerizing glue, accidental spills of glue drops may occur (higher flow resistance at the tip and spitting). As the glue does not stick for several seconds, it can be peeled off the underlying tissue; care is indicated when removing glue from the intestinal serosa.
Additional observations
Fogging: occasionally in the past, the endoscope fogged up at the time of peritoneal closure. By pulling the endoscope back into the trocar tube the fog disappeared in seconds. This was probably due to the temperature difference resulting from the exothermic reaction in the glue polymerization. Curiously, this effect has disappeared in recent years.
Sticky instruments
Should the glue come into contact with surgical instruments or other materials, it can be removed mechanically or with acetone.
Variable efficacy of glue on different tissues and different meshes
The glue is best suited to fatty tissues and least suited to pubic bone. The three-dimensional structure of different mesh types seems to influence the bond quality. Attempting to fix two overlapping meshes to the underlying tissue in the midline is a frustrating exercise. Therefore we fix the most medial part of the first mesh, and the second mesh at the level where the overlap begins.
Discussion
The extended toxicological and implantation studies prove the good local tolerance and biocompatibility of Histoacryl®. This is additionally supported by the long-term implantation of meshes fixed with Histoacryl® in comparison to absorbable suture material. For both fixation methods, formation of granulation and connective tissue shows that the implant is well tolerated. Histoacryl® seems to be absorbed almost completely after 360 days.
However, Fortelny et al. [29] do not corroborate our findings in a study on the use of Glubran-2® in Ti-Mesh fixation in 20 rats. This deserves some comments.
-
They found glue residues in all samples. But the longest follow-up was 3 months. This shows only that the degradation of the glue takes longer than 3 months.
-
Regarding tensile strength, they noted less resistance to tensile forces in the glued areas than in the adjacent areas. But this is normal since the glued areas have limited in-growth of scar tissue until the glue is fully degraded. The moral of the story is to apply the glue spot-wise.
-
In their experiment, they used too much glue per square centimeter, i.e., 0.3 ml Glubran-2® to fix 4 cm2 mesh. In contrast, we used exactly 4 × 10 μl Histoacryl®, i.e., 4 μl/cm2 compared with 75 μl/cm2 in their study. In the case of bilateral repair, we use 1.0 ml glue for 300 cm2 of mesh, i.e., max. 4 μl/cm2.Obviously, 75 μl/cm2 glue forms a thick and compact layer, making the mesh too rigid, and will definitely inhibit the in-growth of fibroblasts. This explains the non-vascularization of the mesh very well.
-
Furthermore, they found multiple micro-abscedations in all samples. But, in our series, we did not observe—experimentally or clinically—a single case of mesh or wound infection in any animal or in any patient since 1992 when we started to use the TAPP technique, or since 2001 when we started to use glue.
Clinical evaluation
It is difficult, if not impossible, to determine the real impact of only one among a complex of factors (e.g., prosthetic material, suture closure of peritoneum, monopolar coagulation, etc.) in terms of patient outcomes. We have experienced higher patient satisfaction since using glue fixation. To demonstrate reduced postoperative pain expressed in VAS scores, less analgesics or shortened hospital stay, studies would have to be carried out on smaller patient collectives, in limited time frames, and with a constant surgical team performing a standardized procedure. The higher patient satisfaction in our experience may be due to one or all of the following factors: advanced learning curve, better tissue dissection, consequent parietalization, bigger meshes, light-weight meshes, avoidance of penetrating mesh fixation, peritoneal closure with running instead of tacking suture, prudent use of monopolar cautery, infiltration of trocar wounds with long acting local anesthetic, awareness of chronic pain, better information, preparation and motivation of the patient.
The overall patient satisfaction we recorded is supported by similar findings of randomized controlled trials reported by Helbling [30], Testini [31] or Nowobilski [32]. Very low pain levels, shorter hospital stay, immediate unrestricted physical activity and shorter off-work period are the best indicators.
The sporadic cases of long-term preperitoneal mesh implantation (Figs. 7, 8) we encountered confirm the overall outcome.
General considerations
Novik et al. [33] point out that cyanoacrylate glues may stick to surgical instruments, impairing handling during the operation, and necessitating special chemical treatment for cleaning and resterilization [29]. But the glue residues can be removed mechanically or with acetone in difficult cases. There is no reason why reusable instruments should not be sterilized normally.
Costs
-
Suture fixation of the mesh is the cheapest in terms of material cost, but time consuming. The operative time cost (ca. 15 €/min) must be included in the overall cost of the operation. Three single sutures stabilize the mesh, but do not prevent the lateral caudal corner from lifting up.
-
Tacking devices are the most costly, they are rapid in application, but may cause injury and pain, and are thus not really suitable to close the peritoneal gap.
-
Fibrin glues are expensive, they are slow in application, and provide a weak and frail bond.
-
n-butyl cyanoacrylate glue is cheaper than tacking devices or fibrin glues, is fast acting and provides a strong bond; it is faster and stronger than fibrin and less painful than sutures and tacks.
Conclusions
In extended preclinical and clinical studies, n-butyl cyanoacrylate has been shown to be safe for both external and internal applications if some elementary rules are observed. The properties of this synthetic glue make it a very attractive alternative to other methods to fix the prosthetic meshes in laparoscopic hernia repair if it is used spot-wise and sparingly. We consider 6–8 drops of cyanoacrylate glue sufficient to fix a mesh of 15 × 10 cm. This amount does not impair the necessary host tissue in-growth until its complete degradation. Its positive cost-benefit ratio could make it a first choice for this indication.
References
Amiel GE, Sukhotnik I, Kawar B, Siplovich L (1999) Use of N-butyl-2-cyanoacrylate in elective surgical incisions—long term outcomes. J Am Coll Surg 189(1):21–25
Esposito C, Damiano R, Settimi A, De Marco M, Maglio P, Centonze A (2004) Experience with the use of tissue adhesives in pediatric endoscopic surgery. Surg Endosc 18:290–292. doi:10.1007/s00464-003-9032-x
Miyano G, Yamataka A, Kato Y, Tei E, Lane GJ, Kobayashi H, Sueyoshi N, Miyano T (2004) Laparoscopic injection of dermabond tissue adhesive for the repair of inguinal hernia: short- and long-term follow-up. J Pediatr Surg 39(12):1867–1870
Seewald S, Seitz U, Yang AM, Soehendra N (2001) Variceal bleeding and portal hypertension: still a therapeutic challenge? Endoscopy 33(2):126–139
Stoppa R, Henry X, Odimba E, Verhaeghe P, Largueche S, Myon Y (1980) Dacron tulle prosthesis and biological glue in the surgical treatment of incisional hernias (author’s translation). Nouv Presse Med 9(46):3541–3545
Cuschieri A (2001) Tissue adhesives in endosurgery. Semin Laparosc Surg 8:63–68
Jourdan IC, Bailey ME (1998) Initial experience with the use of N-butyl 2-cyanoacrylate glue for the fixation of polypropylene mesh in laparoscopic hernia repair. Surg Laparosc Endosc 8:291–293
Birch DW, Park A (2001) Octylcyanoacrylate tissue adhesive as an alternative to mechanical fixation of expanded polytetrafluoroethylene prosthesis. Am Surg 67:974–978
Costa HJ, Pereira CS, Costa MP, Fabri FS, Lancellotti CL, Dolci JE (2006) Comparison of butyl-2-cyanoacrylate, gelatin-resorcin-formaldehyde (GRF) compound and suture in stabilization of cartilage grafts in rabbits. Rev Bras Otorrinolaringol (Engl Ed) 72(1):61–71
Jain SK, Vindal A (2009) Gelatin-resorcin-formalin (GRF) tissue glue as a novel technique for fixing prosthetic mesh in open hernia repair. Hernia 13(3):299–304
Katkhouda N, Mavor E, Friedlander MH, Mason RJ, Kiyabu M, Grant SW, Achanta K, Kirkman EL, Narayanan K, Essani R (2001) Use of fibrin sealant for prosthetic mesh fixation in laparoscopic extraperitoneal inguinal hernia repair. Ann Surg 233:18–25
Kapiris SA, Brough WA, Royston CMS, O’Boyle C, Sedman PC (2001) Laparoscopic transabdominal preperitoneal (TAPP) hernia repair. Surg Endosc 15:972–975
Khajanchee YS, Urbach DR, Swanstrom LL, Hansen PD (2001) Outcomes of laparoscopic herniorrhaphy without fixation of mesh to the abdominal wall. Surg Endosc 15(10):1102–1107
Smith AI, Royston CMS, Sedman PC (1999) Stapled and non stapled laparoscopic transabdominal (TAPP) inguinal hernia repair. Surg Endosc 13:804–806
Spitz JD, Arregui ME (2000) Sutureless laparoscopic extraperitoneal inguinal herniorrhaphy using reusable instruments: two hundred three repairs without recurrence. Surg Laparosc Endosc Percutan Tech 10(1):24–29
Ferzli GS, Frezza EE, Pecoraro AM Jr, Ahern KD (1999) Prospective randomized study of stapled versus unstapled mesh in a laparoscopic preperitoneal inguinal hernia repair. J Am Coll Surg 188(5):461–465
Moreno-Egea A, Torralba Martinez JA, Morales Cuenca G, Aguayo Albasini JL (2004) Randomized clinical trial of fixation vs nonfixation of mesh in total extraperitoneal inguinal hernioplasty. Arch Surg 139(12):1376–1379
Ellner S, Daoud I, Gulleth Y (2006) Over five hundred laparoscopic totally extraperitoneal hernia repairs using mesh without fixation. Oral presentation (S061) at society of American gastrointestinal and endoscopic surgeons annual meeting Dallas, Texas, 26–29 April 2006
Beattie GC, Kumar S, Nixon SJ (2000) Laparoscopic total extraperitoneal hernia repair: mesh fixation is unnecessary. J Laparoendosc Adv Surg Tech A 10(2):71–73
Knook MTT, Weidema WF, Stassen LPS, van Steensel CJ (1999) Endoscopic total extraperitoneal repair of primary and recurrent inguinal hernias. Surg Endosc 13(5):507–511
Akolekar D, Kumar S, Khan LR, De Baux A, Nixon S (2008) Comparison of recurrence with lightweight composite polypropylene mesh and heavyweight mesh in laparoscopic totally extraperitoneal inguinal hernia repair: an audit of 1,232 repairs. Hernia 12:39–43
Bittner R, Sauerland S, Schmedt CG (2005) Comparison of endoscopic techniques vs Shouldice and other open non-mesh techniques for inguinal hernia repair: a meta-analysis of randomized controlled trials. Surg Endosc 19(5):605–615 (Epub 2005 Mar 28)
The EU Hernia Trialist Collaboration (2002) Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg 235:322–332
Koch CA, Greenlee SM, Larson DR, Harrington JR, Farley DR (2006) Randomized prospective study of totally extraperitoneal inguinal hernia repair: fixation versus no fixation of mesh. JSLS 10(4):457–460
Lovisetto F, Zonta S, Rota E, Mazzilli M, Bardone M, Bottero L, Faillace G, Longoni M (2007) Use of human fibrin glue (Tissucol) versus staples for mesh fixation in laparoscopic transabdominal preperitoneal hernioplasty: a prospective, randomized study. Ann Surg 245(2):222–231
International Organization for Standardization (ISO) 10993, Biological evaluation of medical devices part 1: evaluation and testing within a risk management system (ISO 10993-1:2009) and references therein. ISO, Geneva
Magnusson B, Kligman AM (1969) The identification of contact allergens by animal assay, the guinea pig maximization test. J Investig Dermatol 52:268–276
Organisation for Economic Cooperation and Development (OECD) (1997) Guidelines for the testing of chemicals, test no. 471: bacterial reverse mutation test. http://www.oecd-ilibrary.org/environment/test-no-471-bacterial-reverse-mutation-test_9789264071247-en
Fortelny RH, Petter-Puchner AH, Walder N, Mittermayr R, Oehlinger W, Heinze A, Redl H (2007) Cyanoacrylate sealant impairs tissue integration of macroporous mesh in experimental hernia repair. Surg Endosc 21:1781–1785. doi:10.1007/s00464-007-9243-7
Helbling C, Schlumpf R (2003) Sutureless Lichtenstein: first results of a prospective randomised clinical trial. Hernia 7:80–84
Testini M, Lissidini G, Poli E, Gurrado A, Lardo D, Piccinni G (2010) A single-surgeon randomized trial comparing sutures, N-butyl-2-cyanoacrylate and human fibrin glue for mesh fixation during primary inguinal hernia repair. Can J Surg 53(3):155–160
Nowobilski W, Dobosz M, Wojciechowicz T, Mionskowska L (2004) Lichtenstein inguinal hernioplasty using butyl-2-cyanoacrylate versus sutures: preliminary experience of a prospective randomized trial. Eur Surg Res 36:367–370
Novik B, Hagedorn S, Mork UB, Dahlin K, Skullman S, Dalenback J (2006) Fibrin glue for securing the mesh in laparoscopic totally extraperitoneal inguinal hernia repair: a study with a 40-month prospective follow-up period. Surg Endosc 20:462–467
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kukleta, J.F., Freytag, C. & Weber, M. Efficiency and safety of mesh fixation in laparoscopic inguinal hernia repair using n-butyl cyanoacrylate: long-term biocompatibility in over 1,300 mesh fixations. Hernia 16, 153–162 (2012). https://doi.org/10.1007/s10029-011-0887-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-011-0887-9