Abstract
Purpose
To evaluate whether a perioperative open-lung ventilation strategy prevents postoperative pulmonary complications after elective on-pump cardiac surgery.
Methods
In a pragmatic, randomized, multicenter, controlled trial, we assigned patients planned for on-pump cardiac surgery to either a conventional ventilation strategy with no ventilation during cardiopulmonary bypass (CPB) and lower perioperative positive end-expiratory pressure (PEEP) levels (2 cm H2O) or an open-lung ventilation strategy that included maintaining ventilation during CPB along with perioperative recruitment maneuvers and higher PEEP levels (8 cm H2O). All study patients were ventilated with low-tidal volumes before and after CPB (6 to 8 ml/kg of predicted body weight). The primary end point was a composite of pulmonary complications occurring within the first 7 postoperative days.
Results
Among 493 randomized patients, 488 completed the study (mean age, 65.7 years; 360 (73.7%) men; 230 (47.1%) underwent isolated valve surgery). Postoperative pulmonary complications occurred in 133 of 243 patients (54.7%) assigned to open-lung ventilation and in 145 of 245 patients (59.2%) assigned to conventional ventilation (p = 0.32). Open-lung ventilation did not significantly reduce the use of high-flow nasal oxygenotherapy (8.6% vs 9.4%; p = 0.77), non-invasive ventilation (13.2% vs 15.5%; p = 0.46) or new invasive mechanical ventilation (0.8% vs 2.4%, p = 0.28). Mean alive ICU-free days at postoperative day 7 was 4.4 ± 1.3 days in the open-lung group vs 4.3 ± 1.3 days in the conventional group (mean difference, 0.1 ± 0.1 day, p = 0.51). Extra-pulmonary complications and adverse events did not significantly differ between groups.
Conclusions
A perioperative open-lung ventilation including ventilation during CPB does not reduce the incidence of postoperative pulmonary complications as compared with usual care. This finding does not support the use of such a strategy in patients undergoing on-pump cardiac surgery.
Trial registration
Clinicaltrials.gov Identifier: NCT 02866578. https://clinicaltrials.gov/ct2/show/NCT02866578
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Maintaining ventilation during cardiopulmonary bypass along with perioperative recruitment maneuvers and higher levels of positive end-expiratory pressure was not effective in reducing postoperative pulmonary complications after on-pump cardiac surgery. This finding does not support the systematic use of such perioperative open-lung procedures in cardiac surgery patients. |
Introduction
Every year an estimated 1.25 million patients undergo cardiac surgery with cardiopulmonary bypass (CPB) worldwide. Despite fast-track protocols, postoperative pulmonary complications, ranging from mild hypoxemia [1] to acute respiratory distress syndrome [2], are common after on-pump cardiac surgery [3]. Such postoperative complications have been shown to extend intensive care unit (ICU) stays [4], increase in-hospital mortality [5], and lead to adverse financial outcomes in health care [6].
Preventing postoperative pulmonary complications with the use of low-tidal-volume ventilation (6 to 8 ml per kilogram of predicted body weight [PBW]) is now an established consensus (protective ventilation) [7]. However, low tidal volumes promote alveolar collapse in poorly ventilated, dependent regions of the lung [8]. As a result, atelectrauma, secondary to the repetitive collapse and reopening of alveolar units, contributes to ventilator-induced lung injury [9]. The open-lung ventilation strategy corresponds to the use of recruitment maneuvers (‘open the lung’) associated with high levels of positive end-expiratory pressure (PEEP) in order to prevent alveolar collapse (‘keep it open’) [10, 11]. This approach has been shown to improve pulmonary mechanics [12]. However, its clinical benefit is still uncertain in surgical patients [13, 14].
In cardiac surgery, recruitment maneuvers and high levels of PEEP have traditionally been avoided [15]. In addition, mechanical ventilation is frequently interrupted during CPB, with or without disconnection of the breathing circuit [15, 16]. This conventional approach, along with specific risk factors for lung injury, such as lung ischemia–reperfusion [17], inflammation [18], and postoperative diaphragmatic dysfunction [19], could compound to worsen pulmonary atelectasis and the risk of postoperative pulmonary complications [20]. Notably, maintaining mechanical ventilation or a positive airway pressure during CPB improves gas exchange in the first postoperative hours [21] with beneficial effects on inflammatory response [22] and immune function [23]. Likewise, perioperative recruitment maneuvers and higher PEEP levels have been shown to attenuate atelectasis formation [24] and inflammation [25] in cardiac surgery patients. Consequently, maintaining ventilation during CPB in association with open-lung procedures before and after CPB, in order to maximize alveolar recruitment, could be an optimal strategy to prevent postoperative pulmonary complications.
However, such strategies remain highly controversial amongst perioperative physicians [26]. This is due to major surgical concerns related to limited visualization and access to the operative field produced by continuously expanded lungs, and the potential deleterious hemodynamic effects of higher ventilatory pressures in patients with severe cardiac disease [27]. As a result, the effect of an open-lung approach, including alveolar recruitment during the CPB period, on robust clinical outcomes is unknown in patients undergoing on-pump cardiac surgery.
We designed the open-lung Protective Ventilation in Cardiac Surgery (PROVECS) trial to assess whether an open-lung perioperative ventilation strategy, combining mechanical ventilation during CPB, perioperative recruitment maneuvers and higher PEEP levels, protects against postoperative pulmonary complications after elective on-pump cardiac surgery, as compared with a conventional ventilation strategy with no ventilation during CPB and lower PEEP levels.
Methods
Trial design
We conducted a pragmatic, multicenter, randomized, stratified, parallel-group clinical trial in five university hospitals in France. A detailed description of the study protocol has previously been published elsewhere and is available in Supplement 1 [28]. An ethical committee approved the study (CPP Sud Mediterranée I) on February 29, 2016 (ID-RCB 2016-A00352-49). An independent monitoring committee monitored patient data and safety issues. Written informed consent was obtained from all individual participants included in the study. The study was funded by the French Ministry of Health (PHRC-2015). There was no industry support or involvement in the trial.
Randomization and masking
Patients’ data were collected anonymously on an electronic platform, whereby each patient is assigned a unique identification number (CleanWEB™, Telemedicine Technologies S.A.S., Boulogne-Billancourt, France). Randomization was performed with a computer-generated list, using permuted block of 4 design, which was drawn up by an independent operator before the beginning of the study. The allocation sequence was stratified by center. Local investigators performed the allocation before induction of general anesthesia using a web-based, secured system, centralized on the electronic platform. Participants and postoperative outcome assessors were blinded to the treatment arm. At the end of surgery, all the intraoperative data (including ventilator settings) were hidden on the electronic case report form by the intraoperative assessor.
Participants
We screened patients 18 years of age or older who were scheduled for elective cardiac surgery with general anesthesia, invasive mechanical ventilation, complete median sternotomy, conventional CPB, and aortic cross clamp. Patients were excluded in case of emergent or redo surgeries, preoperative hypoxemia, body mass index > 35 kg/m2 or obstructive sleep apnea syndrome. Full list of exclusion criteria is available in the Supplement 2.
Interventions
Mechanical ventilation was performed using volume-controlled ventilation. All study patients were ventilated with low-tidal volumes before and after CPB (6 to 8 ml per kilogram of PBW, calculated using standard formula). Patients were assigned to one of the two strategies: in the open-lung ventilation strategy, ventilation was maintained during CPB (tidal volumes of 3 ml per kilogram of PBW, respiratory rate at 12 cycles per minute and fraction of inspired oxygen of 40%), PEEP level was set at 8 cm H2O from intubation in the operating room to extubation in the ICU, and recruitment maneuvers (continuous positive airway pressure maintained at 30 cm H2O for 30 s) were systematically implemented at predefined stages in the surgical procedure; in the conventional ventilation strategy, mechanical ventilation was suspended during CPB, and PEEP level was set at 2 cm H2O from intubation to extubation (Table 1).
Because surgeon’s discomfort and arterial hypotension were expected, we pragmatically standardized adjustments of intraoperative ventilatory settings in response to these. In case of surgical requirements, or because of a systolic arterial pressure lower than 80 mm Hg despite the adequate use of fluids and/or vasoactive drugs, interruption of a planned recruitment maneuver and transient lung deflation by lowering PEEP levels in stages of 1 cm H2O were permitted in both ventilation strategies. The use of temporary apnea (continuous positive airway pressure set at the pre-apnea PEEP level) before, during or after CPB was also permitted on surgical demand. In both arms of the study, unplanned recruitment maneuvers and/or increased PEEP levels were permitted, as a rescue strategy, in case of critical intraoperative hypoxemia (peripheral capillary oxygen saturation < 92% with inspired oxygen fraction of 0.8).
During sternal sawing, PEEP was temporarily set to 0 cm H2O to prevent pleural injury. Before aortic declamping, de-airing maneuvers by manual balloon ventilation were performed in both groups according to local protocols, with or without the use of transesophageal echocardiography, and under surgical guidance. During transport from the operating room to the ICU, ventilation was performed with a self-inflating balloon or transport ventilator with parameters set according to the treatment arm. All other ventilation procedures were identical in the two study groups (Table 1).
A fast-track extubation protocol, defined as extubation performed before the 6th postoperative hour, was followed. Perioperative care, including anesthesia and analgesia protocols, fluid management, transfusion strategy, or respiratory physiotherapy was performed at the discretion of the physician in charge. The use of noninvasive ventilation or nasal high-flow oxygen therapy was implemented according to local protocols. The prophylactic use (before any postoperative pulmonary complication) of these techniques was not permitted.
End points
The primary end point was a collapsed composite of postoperative pulmonary complications within the first 7 postoperative days. It included postextubation respiratory failure (graded as mild, moderate, or severe); bronchospasm, severe trachea–bronchial congestion, respiratory acidosis, suspected or confirmed pneumonia, pleural effusion, radiological atelectasis, acute respiratory distress syndrome, and fast-track extubation failure or the need for new invasive ventilation associated with hypoxemia (partial pressure of oxygen:fraction of inspired oxygen ratio of less than 300) (Supplement 2).
The secondary end points were each component of the primary end point analyzed individually; postoperative extrapulmonary complications, which included systemic inflammatory response syndrome, sepsis or septic shock, wound infection, pericardial tamponade, atrial fibrillation, cardiogenic pulmonary edema, acute kidney injury and delirium; and adverse events, defined as acute bleeding requiring reintervention, pneumothorax, and need for vasoactive drugs or high doses of inotropes (Supplement 2). Other secondary end points were use of high-flow nasal oxygen therapy, use of noninvasive or invasive ventilation, alive ICU-free days at day 7, and death at day 7. Alive ICU-free days at day 7 was defined as the difference, in days, of seven and ICU length of stay.
Statistical analysis
The sample size was determined to obtain 80% power to detect a 10-point difference in occurrence of postoperative pulmonary complications within 7 days after surgery between the two groups (25% in the control group vs 15% in the experimental group), based on previous reports [1]. With the threshold for statistical significance set at a p value of 0.05, 494 patients were needed (247 per group). The statistical analysis plan is available in Supplement 1. The primary analysis was performed on the modified intention-to-treat population (including all subjects who were randomized and were at least evaluated at baseline; patients who withdrew their consent were not included in the final analysis). No interim analysis was planned. The tests were two-tailed with a 5% significance level. The proportions of postoperative pulmonary complications within 7 days after surgery were compared between the two groups using the Chi square test (primary analysis), and the relative risk was presented with its 95% confidence interval (95% CI). Comparisons between the two groups were performed for the secondary end points: Chi square or Fisher’s exact test for proportions (multiple comparison corrections were performed using false discovery rate according to the number of comparisons), and Student t test for continuous variables (Alive ICU-free days). For the binary outcomes, relative risks and 95% CIs were calculated using the Wald likelihood ratio approximation test. The effect estimates were also presented as absolute difference (95% CI) or mean (standard deviation) difference (ICU-free days). Survival estimates were calculated according to the Kaplan–Meier method and compared using a log-rank test. Two post hoc analyses of the primary end point were performed: a potential center effect was assessed by mixed-effects modeling using the GLIMMIX procedure (SAS software, 9.4 version; center as a random effect, a logit-link function, and a binomial distribution function); heterogeneity of the strategy effect among pre-specified subgroups (sex, age, body mass index, type of surgery) using an interaction term between arm and subgroup in a generalized linear model considering a binomial distribution.
Results
Study population
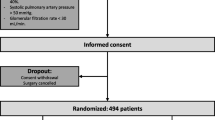
From September 2016 through July 2018, a total of 1025 patients were assessed for eligibility. A total of 494 patients were randomly assigned to one of the two ventilation strategies (247 patients in each group). Three patients in the open-lung ventilation group and two patients in the conventional ventilation group did not receive the allocated intervention. One patient who was assigned to the open-lung ventilation strategy was secondarily excluded because of consent withdrawal for the use of data. Therefore, the primary analysis was performed on a modified intention-to-treat population: 246 patients in the open-lung ventilation group and 247 patients in the conventional ventilation group (Fig. 1). Baseline characteristics are reported in Table 2.
Intraoperative procedures
In the open-lung ventilation group, median (IQR) was 8 (8–8) cm H2O for highest and 8 (5–8) cm H2O for mode PEEP levels; and 5 (2–8) cm H2O for lowest PEEP level. In patients assigned to the conventional ventilation strategy, analysis of applied intraoperative PEEP revealed a median (IQR) of 2 (2–2) cm H2O for lowest, highest and mode levels. In the open-lung ventilation group, 89.7% of the patients received at least three recruitment maneuvers and 76.5% of the patients received more than three recruitment maneuvers. Open-lung ventilation strategy significantly increases the use of adjustments because of arterial hypotension (difference, 17.7% [95% CI, 12.7% to 22.7%]; p < 0.001) or surgical requirements (difference, 58.9% [95% CI, 50.6% to 67.2%]; p < 0.001). CPB durations (p = 0.05) and incidence of platelets transfusion (difference, 5.0% [95% CI, 0.3% to 9.7%]; p = 0.04) were higher in the open-lung ventilation group. Use of rescue strategy for critical intraoperative hypoxemia was significantly lower in the open-lung ventilation group (difference, − 12.7% [95% CI, − 17.5% to − 7.9%]; p < 0.001). No significant differences were found with respect to the use of fluids, vasopressors or inotropes. At the end of surgery, dynamic and static respiratory compliance were greater in the open-lung ventilation than in the conventional ventilation group (Table 3).
Primary end point
Within the first 7 days, postoperative pulmonary complications occurred in 133 patients (54.7%) in the open-lung ventilation group and in 145 patients (59.2%) in the conventional ventilation group (difference, − 4.5% [95% CI, − 13.1% to 4.3%]; relative risk, 0.83 [95% CI, 0.58–1.19]; p = 0.32) (Table 4 and Fig. 2). The effect of ventilation strategy on the occurrence of the primary outcome was consistent across subgroups, including male vs female, age less than 65 vs 65 or greater, body mass index less than 30 vs 30 or greater, and isolated valve surgery or isolated CABG vs other types of surgery (See Table S1 in Supplement 2). No center effect was identified using mixed effects models (odd ratio, 0.83; 95% CI, 0.50–1.37; p = 0.36).
Secondary end points
At day 7, the proportion of patients who presented with an extrapulmonary complication, analyzed separately, did not differ between groups. There was no between-groups difference with regard to the occurrence of adverse events, the use of high-flow nasal oxygen therapy (difference,− 0.8% [95% CI, − 6.0% to 4.5%]; relative risk, 0.91 [95% CI, 0.49–1.70]; p = 0.77), noninvasive ventilation (difference, − 2.3% [95% CI, − 8.6% to 3.9%]; relative risk, 0.83 [95% CI, 0.50–1.37]; p = 0.46) or new invasive mechanical ventilation (difference, − 1.6% [95% CI, − 4.5% to 0.8%]; relative risk, 0.33 [95% CI, 0.07–1.65]; p = 0.28). No significance between group difference was identified, with regard to secondary outcomes, after multiple comparison corrections. At day 7, the mean number of alive ICU-free days did not differ between groups (4.4 days in the open-lung group vs 4.3 days in the conventional group; mean ± SD difference, 0.1 ± 0.1 day; p = 0.51) (Table 4 and Fig. S2 in Supplement 2).
Discussion
Maintaining mechanical ventilation during CPB in association with perioperative recruitment maneuvers and higher PEEP levels did not reduce the incidence of postoperative pulmonary complications in patients undergoing on-pump cardiac surgery, as compared with a conventional strategy with no ventilation during CPB and lower PEEP levels.
Atelectasis has been associated with lung infection and could promote further mechanical lung injury from cyclic alveolar recruitment during ventilation [9]. Accordingly, an open-lung approach, by preventing atelectrauma, would be expected to result in a reduction in postoperative pulmonary complications in high-risk settings such as cardiac surgery. Indeed, previous studies indicated that such an approach improved functional residual capacity after extubation [29] and reduced inflammation after cardiac surgery with CPB [25].
As suggested by the improvement in pulmonary compliance and the reduced need to rescue critical intraoperative hypoxemia, open-lung ventilation, as applied in our trial, improved alveolar recruitment during general anesthesia. However, our results indicate that enhancing gas exchange and pulmonary mechanics during mechanical ventilation does not by itself improve postoperative clinical outcomes. Several factors could have contributed to this result. Apart from ventilation management, pain, fluid overload, prolonged bed rest, or diaphragmatic dysfunction may facilitate the development of pulmonary complications in the postextubation period [30]. Moreover, it has been suggested that the open-lung ventilation strategy may be harmful in terms of increased alveolar distension [31] and tidal strain [32]. This effect could mitigate the benefit of improved alveolar recruitment. Notably, anterior chest and pleural opening, intrinsic to open cardiac surgery, may amplify transpulmonary pressures [33, 34] in the non-dependent regions of the lung. Finally, besides the alveolar recruitment issue, the beneficial effect of maintaining mechanical ventilation during CPB, when lungs are no longer perfused, is controversial [35,36,37].
As expected, the open-lung ventilation strategy was associated with more frequent need for temporary adjustments due to iatrogenic arterial hypotension and surgical requirements. As a result, 23.5% of the patients assigned to the open-lung ventilation group did not receive more than three complete recruitment maneuvers. However, the number of recruitment maneuvers and the levels of PEEP have been empirically designed in the protocol and it is unclear if the recruiting efficacy of such maneuvers depends on a quantitative effect. In contrast, the conventional ventilation strategy minimizes interference with the surgical field and prevents hypotensive events. Importantly, the use of adjustments or rescue strategies did not compromise the reliability of our study, as between-groups differences on completed recruitment maneuvers and applied PEEP levels were both statistically and clinically (lower critical intraoperative hypoxemia in the open-lung group) significant.
Our findings are consistent with the previous results of two multicenter clinical trials [14, 38] in non-cardiac surgery that failed to demonstrate the superiority of systematic open-lung approaches in patients with normal lungs. Such previous studies and our results imply that use of intensive alveolar recruitment procedures should be reserved to selected patients presenting hypoxemia consistent with significant lung atelectasis, as a curative rather than a preventive approach. Moreover, timing of alveolar recruitment within the perioperative period is probably an underestimated factor. A delayed recruitment strategy, in patients presenting a high-degree of alveolar collapse, appears to be more relevant than starting alveolar recruitment procedures in the early stages of surgery, before any collapse occurs. This has been recently suggested by Costa Leme et al. [39] who reported a significant reduction in postoperative pulmonary complications when alveolar recruitment was applied postoperatively in cardiac surgery patients presenting hypoxemia at ICU arrival (partial pressure of oxygen:fraction of inspired oxygen ratio of less than 250 mmHg).
There are limitations to our trial. First, the observed rate of postoperative pulmonary complications, defined by a binary collapsed composite end point, was higher than previously advanced [1]. Nonetheless, this is the first prospective study to systematically assess lung function with daily, highly relevant to clinical practice, room air trials and to show the large proportion of patients presenting measurements consistent with respiratory failure after on-pump cardiac surgery. Such a difference between expected and observed incidence of pulmonary complications may have implications in the required sample size and the adequation of study power. Second, the use of composite outcomes offers the interest to reduce sample sizes; however, it may be responsible for difficulties in the interpretation of the results. Particularly, for each component included in our composite outcome, the differences in the degree of severity and the incidence represent a limitation. Nonetheless, postoperative pulmonary complications have been well described using composite outcomes in previous preeminent studies. Most importantly, our definitions of postoperative pulmonary complications are consistent with those previously used in such clinical trials [7, 14, 38]. Third, although present, the statistically significant difference in intraoperative tidal volume of 0.1 ml/kg of PBW between groups would be expected to be clinically and physiologically negligible. Fourth, we did not standardize perioperative fluid administration. However, no significant difference was found for administered intraoperative fluid volume and occurrence of cardiogenic pulmonary edema. Fifth, as use of noninvasive ventilation or high-flow nasal oxygen therapy might impact pulmonary complications [4, 40], the nonstandardization of their use is another limitation. However, preventive use of these techniques was excluded per protocol. Also, the proportion of patients that required these techniques was similar between groups, indicating minimal impact on the primary outcome. Finally, even if outcome assessors were blinded to the allocated treatment, the study was not strictly double-blind because intraoperative management was operated by unblinded investigators.
In conclusion, maintaining ventilation during CPB in association with perioperative recruitment maneuvers and higher PEEP levels to optimize perioperative lung recruitment does not reduce the incidence of postoperative pulmonary complications after on-pump cardiac surgery, as compared with use of no ventilation during CPB and lower perioperative PEEP levels.
References
Ranucci M, Ballotta A, La Rovere MT, Castelvecchio S, Surgical and Clinical Outcome Research (SCORE) Group (2014) Postoperative hypoxia and length of intensive care unit stay after cardiac surgery: the underweight paradox? PLoS One 9:e93992
Milot J, Perron J, Lacasse Y, Letourneau L, Cartier PC, Maltais F (2001) Incidence and predictors of ARDS after cardiac surgery. Chest 119:884–888
Ng CS, Wan S, Yim AP, Arifi AA (2002) Pulmonary dysfunction after cardiac surgery. Chest 121:1269–1277
Stephan F, Barrucand B, Petit P, Rezaiguia-Delclaux S, Medard A, Delannoy B, Cosserant B, Flicoteaux G, Imbert A, Pilorge C, Berard L, Bi POPSG (2015) High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA 313:2331–2339
Serpa Neto A, Hemmes SN, Barbas CS, Beiderlinden M, Fernandez-Bustamante A, Futier E, Hollmann MW, Jaber S, Kozian A, Licker M, Lin WQ, Moine P, Scavonetto F, Schilling T, Selmo G, Severgnini P, Sprung J, Treschan T, Unzueta C, Weingarten TN, Wolthuis EK, Wrigge H, Gama de Abreu M, Pelosi P, Schultz MJ, investigators PN (2014) Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: a systematic review and meta-analysis. Lancet Respir Med 2:1007–1015
Shander A, Fleisher LA, Barie PS, Bigatello LM, Sladen RN, Watson CB (2011) Clinical and economic burden of postoperative pulmonary complications: patient safety summit on definition, risk-reducing interventions, and preventive strategies. Crit Care Med 39:2163–2172
Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, Marret E, Beaussier M, Gutton C, Lefrant JY, Allaouchiche B, Verzilli D, Leone M, De Jong A, Bazin JE, Pereira B, Jaber S, Group IS (2013) A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 369:428–437
Bendixen HH, Hedley-Whyte J, Laver MB (1963) Impaired oxygenation in surgical patients during general anesthesia with controlled ventilation. A concept of atelectasis. N Engl J Med 269:991–996
Slutsky AS, Ranieri VM (2013) Ventilator-induced lung injury. N Engl J Med 369:2126–2136
Futier E, Marret E, Jaber S (2014) Perioperative positive pressure ventilation: an integrated approach to improve pulmonary care. Anesthesiology 121:400–408
Lachmann B (1992) Open up the lung and keep the lung open. Intensive Care Med 18:319–321
D’Antini D, Huhle R, Herrmann J, Sulemanji DS, Oto J, Raimondo P, Mirabella L, Hemmes SNT, Schultz MJ, Pelosi P, Kaczka DW, Vidal Melo MF, Gama de Abreu M, Cinnella G, European Society of A, the PVN (2018) Respiratory system mechanics during low versus high positive end-expiratory pressure in open abdominal surgery: a substudy of provhilo randomized controlled trial. Anesth Analg 126:143–149
Pelosi P, Rocco PRM, Gama de Abreu M (2018) Close down the lungs and keep them resting to minimize ventilator-induced lung injury. Crit Care 22:72
PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology, Hemmes SN, Gama de Abreu M, Pelosi P, Schultz MJ (2014) High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 384:495–503
Fischer MO, Courteille B, Guinot PG, Dupont H, Gerard JL, Hanouz JL, Lorne E, Arcothova CG (2016) Perioperative ventilatory management in cardiac surgery: a french nationwide survey. Medicine (Baltimore) 95:e2655
Bouchez S (2012) Current ventilation practice during and after cardiopulmonary bypass. BJA: British Journal of Anaesthesia 109: Issue eLetters Supplement
Dodd-o JM, Welsh LE, Salazar JD, Walinsky PL, Peck EA, Shake JG, Caparrelli DJ, Bethea BT, Cattaneo SM, Baumgartner WA, Pearse DB (2004) Effect of bronchial artery blood flow on cardiopulmonary bypass-induced lung injury. Am J Physiol Heart Circ Physiol 286:H693–H700
Massoudy P, Zahler S, Becker BF, Braun SL, Barankay A, Meisner H (2001) Evidence for inflammatory responses of the lungs during coronary artery bypass grafting with cardiopulmonary bypass. Chest 119:31–36
Lerolle N, Guerot E, Dimassi S, Zegdi R, Faisy C, Fagon JY, Diehl JL (2009) Ultrasonographic diagnostic criterion for severe diaphragmatic dysfunction after cardiac surgery. Chest 135:401–407
Neves FH, Carmona MJ, Auler JO Jr, Rodrigues RR, Rouby JJ, Malbouisson LM (2013) Cardiac compression of lung lower lobes after coronary artery bypass graft with cardiopulmonary bypass. PLoS One 8:e78643
Chi D, Chen C, Shi Y, Wang W, Ma Y, Zhou R, Yu H, Liu B (2017) Ventilation during cardiopulmonary bypass for prevention of respiratory insufficiency: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 96:e6454
Ng CS, Arifi AA, Wan S, Ho AM, Wan IY, Wong EM, Yim AP (2008) Ventilation during cardiopulmonary bypass: impact on cytokine response and cardiopulmonary function. Ann Thorac Surg 85:154–162
Gaudriot B, Uhel F, Gregoire M, Gacouin A, Biedermann S, Roisne A, Flecher E, Le Tulzo Y, Tarte K, Tadie JM (2015) Immune dysfunction after cardiac surgery with cardiopulmonary bypass: beneficial effects of maintaining mechanical ventilation. Shock 44:228–233
Magnusson L, Zemgulis V, Tenling A, Wernlund J, Tyden H, Thelin S, Hedenstierna G (1998) Use of a vital capacity maneuver to prevent atelectasis after cardiopulmonary bypass: an experimental study. Anesthesiology 88:134–142
Reis Miranda D, Gommers D, Struijs A, Dekker R, Mekel J, Feelders R, Lachmann B, Bogers AJ (2005) Ventilation according to the open lung concept attenuates pulmonary inflammatory response in cardiac surgery. Eur J Cardiothorac Surg 28:889–895
Bignami E, Guarnieri M, Saglietti F, Belletti A, Trumello C, Giambuzzi I, Monaco F, Alfieri O (2016) Mechanical ventilation during cardiopulmonary bypass. J Cardiothorac Vasc Anesth 30:1668–1675
Mahmood SS, Pinsky MR (2018) Heart-lung interactions during mechanical ventilation: the basics. Ann Transl Med 6:349
Lagier D, Fischer F, Fornier W, Fellahi JL, Colson P, Cholley B, Jaber S, Baumstarck K, Guidon C, investigators P, the Ag (2018) A perioperative surgeon-controlled open-lung approach versus conventional protective ventilation with low positive end-expiratory pressure in cardiac surgery with cardiopulmonary bypass (PROVECS): study protocol for a randomized controlled trial. Trials 19:624
Reis Miranda D, Struijs A, Koetsier P, van Thiel R, Schepp R, Hop W, Klein J, Lachmann B, Bogers AJ, Gommers D (2005) Open lung ventilation improves functional residual capacity after extubation in cardiac surgery. Crit Care Med 33:2253–2258
Huffmyer JL, Groves DS (2015) Pulmonary complications of cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol 29:163–175
Puybasset L, Cluzel P, Chao N, Slutsky AS, Coriat P, Rouby JJ (1998) A computed tomography scan assessment of regional lung volume in acute lung injury. The CT Scan ARDS Study Group. Am J Respir Crit Care Med 158:1644–1655
Guldner A, Braune A, Ball L, Silva PL, Samary C, Insorsi A, Huhle R, Rentzsch I, Becker C, Oehme L, Andreeff M, Vidal Melo MF, Winkler T, Pelosi P, Rocco PR, Kotzerke J, Gama de Abreu M (2016) Comparative effects of volutrauma and atelectrauma on lung inflammation in experimental acute respiratory distress syndrome. Crit Care Med 44:e854–e865
Beitler JR, Sarge T, Banner-Goodspeed VM, Gong MN, Cook D, Novack V, Loring SH, Talmor D, Group EP-S (2019) Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-Fio2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA 321(9):846–857
Lai-Fook SJ, Rodarte JR (1985) Pleural pressure distribution and its relationship to lung volume and interstitial pressure. J Appl Physiol 70:967–978
Loer SA, Kalweit G, Tarnow J (2000) Effects of ventilation and nonventilation on pulmonary venous blood gases and markers of lung hypoxia in humans undergoing total cardiopulmonary bypass. Crit Care Med 28:1336–1340
Khimenko PL, Bagby GJ, Fuseler J, Taylor AE (1985) Tumor necrosis factor-alpha in ischemia and reperfusion injury in rat lungs. J Appl Physiol 85:2005–2011
Dryer C, Tolpin D, Anton J (2018) Con: mechanical ventilation during cardiopulmonary bypass does not improve outcomes after cardiac surgery. J Cardiothorac Vasc Anesth 32:2001–2004
Ferrando C, Soro M, Unzueta C, Suarez-Sipmann F, Canet J, Librero J, Pozo N, Peiro S, Llombart A, Leon I, India I, Aldecoa C, Diaz-Cambronero O, Pestana D, Redondo FJ, Garutti I, Balust J, Garcia JI, Ibanez M, Granell M, Rodriguez A, Gallego L, de la Matta M, Gonzalez R, Brunelli A, Garcia J, Rovira L, Barrios F, Torres V, Hernandez S, Gracia E, Gine M, Garcia M, Garcia N, Miguel L, Sanchez S, Pineiro P, Pujol R, Garcia-Del-Valle S, Valdivia J, Hernandez MJ, Padron O, Colas A, Puig J, Azparren G, Tusman G, Villar J, Belda J, Individualized PeRioperative Open-lung VN (2018) Individualised perioperative open-lung approach versus standard protective ventilation in abdominal surgery (iPROVE): a randomised controlled trial. Lancet Respir Med 6(3):193–203
Costa Leme A, Hajjar LA, Volpe MS, Fukushima JT, De Santis Santiago RR, Osawa EA, Pinheiro de Almeida J, Gerent AM, Franco RA, Zanetti Feltrim MI, Nozawa E, de Moraes Coimbra VR, de Moraes Ianotti R, Hashizume CS, Kalil Filho R, Auler JO Jr, Jatene FB, Gomes Galas FR, Amato MB (2017) Effect of intensive vs moderate alveolar recruitment strategies added to lung-protective ventilation on postoperative pulmonary complications: a randomized clinical trial. JAMA 317:1422–1432
Jaber S, Lescot T, Futier E, Paugam-Burtz C, Seguin P, Ferrandiere M, Lasocki S, Mimoz O, Hengy B, Sannini A, Pottecher J, Abback PS, Riu B, Belafia F, Constantin JM, Masseret E, Beaussier M, Verzilli D, De Jong A, Chanques G, Brochard L, Molinari N, Group NS (2016) Effect of Noninvasive Ventilation on Tracheal Reintubation Among Patients With Hypoxemic Respiratory Failure Following Abdominal Surgery: A Randomized Clinical Trial. JAMA 315:1345–1353
Acknowledgements
This study was funded by the Programme Hospitalier de Recherche Clinique of the French Ministry of Health (PHRCI-15-055). The authors thank the team of the Health Research Department of the Assistance Publique Hôpitaux de Marseille, and particularly Mrs Valentine Verdier and Mr Patrick Sudour for their contributions to the study management,and Prof Nicolas Bruder, Prof Marc Leone and Prof Laurent Papazian for their scientific advices. They thank the Association des Anesthésistes-Réanimateurs en Chirurgie Cardio-Thoracique and Vasculaire (ARCOTHOVA) for facilitating the organization of this study by connecting several French cardiac surgical centers.
Author information
Authors and Affiliations
Consortia
Contributions
DL and KB had full access to all the data and take responsibility for the integrity of the data and accuracy of data analysis. Concept and design: DL, SJ, KB, CG; Acquisition, analysis, or interpretation of data: All authors; Drafting of the manuscript: DL, FF, J-LF, BC, MFVM, KB; Critical revision of the manuscript for important intellectual content: MFVM, SJ, LJV, KB; Statistical analysis: KB; Obtained funding: DL; Administrative, technical, or material support: SB, LJV.
Corresponding author
Ethics declarations
Conflicts of interest
Pr Jaber reports consulting fees from Drager, Xenios, Medtronic and Fisher and Paykel. Pr Vidal Melo was supported by NIH/NHLBI grant UG3 HL140177. No other disclosures were reported.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lagier, D., Fischer, F., Fornier, W. et al. Effect of open-lung vs conventional perioperative ventilation strategies on postoperative pulmonary complications after on-pump cardiac surgery: the PROVECS randomized clinical trial. Intensive Care Med 45, 1401–1412 (2019). https://doi.org/10.1007/s00134-019-05741-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-019-05741-8