Abstract
Introduction
Computed tomography (CT)-guided coeliac plexus neurolysis (CPN) is considered effective at controlling pain in patients with intra-abdominal malignancies. The primary objective was to correlate pain outcomes with the spread of neurolytic solution in the coeliac area and to evaluate the predictive value for the spread of injectate for pain outcomes and side effects.
Methods
Blinded CT scans were reviewed. The coeliac area was divided into nine quadrants. Assessors evaluated quadrants according to contrast spread, needle tip position, and the contact between the injectate and other organs and plexuses. Efficacy of CPN and complications were estimated.
Results
In 54.9% there was complete spread of the neurolytic in the coeliac area with no correlation between pain relief and spread of injectate. In 85% the neurolytic had contact with viscera with no correlation with pain relief or complications. There was no correlation between needle tip position and spread of the neurolytic and contact of the neurolytic with viscera. In 71.6% the injectate was found to have spread into “other” plexuses. In 13.3% hampered spread of the injectate was observed. There was no correlation between patterns of injectate spread and pain relief, pain relief and spread of injectate in any particular quadrants, and expected and documented post-procedural pain scores.
Conclusions
Based on the spread of contrast medium clinicians can neither correctly anticipate the pain relief or post-procedural NRS, nor the duration of pain relief and complications. It is not essential to have the perfect sickle-shaped spread of the injectate for adequate pain control.
Plain language summary
CT-guided coeliac plexus neurolysis is considered effective at controlling pain in patients with intra-abdominal malignancies. Based on the spread of contrast medium clinicians can neither correctly anticipate the pain relief or post-procedural NRS, nor the duration of pain relief and complications. It is not essential to have the perfect sickle-shaped spread of the injectate for adequate pain control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Computed tomography (CT)-guided coeliac plexus neurolysis (CPN), a percutaneous interventional technique, is commonly considered effective at controlling background pain and breakthrough cancer pain in patients with intra-abdominal malignancies. |
The primary objective of this present retrospective evaluation was to correlate pain outcomes in patients with intra-abdominal malignancies with the spread of neurolytic solution in the coeliac area and to evaluate the predictive value for the spread of contrast medium regarding pain relief, duration of pain relief, pain intensity after intervention, and side effects after CPN. |
What was learned from the study? |
Based on the spread of contrast medium in the coeliac area, the needle tip position in relation to the coeliac trunk, the patterns of injectate spread, and the calculated area of injectate, clinicians can neither correctly anticipate the pain relief or post-procedural NRS, nor the duration of pain relief after the procedure. |
Given the potential contact between the neurolytic solution and intra-abdominal organs or plexuses it is not possible for clinicians to predict different side effects or complications correctly. |
Incomplete spread of the neurolytic solution in the coeliac area could lead to good pain relief. Therefore, it is not essential to have the perfect sickle-shaped spread of the neurolytic solution in CPN in palliative patients with intra-abdominal malignancies for adequate pain control. |
Introduction
Computed tomography (CT)-guided coeliac plexus neurolysis (CPN), a percutaneous interventional technique, is commonly considered effective at controlling background pain and breakthrough cancer pain (BTcP) in patients with intra-abdominal malignancies [11, 19, 26]. The coeliac plexus (one of the largest autonomic plexuses) is located cranial to the anterior and lateral surface of the aorta, next to the origin of the superior mesenteric and renal arteries and the coeliac trunk, dorsally to the pancreas and stomach [9]. Located in retroperitoneal fat and therefore in the retroperitoneal space, it is flanked by the diaphragmatic crura, and composed of two large paired ganglia right and left from the origin of the coeliac trunk, and many smaller ganglia and nerve fibres [9, 26]. Preganglionic sympathetic efferent nerve fibres originating from the lower thoracic and upper lumbar ganglia, the posterior vagal trunk of the oesophageal plexus and the splanchnic nerves join the coeliac plexus together with preganglionic para-sympathetic efferent fibres from the posterior vagal trunk [9]. Visceral afferent fibres providing nociceptive stimuli from the distal oesophagus to the colon pass through the coeliac plexus and thus make the coeliac plexus a veritable target for controlling pain from upper abdominal organs [19, 26].
The neurolytic coeliac plexus block can be performed under multidetector computed tomography guidance using an anterior para-aortic [11, 19, 22], a bilateral posterior para-aortic [2, 26], a posterior transaortic [11, 13], or a trans-intervertebral disc approach [10, 26]. Given that patient anatomy and the needle tip are clearly visible, complication rates are low [11, 18, 19, 26].
Studies have shown that in patients with intra-abdominal malignancies CPN may provide good pain relief, increase quality of life and reduce opioid therapy, although different volumes of neurolytic solutions have been used [26, 30].
Furthermore, it has been shown that spread of neurolytic solution in the coeliac area is crucial [5, 6]: the success of CPN and the guarantee of long-lasting pain relief depends on the adequate and complete spread of the neurolytic solution in the coeliac area. In addition, one should expect poor pain relief when only parts of the coeliac area are reached by the injectate.
The primary objective of this present retrospective evaluation, which stems from an earlier investigation of our group [19], was to correlate pain outcomes in patients with intra-abdominal malignancies with the spread of neurolytic solution in the coeliac area and to evaluate the predictive value for the spread of contrast medium regarding pain relief, duration of pain relief, pain intensity after intervention, and side effects after CPN.
Methods
Compliance with Ethics Guidelines
This retrospective study was approved by the Ethics Committee of Carinthia (S2021-32, 20th November 2021). The requirement for written informed consent was waived by the Ethics Committee. This study was conducted according to the Helsinki declaration and IASP’s guidelines for pain research in animals and humans, and authorized by the hospital general management.
Setting
In a 9-year period, 52 patients with intra-abdominal malignancies underwent diagnostic coeliac plexus block (CPB) and/or CPN for pain control at the general hospital Klagenfurt am Wörthersee. These patients were identified using our clinical data retrieval system. Hospital charts of these patients were reviewed to extract demographic data and baseline clinical characteristics. Patients with incorrect medical records or ambiguous documentation and outcome measures were not included in this evaluation.
Procedure
The technique of coeliac plexus block and neurolysis used was as follows [19]: using a standardized protocol and technique, all CPB and CPN were performed by one physician (R.L.) only, who is an expert in this field with 25 years of experience in this technique. No premedication and no sedation during the procedure was given to prevent potential hypotensive events. Patients were positioned in a supine position on the CT table, and an anterior approach [11, 26] to the coeliac plexus was planned.
Preliminary upper abdomen computed tomography with a slice thickness of 5 mm was initially obtained to identify the coeliac trunk and the superior mesenteric artery, and the depth from skin to the needle tip target was calculated. The intervention was carried out with a layer thickness of 4.8 mm, which corresponds to a range of 9.6 mm in a threefold division.
After skin anaesthesia with 2% lignocaine, a single needle (Sterican® G23 × 3 1/8″, Ø 0.60 × 80 mm, B. Braun Austria Ges.m.b.H; Chiba-needle G22, Ø 0.7 × 150 mm, Angiomed GmbH & Co. Medizintechnik KG) was inserted through the anterior abdominal wall into the retro-pancreatic space [27]. The needle tip was placed in midline just anterior to the aorta cephalad to the root of the coeliac trunk. When organ puncture (e.g. liver, stomach, colon) could not be avoided the shortest needle path was chosen to minimize possible complications.
Once the needle tip was in the targeted position, extension tubing was connected and suction was applied to detect an unexpected intravasal position.
After confirmation of the needle tip in the appropriate position, 2 ml nonionic contrast medium (Jopamidol/Jopamiro®) mixed with 10 ml of Bupivacain 0.25% (Bucain® 0.25%) was injected for diagnostic CPB.
For CPN, 2 ml nonionic contrast medium (Jopamidol/Jopamiro®) mixed with 10 ml of Bupivacain 0.25% (Bucain® 0.25%) and then 10 ml of ethanol 95% were injected during a 2-min period into the so-called antecrural (alias retroperitoneal) space. After CPN, 2 ml of 2% lignocaine was injected before and during needle removal, to prevent the neurolytic solution from tracking back and causing necrosis along the needle pass.
Evaluation
CT scans were taken during all phases of the procedure. Retrospectively, these blinded CT scans were reviewed by an anaesthetist and pain specialist (S.N-S.), an anatomist (G.F.), and a radiologist (M.F.) separately. The coeliac area was divided, in the frontal plane, into nine almost equal quadrants, with the origin of the coeliac trunk as the central structure (see Fig. 1): (1) superodexter, (2) superior, (3) superosinister, (4) dexter, (C) central, (5) sinister, (6) inferiodexter, (7) inferior, (8) inferiosinister [5, 21].
The spread of the injected solution was evaluated based on the visible presence of the contrast medium on the CT scans (see Figs. 2 and 3).
Assessors evaluated the quadrants according to contrast spread, the furthermost spread of the injectate from the needle tip to anterior, posterior, left, right, cranial, and caudal direction in mm, and any hampered spread of the injectate due to regional anatomical alterations (e.g. tumour masses).
The spread of the injectate in the coeliac area, the needle tip position according to the coeliac trunk, the distance in mm from the needle tip to the coeliac trunk, and contact between the injected solution and other organs and plexuses were also evaluated.
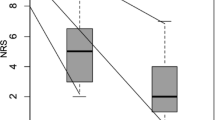
Based on this information, the assessors estimated the efficacy of CPN on pain relief on day one after CPN, the duration of pain relief, pain scores after intervention (decreasing, increasing, constant), and side effects. Pain scores were evaluated using an 11-item NRS (numerical rating scale; 0 “no pain” – 10 “worst pain imaginable). We judged pain relief of 50–100% as high, pain relief of 30–50% as intermediate, and pain relief of < 30% as low. Pain relief for > 30 days after the intervention was judged as long-lasting, pain relief for < 30 days after the intervention was judged as short-term [5].
Statistical Analysis
Descriptive statistics (Mean and Median, Minimum, Maximum, Standard deviation, SEM) were used for characterization of the observed variables. Appropriate parametric or non-parametric tests were performed. Outliers were identified by Grubbs test and excluded from the analysis. Different variables were compared using the U test (Wilcoxon Mann–Whitney test for two variables) and the H test (Kruskal–Wallis test for more than two variables). To determine the association between ordered variables the nonparametric Spearman rank correlation coefficient and its significance pS were calculated. Associations in cross-tables were investigated by Chi-square-test, and in the case of 2 × 2 tables also by exact Fisher test. To estimate the dependence of probabilities of events on influencing variables the logistic regression model was also used. Significance was defined as a p value less than 0.05, and weak significance was defined as a p value less than 0.1, but not less than 0.05. P values are reported to a maximum of three decimal places. The statistical package R version 4.0.3 resp. Hewlett–Packard RPL version 2.15 was used for all analyses.
Results
Demographic data, primary malignancies, baseline patient characteristics, pain-related data, pain outcomes, duration of pain relief, complications, and time frames from both diagnosis and onset of pain to CPN stem from an earlier investigation by our group [19], and are shown in Table 1.
Main results of spread of neurolytic solution are shown in Table 2.
Needle Tip Position in Relation to the Coeliac Trunk
In 83 procedures, the needle tip was placed midline just anterior to the aorta cephalad to the root of the coeliac trunk. In 1 CPN the needle tip was placed caudal of the coeliac trunk.
The distance between the needle tip and the coeliac trunk ranged from 1 to 45 mm (with a median of 4 mm and less than 10% over 25 mm). Increased distance to the coeliac trunk showed a trend for decreased pain relief (p = 0.16).
Needle Tip Position and Spread of Neurolytic Solution
There was no significant correlation between needle tip position and spread of the neurolytic solution to the anterior, posterior, left, and right. The furthest neurolytic solution spread to the anterior was 50 mm, to the posterior the furthest was 38 mm, to the left the furthest was 60 mm, and to the right the furthest was 57 mm. Due to missing values, the cranial and caudal spread of injectate could not be evaluated.
Spread of Neurolytic Solution in “Other” Plexuses
In 71.6% of procedures the contrast medium was found to have spread into “other” plexuses e.g. plexus hepaticus (62.5%), plexus gastricus (20.9%), left plexus suprarenalis (12.5%), right plexus suprarenalis (12.5%), plexus mesentericus superior (4.2%), plexus lienalis (4.2%), and plexus intermesentericus (2.1%). There was a statistical trend (p = 0.0996) for improved pain relief without spread of contrast medium into “other” plexuses.
There was a significant correlation (p = 0.044) between neurolytic solution spread in “other” plexuses and needle tip position in relation to the coeliac. When the distance of the needle tip from the coeliac trunk increased, “other” plexuses were less often affected.
Contact Between Neurolytic Solution and Intra-Abdominal Organs
In 85% of cases the neurolytic solution had contact with intra-abdominal organs.
In these cases, the liver (43.5%) and stomach (23.2%) were most affected, followed by the right adrenal gland (11.6%), left adrenal gland (10.1%) pancreas (4.3%), duodenum (2.9%), spleen (2.9%), transverse colon (1.4%), and left kidney (1.4%).
There was no significant correlation (p = 0.16) between contact of neurolytic solution to intra-abdominal organs and pain relief or complications during or after the intervention (p = 0.17).
There was no correlation (p = 0.12) between the needle tip position in relation to the coeliac trunk and the contact of the contrast medium with intra-abdominal organs.
Regional Anatomical Distortions
In 13.3% of the procedures hampered spread of the injectate was observed due to tumour masses. Surprisingly, there was a weakly significant correlation (p = 0.081) between better pain relief in patients and hampered spread of contrast medium. The difference in the median was 1.5 points.
Spread of Neurolytic Solution in the Coeliac Plexus
In 54.9% of procedures there was complete spread of the neurolytic solution in the coeliac area. In 39.0% of procedures the contrast medium partially reached the coeliac plexus and in 6.1% of procedures the neurolytic solution did not affect the coeliac plexus. There was a non-significant trend for slightly better pain relief in patients with complete spread of the neurolytic solution in the coeliac plexus compared with partial spread of the neurolytic solution (p = 0.18). Furthermore, there was no correlation between pain relief and spread of neurolytic solution in the coeliac plexus.
Spread of Neurolytic Solution and Pain Control
In 4.9% of the procedures the contrast medium was found only on the left, in 29.7% procedures only on the right, and in 56.8% the contrast medium was found bilateral to the aorta. In 8.6% of procedures spread of the neurolytic solution was not classifiable.
Patients with spread of the neurolytic solution bilateral to the aorta showed significantly improved pain relief compared to unilateral right contrast medium spread (p = 0.014).
Patterns of Injectate Spread
There was no significant correlation between the patterns of injectate spread based on the numbers of quadrants with neurolytic solution and pain relief.
The trend for better pain relief in patients with increased numbers of quadrants with contrast was not significant (p = 0.41).
There was no correlation between pain relief and spread of contrast medium in any particular quadrants (p = 0.87) or different combinations of quadrants with neurolytic solution (p = 0.97).
Predictive Value of Spread of Contrast Medium for Post-Procedural Pain Relief
Expected Post-Procedural Pain Scores
Based on the spread of contrast medium in the coeliac area, calculated area of injectate, and patterns of injectate spread based on the numbers of quadrants with neurolytic solution, we expected a correlation between the area with contrast medium and documented post-procedural pain scores.
Expected Pain Relief vs. Documented Pain Relief
First CPN
The expected post-procedural pain relief differed greatly from the documented post-procedural pain relief. In 9 patients there was “low” documented pain relief, but we expected “high” post-procedural pain relief. In 4 patients, we expected “low” pain relief after the procedure, but “high” post-procedural pain relief was documented.
A weak significant (p = 0.077) correlation between contrast spread to the right side and pain relief in the first CPN was shown, but there was no significant correlation (p = 0.15) between expected post-procedural NRS according to furthermost spread and the calculated area and documented post-procedural NRS after the first CPN.
Second CPN
Similar results were observed for expected and documented pain relief after second CPN: no statistically significant (p = 0.29) correlation between expected post-procedural NRS according to furthermost spread and calculated area and documented post-procedural NRS after the second CPN.
Similar to expected pain relief after the first and second CPN there was no significant correlation between expected post-procedural NRS (increasing, decreasing, constant) and documented post-procedural NRS.
Predictive Value of Spread of Contrast Medium on Expected Duration of Pain Relief vs. Documented Duration of Pain Relief
Based on spread of contrast medium in the coeliac area, calculated area of injectate, and patterns of injectate spread based on the numbers of quadrants with neurolytic solution, we estimated the duration of pain relief after CPN.
First CPN
In 63% of 41 procedures, we estimated the duration of pain relief correctly (95% CI: 0.47–0.78). This result was not significant > 0.5 and so accidental assignment is possible.
In 29 procedures long-lasting pain relief was documented and in this group we expected long-lasting pain relief in 76% of cases. In contrast, we expected long-lasting pain relief in 67% of patients, who actually had a short-term pain relief. Statistically (p = 0.4) we found that the assessor cannot correctly anticipate the duration of pain relief after the first CPN.
Second CPN
Similar to the results of the first CPN, one cannot correctly anticipate the duration of pain relief after the second CPN either (p = 0.55).
Predictive Value of Spread of Contrast Medium for Complications/Side Effects
Based on spread of contrast medium in the coeliac area, the needle tip position, contact between the neurolytic solution and intra-abdominal organs or “other” plexuses, and regional anatomic distortions, we estimated possible side effects or complications.
Out of 12 observed complications/side effects only 2 were correctly predicted. In 40 procedures without complications/side effects, 3 were incorrectly predicted. To make a statistically valid forecast of complications the percentage of predicted complications out of the observed complications should be significantly higher than the percentage of predicted complications of procedures without complications. Although we found a 10% higher rate of correctly predicted complications compared to false predictions the difference was not significant (p = 0.35).
Discussion
In patients with intra-abdominal malignancies, chronic abdominal pain (CAP) has a prevalence up to 50% [26]. Furthermore, patients with uncontrolled background pain may develop breakthrough cancer pain in up to 70% of cases, with huge impact on patients’ quality of life and disability [3].
Due to its pain transmission the coeliac plexus is a veritable target for controlling pain from upper abdominal organs. As part of a multimodal approach, CPN is a commonly used interventional pain management strategy to decrease pain, increase quality of life and reduce opioid therapy in patients with intra-abdominal malignancies [26, 30].
Back in 1979, Ward and colleagues [28] defined the coeliac artery as the most reliable landmark for locating the coeliac plexus and it has been reported that the injection site of the neurolytic solution should be cephalad to the coeliac trunk [5, 6]. Given the variations in patients’ anatomy, possible anatomic alterations by tumour masses or previous surgery or radiation therapy, clearly visible needle tip and contrast spread, CT-guidance offers several advantages. Different techniques (single needle or bilateral injections), approaches (anterior para-aortic [11, 19, 22], bilateral posterior para-aortic [2, 26], posterior transaortic [11, 13], trans-intervertebral disc [10, 26]) and patient positions (prone or supine [26]) have been described, with no clear superiority for optimal results in any of these techniques.
However, De Cicco et al. [5] reported that injections cephalad of the coeliac trunk should be performed to obtain wider spread of the injected solution. Furthermore, they divided the coeliac area in the frontal plane into four quadrants defined by a horizontal line passing caudad to the root of the coeliac trunk and by a vertical line in the midline of the ventral wall of the aorta. They showed that only complete spread of the neurolytic in the upper and lower right and left quadrants seems to guarantee optimal and long-lasting pain control. They found that when the neurolytic solution spreads to only one quadrant, poor pain relief should be expected and when fewer than 4 quadrants are reached by the neurolytic only a small percentage of patients will experience adequate pain relief.
Although the goal of CPN is complete spread of the injectate to achieve long-lasting analgesia [5], irregular spread of neurolytic solution may occur because of regional infiltration by tumour masses, or anatomic alterations by previous radiation therapy and/or surgery. In a retrospective evaluation [6] of patients whose coeliac area was infiltrated by tumour masses or distorted by previous radiation therapy or surgery, over 90% of patients showed completely hampered spread of the injectate. In these patients one can expect poor or even no pain relief when the injectate reaches only parts of the coeliac area.
We performed a CT-guided anterior approach with a targeted needle tip position in the midline just anterior to the ventral wall of the aorta cephalad to the root of the coeliac trunk.
Similar to De Cicco et al. [5] the coeliac area was divided, in the frontal plane, into 9 almost equal quadrants, with the origin of the coeliac trunk as the central structure (see Fig. 1). Although we found that spread of the neurolytic solution bilaterally of the aorta leads to improved pain relief compared to unilateral right contrast spread, we did not find any other correlation between pain relief and spread of neurolytic solution in the coeliac plexus. Furthermore, we did not observe any association between the quadrants of contrast spread and pain relief, neither in the number of quadrants reached by the neurolytic solution, nor in any particular quadrant with contrast or different combinations of quadrants with neurolytic solution. Also, in patients with complete spread of the contrast medium in the coeliac plexus there was only a trend for slightly better pain relief compared with partial spread of the neurolytic in the coeliac area.
In our evaluation we found hampered spread of the neurolytic solution due to tumour masses in 13.3% of cases. Interestingly, there was a weak significant correlation between better pain relief in patients and hampered spread of contrast medium.
Surprisingly, based on the spread of contrast medium in the coeliac area, patterns of injectate spread, and calculated area of injectate, the assessors could not correctly anticipate the pain relief or post-procedural NRS, nor expect the duration of pain relief after CPN. In addition, there was only a statistical trend for decreased pain relief with increased distance of the needle tip to the coeliac trunk.
Our findings stand in contrast to the findings of De Cicco et al. [5, 6] who reported that only complete neurolytic spread in the coeliac area can guarantee long-lasting analgesia [5] and secondly that the decision to perform CPN must be based on the anatomic conditions of the coeliac area in each patient [6].
A possible explanation is that less advanced cancer infiltration, which indeed is connected with pain, but is mostly visceral without a multifactorial component, may be more easily suppressed by CPN [23] and thus incomplete neurolytic spread in the coeliac area or even hampered spread of the neurolytic solution could lead to good and long-lasting pain control.
On the other hand, pain evolution is unpredictable [16]. If other areas of neural or somatic structures are involved, the efficacy of the CPN may be decreased as the intervention aims to block the sympathetic pathways, rather than somatic afferents [16, 17]. Furthermore, pancreatic cancer-related pain is a complex condition involving many different pathophysiological mechanisms [1]: tumour location [4], autonomic plexus invasion [7], locoregional and distant tumour spread [20], malignant obstruction and intraluminal activation of pancreatic enzymes [15], small bowel distension [14], perineural tumour invasion of intrapancreatic nerves and neurogenic inflammation [12, 25].
These findings lead to the hypothesis that it is not essential to have the perfect sickle-shaped spread of neurolytic solution in CPN in palliative patients with intra-abdominal malignancies for adequate pain control. In this respect, when needle positioning is difficult, hampered spread of the neurolytic or incomplete or partial spread of the neurolytic in the coeliac area is found, clinicians may relinquish a further attempt at needle positioning or complete spread of contrast medium. Furthermore, we found that it is not essential to place the needle tip as close as possible to the coeliac trunk to achieve good pain relief.
We speculate that these findings will be a relief to both, clinicians and patients, because it does not seem not to be necessary to make multiple attempts at complete spread of contrast medium in the coeliac area for adequate pain relief. It may be possible to shorten the duration of the procedure and so increase patient acceptance and satisfaction due to a more rapid and less afflicted intervention. Furthermore, as good pain relief can be achieved form a safe distance to the coeliac trunk, the possible risk of bleeding complications may be further reduced.
If irregular spread of neurolytic solution occurs, one cannot predict the direction of the injectate spread in the coeliac area. It is possible that different pressure areas, textures and grain may prevent or promote the spread of the injectate [6]. Interestingly, there was no correlation between the needle tip position and spread of neurolytic solution in any anatomical direction or contact between the neurolytic solution and intra-abdominal organs respectively.
One typical side effect of CPN is back pain, which mostly radiates to the shoulder, resulting from neurolysis of sensory nerve fibres [11]. One can also expect transient pain at the injection site, or diarrhoea and hypotension due to sympathetic blockade [2]. Thrombosis of the coeliac trunk or vasospasm of the coeliac trunk leading to hepatic, splenic, gastric, or bowel infarction [8, 26] are rare complications, as are major bleeding [29], retroperitoneal haematoma [18], or lower extremity paralysis [24]. Complications due to poor needle placement, e.g. kidney injuries or neurological complications due to inadvertent injection of neurolytic agent are scarce due to CT-guidance [11].
Given the spread of contrast medium and contact between the neurolytic and different intra-abdominal organs or plexuses we tried to predict different kinds of side effects of CPN, as described above. Although the liver, stomach and adrenal glands with the associated plexuses where most likely reached by the contrast medium, no special complications or side effects occurred. We found that “other” plexuses were less often reached by the neurolytic when the distance of the needle tip from the coeliac trunk increased.
We mainly expected intestinal hypomotility or alteration of stomach peristalsis as possible side effects of our CPN. Although we found a 10% higher rate of correctly predicted complications compared to false predictions, the statistical difference was not significance and it was not possible for assessors to predict different side effects correctly.
There are several important limitations in our study that deserve mention. This study was retrospectively performed in a single centre and used a real-world clinical practice model. Therefore, different pain physicians planned the pain management strategies. The final decision to conduct CPN could have been influenced by their own preconceptions and may have biased the study population. One potential limitation was that after the intervention, no CT scan of the complete coeliac area was performed. So, spread of the cranial and caudal injectate could not be evaluated. It remains unclear, whether the furthermost cranial or caudal spread of the neurolytic in the coeliac area is correlated with pain outcomes. Here further studies with post-interventional CT scans are needed, although their significance in clinical routine is questionable and would increase the radiation exposure of these patients.
Despite these limitations, we feel that our large study population, our standardized procedure as well as the separate review of these blinded CT scans by an anaesthetist and pain specialist, an anatomist, and a radiologist allow us to reach valid clinically relevant conclusions.
Conclusions
As part of a multimodal approach for pain management, CPN is a safe and effective intervention that can provide long-lasting pain relief and should be offered early to patients with intra-abdominal malignancy-related abdominal pain.
Based on the spread of contrast medium in the coeliac area, the needle tip position in relation to the coeliac trunk, the patterns of injectate spread, and the calculated area of injectate, clinicians can neither correctly anticipate the pain relief or post-procedural NRS, nor the duration of pain relief after the procedure. Furthermore, given the potential contact between the neurolytic solution and intra-abdominal organs or plexuses it is not possible for clinicians to predict different side effects or complications correctly.
The finding of the present study also suggests that incomplete spread of the neurolytic solution in the coeliac area could lead to good pain relief. Therefore, it is not essential to have the perfect sickle-shaped spread of the neurolytic solution in CPN in palliative patients with intra-abdominal malignancies for adequate pain control.
References
Carvajal G. Pancreatic cancer related pain: review of pathophysiology and intrathecal drug delivery systems for pain management. Pain Physician. 2021;24:E583–94.
Cornman-Homonoff J, Holzwanger DJ, Lee KS, et al. Celiac plexus block and neurolysis in the management of chronic upper abdominal pain. Semin Intervent Radiol. 2017;34:376–86.
Cuomo A, Cascella M, Forte CA et al. (2020) Careful breakthrough cancer pain treatment through rapid-onset transmucosal fentanyl improves the quality of life in cancer patients: results from the BEST multicenter study. J Clin Med 9
D’haese JG, Hartel M, Demir IE, et al. Pain sensation in pancreatic diseases is not uniform: the different facets of pancreatic pain. World J Gastroenterol. 2014;20:9154–61.
De Cicco M, Matovic M, Balestreri L, et al. Single-needle celiac plexus block: is needle tip position critical in patients with no regional anatomic distortions? Anesthesiology. 1997;87:1301–8.
De Cicco M, Matovic M, Bortolussi R, et al. Celiac plexus block: injectate spread and pain relief in patients with regional anatomic distortions. Anesthesiology. 2001;94:561–5.
Demir IE, Ceyhan GO, Liebl F, et al. Neural invasion in pancreatic cancer: the past, present and future. Cancers (Basel). 2010;2:1513–27.
Gimeno-Garcia AZ, Elwassief A, Paquin SC, et al. Fatal complication after endoscopic ultrasound-guided celiac plexus neurolysis. Endoscopy. 2012;44(Suppl 2):UCTN:E267.
Hafferl A. Lehrbuch der topographischen Anatomie. Berlin Heidelberg: Springer; 1969.
Ina H, Kitoh T, Kobayashi M, et al. New technique for the neurolytic celiac plexus block: the transintervertebral disc approach. Anesthesiology. 1996;85:212–7.
Kambadakone A, Thabet A, Gervais DA, et al. CT-guided celiac plexus neurolysis: a review of anatomy, indications, technique, and tips for successful treatment. Radiographics. 2011;31:1599–621.
Koulouris AI, Banim P, Hart AR. Pain in patients with pancreatic cancer: prevalence, mechanisms, management and future developments. Dig Dis Sci. 2017;62:861–70.
Lieberman RP, Waldman SD. Celiac plexus neurolysis with the modified transaortic approach. Radiology. 1990;175:274–6.
Maire F, Sauvanet A. Palliation of biliary and duodenal obstruction in patients with unresectable pancreatic cancer: endoscopy or surgery? J Visc Surg. 2013;150:S27-31.
Mekaroonkamol P, Willingham FF, Chawla S. Endoscopic management of pain in pancreatic cancer. JOP. 2015;16:33–40.
Mercadante S. Commentary: interpreting data of celiac plexus block in patients with pancreatic pain: timing, patients, survival. Pain Ther. 2022
Mercadante S, Fulfaro F, Casuccio A. Pain mechanisms involved and outcome in advanced cancer patients with possible indications for celiac plexus block and superior hypogastric plexus block. Tumori. 2002;88:243–5.
Moore DC. The dreaded complications from neurolytic celiac plexus blocks are preventable! Reg Anesth Pain Med. 2004;29:377–8.
Neuwersch-Sommeregger S, Koestenberger M, Stettner H, et al. CT-guided coeliac plexus neurolysis in patients with intra-abdominal malignancy: a retrospective evaluation of 52 palliative in-patients. Pain Ther. 2021. https://doi.org/10.1007/s40122-021-00317-1.
Peixoto RD, Speers C, Mcgahan CE, et al. Prognostic factors and sites of metastasis in unresectable locally advanced pancreatic cancer. Cancer Med. 2015;4:1171–7.
Pixner T. Der anteriore plexus coeliacus block und die verteilung des applizierten volumens. Gibt es ein Limit?. In: Lehrstuhl für makroskopische und klinische Anatomie. Medizinische Universität Graz, p 144. 2013
Romanelli DF, Beckmann CF, Heiss FW. Celiac plexus block: efficacy and safety of the anterior approach. AJR Am J Roentgenol. 1993;160:497–500.
Rykowski JJ, Hilgier M. Efficacy of neurolytic celiac plexus block in varying locations of pancreatic cancer: influence on pain relief. Anesthesiology. 2000;92:347–54.
Seicean A. Celiac plexus neurolysis in pancreatic cancer: the endoscopic ultrasound approach. World J Gastroenterol. 2014;20:110–7.
Stopczynski RE, Normolle DP, Hartman DJ, et al. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014;74:1718–27.
Urits I, Jones MR, Orhurhu V, et al. A comprehensive review of the celiac plexus block for the management of chronic abdominal pain. Curr Pain Headache Rep. 2020;24:42.
Wang PJ, Shang MY, Qian Z, et al. CT-guided percutaneous neurolytic celiac plexus block technique. Abdom Imaging. 2006;31:710–8.
Ward EM, Rorie DK, Nauss LA, et al. The celiac ganglia in man: normal anatomic variations. Anesth Analg. 1979;58:461–5.
Warner NS, Moeschler SM, Warner MA, et al. Bleeding complications in patients undergoing celiac plexus block. Reg Anesth Pain Med. 2016;41:488–93.
Zhong W, Yu Z, Zeng JX, et al. Celiac plexus block for treatment of pain associated with pancreatic cancer: a meta-analysis. Pain Pract. 2014;14:43–51.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the Centre for Interdisciplinary Pain Therapy, Oncology and Palliative Care, Klinikum Klagenfurt am Wörthersee, Austria.
Medical Writing and/or editorial assistance
The article has been submitted to Proof-Reading-Service.com for editing and proofreading.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
Stefan Neuwersch-Sommeregger: substantial contribution to conception and design of the study, acquisition of data, analysis and interpretation of data, drafting and revising the article, final approval of the version submitted, agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Markus Köstenberger: substantial contribution to conception and design of the study, acquisition of data, analysis and interpretation of data, drafting and revising the article, final approval of the version submitted, agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Corresponding author.
Haro Stettner: analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version submitted, agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Wolfgang Pipam: analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version submitted, agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Christian Breschan: drafting and revising the article, final approval of the version submitted, agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Markus Egger: substantial contribution to conception and design of the study, acquisition of data, analysis and interpretation of data, drafting and revising the article, final approval of the version submitted, agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Jakob Kraschl: substantial contribution to conception and design of the study, acquisition of data, analysis and interpretation of data, drafting and revising the article, final approval of the version submitted, agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Matthias Fürstner: substantial contribution to conception and design of the study, acquisition of data, analysis and interpretation of data, drafting and revising the article, final approval of the version submitted, agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Rudolf Likar: substantial contribution to conception and design of the study, analysis and interpretation of data, drafting and revising the article, final approval of the version submitted, agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Georg Feigl: substantial contribution to conception and design of the study, acquisition of data, analysis and interpretation of data, drafting and revising the article, final approval of the version submitted, agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosures
Stefan Neuwersch-Sommeregger, Markus Köstenberger, Haro Stettner, Wofgang Pipam, Christian Breschan, Markus Egger, Jakob Kraschl, Matthias Fürstner, Rudolf Likar, Georg Feigl confirmed that no conflicts of interests exist and have nothing to disclose.
Compliance with Ethics Guidelines
This retrospective study was approved by the Ethics Committee of Carinthia (S2021-32, 20th November 2021). The requirement for written informed consent was waived by the Ethics Committee. This study was conducted according to the Helsinki declaration and IASP’s guidelines for pain research in animals and humans, and authorized by the hospital general management.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Neuwersch-Sommeregger, S., Köstenberger, M., Stettner, H. et al. Computed Tomography-Guided Coeliac Plexus Neurolysis in Palliative in-Patients with Intra-Abdominal Malignancy: Retrospective Evaluation of Neurolytic Solution Spread as a Predictive Factor. Pain Ther 11, 1229–1243 (2022). https://doi.org/10.1007/s40122-022-00423-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00423-8