Abstract
Purpose
This study aims to examine granulocyte colony-stimulating factor (G-CSF) prophylaxis by cancer type, chemotherapy regimen, and cycle in a real-world setting to assess if practice conforms to clinical guidelines, which recommend G-CSF prophylaxis every cycle when a patient’s risk of febrile neutropenia (FN) is 20 % or greater, and to describe the incidence of FN among patients who discontinue pegfilgrastim (peg) prophylaxis.
Methods
The cohort was selected from administrative claims data and includes adults diagnosed with non-Hodgkin’s lymphoma (NHL) or breast cancer (BC) who began chemotherapy 2005–2010.
Results
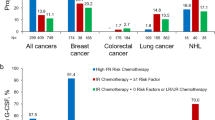
About 83.2 % of the 4,470 patients with BC treated with dose-dense doxorubicin, cyclophosphamide (ddAC), 83.6 % of 2,197 patients with BC treated with docetaxel, doxorubicin, cyclophosphamide (TAC), and about 55.6 % of the 2,722 patients with NHL treated with cyclophosphamide, doxorubicin, vincristine, with or without prednisone for 3-week cycles (CHOP-R Q3W) received peg prophylaxis in cycle 1. Among patients on these regimens who received peg prophylaxis in cycle 1 and were still on the regimen in cycle 4, about 90 % received peg prophylaxis in that cycle. Among patients with BC or NHL who discontinued G-CSF, the incidence proportion of infection or FN varied by regimen and cycle, with a range from 0 to 14 %.
Conclusions
Despite clinical guidelines recommending G-CSF prophylaxis with chemotherapy regimens with a high risk of FN, many NHL and BC patients do not receive FN prophylaxis in cycle 1. However, among patients who receive G-CSF in cycle 1 and remain on the regimen, the majority appear to continue prophylaxis as indicated.


Similar content being viewed by others
References
National Comprehensive Cancer Network (2013) NCCN clinical practice guidelines in oncology. Myeloid growth factors. V.2.2013 (www.nccn.org/professionals/physician_gls/PDF/myeloid_growth.pdf)
Lyman GH, Michels SL, Reynolds MW et al (2010) Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer 116:5555–5563
Crawford J, Dale DC, Kuderer NM et al (2008) Risk and timing of neutropenia events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Cancer Netw 6:109–118
Kuderer NM, Dale DC, Crawford J et al (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106:2258–2266
Kuderer NM, Dale DC, Crawford J, Lyman GH (2007) Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol 25:3158
Timmer-Bonte JN, deBoo TM, Smit HJ et al (2005) Prevention of chemotherapy-induced febrile neutropenia by prophylactic antibiotics plus or minus granulocyte colony-stimulating factor in small-cell lung cancer: a Dutch randomized phase III study. J Clin Oncol 23:7974
Vogel CL, Wojtukiewicz MZ, Carroll RR et al (2005) First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol 23:1178–1184
Holmes FA, O’Shaughnessy JA, Vukelja S et al (2002) Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol 20:727–731
Gerhartz HH, Engelhard M, Meusers P et al (1993) Randomized, double-blind, placebo-controlled, phase III study of recombinant human granulocyte-macrophage colony-stimulating factor as adjunct to induction treatment of high-grade malignant non-Hodgkin’s lymphomas. Blood 82:2329
Crawford J, Ozer H, Stoller R et al (1991) Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med 325:164–170
Crawford J, Caserta C, Roila F, ESMO Guidelines Working Group (2010) Hematopoietic growth factors: ESMO clinical practice guidelines for the applications. Ann Oncol Suppl 5:v248–v251
Aapro MS, Bohlius J, Cameron DA, European Organisation for Research and Treatment of Cancer et al (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47:8–32
Smith TJ, Khatcheressian J, Lyman GH et al (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24:3187–3205
von Minckwitz G, Kümmel S, du Bois A, German Breast Group et al (2008) Pegfilgrastim +/− ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer. Results from the GEPARTRIO study. Ann Oncol 19:292–298
Martín M, Lluch A, Seguí MA et al (2006) Toxicity and health-related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and cyclophosphamide (FAC): impact of adding primary prophylactic granulocyte colony stimulating factor to the TAC regimen. Ann Oncol 17:1205–1212
Aarts MJ, Peters FP, Mandigers CM et al (2013) Primary granulocyte colony-stimulating factor prophylaxis during the first two cycles only or throughout all chemotherapy cycles in patients with breast cancer at risk for febrile neutropenia. J Clin Oncol 31:4290–4296
Health Insurance Portability and Accountability Act (HIPAA) of 1996, 42 USC §1320-d2
US Dept of Health & Human Services (USDHH). Public welfare—protection of human subjects, 45 CFR 46. Effective July 14, 2009. (http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html)
National Comprehensive Cancer Network (2012) NCCN clinical practice guidelines in oncology. Breast cancer. V.3.2012 (http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf)
National Comprehensive Cancer Network (2012) NCCN Clinical practice guidelines in oncology. Non-Hodgkin’s lymphomas. V.3.2012 (http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf)
Tilly H, Vitolo U, Walewski J, da Silva MG, Shpilberg O, André M, Pfreundschuh M, Dreyling M, ESMO Guidelines Working Group (2012) Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):vii78–vii82
Aebi S, Davidson T, Gruber G, Cardoso F, ESMO Guidelines Working Group (2011) Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 22(Suppl 6):vi12–vi24
Baker J, McCune JS, Harvey RD 3rd, Bonsignore C, Lindley CM (2000) Granulocyte colony-stimulating factor use in cancer patients. Ann Pharmacother 34:851–857
Swanson G, Bergstrom K, Stump E, Miyahara T, Herfindal ET (2000) Growth factor usage patterns and outcomes in the community setting: collection through a practice-based computerized clinical information system. J Clin Oncol 18:1764–1770
Du XL, Lairson DR, Begley CE, Fang S (2005) Temporal and geographic variation in the use of hematopoietic growth factors in older women receiving breast cancer chemotherapy: findings from a large population-based cohort. J Clin Oncol 23:8620–8628
Naeim A, Henk HJ, Becker L, Chia V, Badre S, Li X, Deeter R (2013) Pegfilgrastim prophylaxis is associated with a lower risk of hospitalization of cancer patients than filgrastim prophylaxis: a retrospective United States claims analysis of granulocyte colony-stimulating factors (G-CSF). BMC Cancer 13:11
Weycker D, Malin J, Barron R, Edelsberg J, Kartashov A, Oster G (2012) Comparative effectiveness of filgrastim, pegfilgrastim, and sargramostim as prophylaxis against hospitalization for neutropenic complications in patients with cancer receiving chemotherapy. Am J Clin Oncol 35:267–274
Rajan SS, Stearns SC, Lyman GH, Carpenter WR (2011) Effect of primary prophylactic G-CSF use on systemic therapy administration for elderly breast cancer patients. Breast Cancer Res Treat 130:255–266
Tan H, Tomic K, Hurley D et al (2011) Comparative effectiveness of colony-stimulating factors for febrile neutropenia: a retrospective study. Curr Med Res Opin 27:79–86
Heaney ML, Toy EL, Vekeman F, Laliberte F, Dority BL, Perlman D, Barghout V, Duh MS (2009) Comparison of hospitalization risk and associated costs among patients receiving sargramostim, filgrastim, and pegfilgrastim for chemotherapy-induced neutropenia. Cancer 115:4839–4848
Morrison VA, Wong M, Hershman D, Campos LT, Ding B, Malin J (2007) Observational study of the prevalence of febrile neutropenia in patients who received filgrastim or pegfilgrastim associated with 3–4 week chemotherapy regimens in community oncology practices. J Manag Care Pharm 13:337–348
Chen-Hardee S, Chrischilles EA, Voelker MD, Brooks JM, Scott S, Link BK, Delgado D (2006) Population-based assessment of hospitalizations for neutropenia from chemotherapy in older adults with non-Hodgkin’s lymphoma (United States). Cancer Causes Control 17:647–654
Caggiano V, Weiss RV, Rickert TS, Linde-Zwirble WT (2005) Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer 103:1916–1924
Weycker D, Sofrygin O, Seefeld K, Deeter RG, Legg J, Edelsberg J (2013) Technical evaluation of methods for identifying chemotherapy-induced febrile neutropenia in healthcare claims databases. BMC Health Serv Res 13:60
Acknowledgments
We thank Dr. Xiaoyan Li and Dr. Hairong Xu (Amgen Inc., Thousand Oaks, CA, USA) and Dr. Derek Weycker and Dr. Alex Kartashov (Policy Analysis Inc., Brookline, MA, USA), who assisted with diagnosis and drug codes and other aspects of data programming, and Jan Lethen, Lori Cyprien, Vincent Jones, and Jason Yuan (Amgen Inc., Thousand Oaks, CA, USA) who provided independent review of the computer programming.
Conflict of interest
All authors are employed by Amgen Inc., Thousand Oaks, CA, USA, which sponsored this research. The authors had full control of the dataset.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Langeberg, W.J., Siozon, C.C., Page, J.H. et al. Use of pegfilgrastim primary prophylaxis and risk of infection, by chemotherapy cycle and regimen, among patients with breast cancer or non-Hodgkin’s lymphoma. Support Care Cancer 22, 2167–2175 (2014). https://doi.org/10.1007/s00520-014-2184-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2184-5