Published online Mar 27, 2015. doi: 10.4254/wjh.v7.i3.443
Peer-review started: August 30, 2014
First decision: November 1, 2014
Revised: November 8, 2014
Accepted: November 27, 2014
Article in press: November 27, 2014
Published online: March 27, 2015
This review describes the recent developments in the pathobiology of endothelial dysfunction (ED) in the context of cirrhosis with portal hypertension and defines novel strategies and potential targets for therapy. ED has prognostic implications by predicting unfavourable early hepatic events and mortality in patients with portal hypertension and advanced liver diseases. ED characterised by an impaired bioactivity of nitric oxide (NO) within the hepatic circulation and is mainly due to decreased bioavailability of NO and accelerated degradation of NO with reactive oxygen species. Furthermore, elevated inflammatory markers also inhibit NO synthesis and causes ED in cirrhotic liver. Therefore, improvement of NO availability in the hepatic circulation can be beneficial for the improvement of endothelial dysfunction and associated portal hypertension in patients with cirrhosis. Furthermore, therapeutic agents that are identified in increasing NO bioavailability through improvement of hepatic endothelial nitric oxide synthase (eNOS) activity and reduction in hepatic asymmetric dimethylarginine, an endogenous modulator of eNOS and a key mediator of elevated intrahepatic vascular tone in cirrhosis would be interesting therapeutic approaches in patients with endothelial dysfunction and portal hypertension in advanced liver diseases.
Core tip: Endothelial dysfunction (ED) is a key and early relentless event in patients suffering from gastrointestinal bleeding in cirrhosis and involves in response to both vasoactive and vasoconstrictor substances. The one such vasoactive molecule, nitric oxide (NO) plays a prime role in maintaining normal hepatic vascular function and if there any defect in NO availability leads to ED and portal hypertension (PHT) could be of great utility in preventing and curing complications of PHT.
- Citation: Vairappan B. Endothelial dysfunction in cirrhosis: Role of inflammation and oxidative stress. World J Hepatol 2015; 7(3): 443-459
- URL: https://www.wjgnet.com/1948-5182/full/v7/i3/443.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i3.443
The endothelium is the largest organ and encompasses > 1013 endothelial cells in the body can able to generate both vasodilator [nitric oxide (NO), endothelium derived hyperpolarising factor (EDHF) and prostacyclin] and vasoconstrictor (endothelin-1, norepinephrine, leukotriene, thromboxane A2 and angiotensin II) substances and is essential for hepatic vascular homeostasis. Endothelium serves as a barrier to separate blood from the underlined tissue and thus maintains homeostasis at the vascular wall during physiological condition[1,2]. The salient features of normal healthy endothelium which including, regulation of vascular permeability, decrease in vascular tone, reduce in platelets adhesion and aggregation, prevention of thrombosis, inhibition of smooth muscle cell proliferation, inflammation and restricting leukocyte adhesion[3]. Indeed, many of these functions are mediated by endothelium driven NO[4]. The term endothelial dysfunction (ED) implicate a loss of function of numerous activities of the endothelium[5], mainly characterised by impairment of the production and release of endothelium driven vasodilatory factors including NO[4,6]. The hepatic vascular bed of cirrhotic liver exhibits ED and is now considered to play a key role in the initiation and advancement of liver cirrhosis[7]. The intrahepatic vasculature also displays increased sensitivity to vasoconstrictors in cirrhosis[8]. Furthermore, ED is also a common index for a wide variety of pathological conditions such as chronic renal failure, atherosclerosis, hypercholesterolemia, hypertension, diabetes and coronary artery disease[9,10].
Cirrhosis is a complication of many forms of chronic liver diseases and is a late stage of fibrosis, in which regenerative nodular formation surrounded by fibrous bands of the liver. The development of portal hypertension (PHT) (elevated pressure within the hepatic circulation) heralds the onset of most fatal complications of cirrhosis, which carry a poor prognosis and represent the first cause of death and need for liver transplantation in patients with cirrhosis[8,11]. The pathogenesis of PHT is predominantly related to a combination of structural and dynamic components that cause an increase in hepatic vascular resistance to portal blood flow[12]. The structural components such as fibrosis, regenerative nodule formation and vascular remodelling[13].
Impaired endothelial dependent relaxation in the hepatic microcirculation due to reduced bioavailability of vasodilator, NO in cirrhotic liver contributes to increasing intra-hepatic vascular resistance, which culminating portal hypertension[14]. By contrast, in the splanchnic vascular bed, overproduction of NO contributes to increased endothelium dependent relaxation, leading to hyperdynamic circulatory disturbances, which observed in cirrhosis with portal hypertension[15,16]. Furthermore, increased vasoconstrictor agents such as thromboxane A2 (TX A2), a COX-1 derived prostanoids, and endothelin-1 are thought to associated with the pathogenesis of the dynamic component of the augmented intra-hepatic resistance and play a major role in the intrahepatic endothelial dysfunction of the cirrhotic liver[7,17]. Such imbalance between endogenous vasoconstrictor and vasodilator factors observed in the cirrhotic liver is thought to be similar to that found in other cardiovascular diseases[18]. The assessment of NO concentration in cirrhotic liver and systemic circulation is considered to be the prime indicative of endothelial dysfunction (ED).
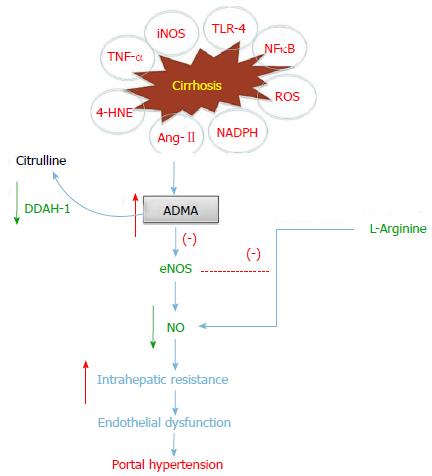
Endothelial dysfunction is thought to be a key event in the development of distinct human vascular diseases, including liver cirrhosis, hypertension, diabetes and atherosclerosis. Classically, ED has been considered to be the result of a decrease in bioavailability of NO in cirrhosis[14,19]. The amino acid, L-arginine, is the substrate of eNOS, the enzyme responsible for NO synthesis (Figure 1). Endothelial nitric oxide synthase (eNOS) driven nitric oxide (NO) is a potent vasodilator that plays a substantial role in maintaining vascular homeostasis in the normal intact liver[14,19], however when the liver fails, reduced intrahepatic eNOS activity triggers endothelial dysfunction contributes to the pathogenesis of PHT (Figure 1)[20]. It is an early and relentless event occurring after all forms of liver injury that leads to substantial morbidity and mortality in individuals with cirrhosis[11,21]. Reduced NO bioavailability makes a major contribution to endothelial dysfunction and is mainly due to reduced NO production or increased NO breakdown due to the chemical reaction with oxidant radicals[22]. Inflammation and oxidative stress are other important pathophysiological consequences that causes endothelial dysfunction and reduced NO bioavailability[22], which make an important contribution to the vascular structural changes in cirrhosis[23]. Furthermore, treatment with exogenous L-arginine has been shown to improve vascular function in liver cirrhosis suggesting that decreased substrate availability contributes to endothelial dysfunction[24]. Thus, impaired vascular uptake of L-arginine may play a key role in the pathogenesis of endothelial dysfunction in cirrhosis and should be explored as a potential therapeutic target[25].
Several mechanisms are involved in regulating NO production following eNOS activation. Studies in relation to decreased hepatic eNOS activity have, to date, focused on inhibitors of eNOS activity such as asymmetric dimethylarginine (ADMA)[26,27] and caveolin-1 (major coat protein of endothelial caveolae)[28], or on the process affecting the post translational modification of eNOS[29]. Furthermore, trafficking and proper subcellular localization of eNOS are also critical for regulation of its activity[29,30]. One such protein, eNOS trafficking inducer (NOSTRIN), identified in a yeast two-hybrid approach, has been demonstrated to interact physically with eNOS via its C-terminal SH3 domain and regulated its function[29-31].
An emerging body of evidence indicated that ADMA, a deleterious endogenous inhibitor of NO synthases and thus presumed to be a marker of hepatic dysfunction in cirrhosis with PHT. One mechanism thought to be partially responsible for the reduction in NO and resultant ED in liver disease is an increase in the levels of the endogenous inhibitor of NOS, ADMA[19,32]. In this regard, our previous studies have shown evidence that increased ADMA contributes to reduced hepatic NO biosynthesis as a consequence of altered hepatic vascular function in cirrhosis[26,27,33]. In critically ill patients to whom admitted in ICU, hepatic dysfunction was associated with elevated ADMA levels and was identified as an independent predictor of mortality[34]. Furthermore, increased plasma ADMA was reported recently in biopsy proven non-alcoholic fatty liver disease (NAFLD) patients[35], hepatic vein of patients with compensated cirrhosis[36] and decreased following liver transplantation; thus the significant improvement of liver function[37] propose an important role for ADMA in clinical medicine. Elevated plasma ADMA has also recognised as an important risk factor for cardiovascular disease[38], coronary heart disease[39] and chronic renal failure[40]. Consequently, increased ADMA may be related to elevated activity of protein methyltransferase (PRMT), which is responsible for the methylation of arginine residues in cellular proteins[41]. Moreover, increased ADMA level was correlated with the severity of inflammation and levels of increased proinflammatory cytokine such as tumor necrosis factor (TNF)[42,43]. Laleman et al[44] showed in cirrhotic animals infusion of ADMA and NG-nitro-L-arginine methyl ester (L-NAME), other known inhibitors of NOS synergistically aggravated and resulted in paradoxical vasoconstriction, which associated with a further decrease in NOx levels. The pathophysiological increase in hepatic ADMA concentration observed in cirrhotic rats manifest decrease of hepatic eNOS activity.
Furthermore, the intracellular levels of these methylated arginines are regulated through their metabolism to citrulline and dimethylamine by its hepatic specific enzyme called, dimethylarginine diaminohydrolase (DDAH)[41,45] (Figure 1). Two isoforms of DDAH have been identified and are widely expressed in human and rodent liver. DDAH 1 is an important isoform for the regulation of hepatic and systemic ADMA concentration and is present higher levels in tissue that expressing neuronal NOS (nNOS)[41,45]. The other DDAH isoform (DDAH 2) has an important effect in regulating NO activity, and is mainly found in tissue expressing eNOS and inducible NOS (iNOS)[41,45,46]. It is well known that increased intracellular DDAH plays a critical role in regulating tissue ADMA concentration[26,27,33,47], therefore alterations of DDAH activity and expression lead to change in intracellular ADMA concentrations and concomitant NO synthesis. In vitro, human umbilical vein endothelial cells (HUVECS) exposed to prolonged (48 h) TNF-α show eight fold increase of ADMA, compared to control medium and associated DDAH activity was decreased to almost 60% of baseline values[48]. DDAH is a redox sensitive enzyme and is thus subject to inhibition by oxidants derived from endothelial superoxide[47,49], and antioxidant treatment corrects DDAH inhibition in in vivo[33,50]. Cirrhotic rat livers showed an increased O2- content compared to control rat livers and was ameliorated by adenoviral gene delivery of superoxide dismutase, increases NO bioavailability, improves intrahepatic endothelial function and reduces portal pressure[51]. In contrast to ADMA, SDMA, its vasoactive stereoisomer, has no effect on inhibition of NO synthases but competes with arginine for cellular transport across the y+ transporter[38,52]. Recently, Siroen and co-workers have shown that the human liver takes up substantial amounts of SDMA from the portal and systemic circulation and suggested that high plasma levels of SDMA may have hemodynamic consequences similar to those reported for ADMA[53]. However, in patients with alcoholic cirrhosis, noted plasma SDMA level was within the normal limit[54].
In cirrhosis, oxidative stress induced mainly by an overproduction of reactive oxygen species (ROS)[55], which is a critical determinant of endothelial dysfunction and is due to disturbed balance between oxidant and antioxidant enzymes. Increased superoxide formation in the presence of equimolar concentrations of NO will lead to the formation of the potent ROS and reactive nitrogen species[56]. Furthermore, decreased NO bioavailability within the cirrhotic liver is mainly attributed by endothelial dysfunction and is mainly due to diminished eNOS activity as well as NO scavenging by increased release of superoxide (O2-)[55]. NO also binds to superoxide anion (O2-) to produce the most powerful oxidant peroxynitrite (ONOO-). Consequently, peroxynitrite can cause oxidative damage, protein nitration and S-nitrosylation of biomolecules such as proteins, lipids and DNA[57,58]. In fact, peroxynitrite can also oxidise tetrahydrobiopterin (BH4), an essential cofactor for eNOS to trihydrobiopterin radical (BH3)[59], which can further disproportionate to dihydrobiopterin (BH2). As a consequence, functional endothelial NOS is converted into a dysfunctional O2- generating enzyme[60] termed as eNOS uncoupling, contributes to ROS overproduction within the intrahepatic circulation. The generation of ROS and superoxide are the key mediators of damage to endothelial cells in cirrhosis[23,61]. BH4 administration to cirrhotic rats increased hepatic NOS activity and cyclic guanosine monophosphate (cGMP) levels and significantly reduced ED, and subsequent portal pressure may represent a new therapy for ED in patients with cirrhosis[62,63]. Overwhelming evidences have also indicate that in cirrhosis with bacterial endotoxemia, excessive NO production by iNOS reacts with molecular oxygen (O2-), leading to the formation of peroxynitrite radical and causes massive hepatic tissue damage as well as fall in blood pressure induced in the vascular wall[27,64]. Furthermore, eNOS uncoupling is also caused by L-arginine depletion, increased endogenous eNOS inhibitors and S-glutathionylation of eNOS[65,66].
Hepatic inflammation is a common trigger of liver diseases, associated with ED and causes increased hepatic vascular risk in cirrhosis[67]. There are several inflammatory mediators involved in downregulation of eNOS activity and NO bioavailability within the hepatic circulation resulting increased intrahepatic resistance and ED thus, PHT in patients with cirrhosis (Table 1).
| Marker | Endothelial dysfunction | Ref. |
| Inflammatory marker | ||
| TNF-α | Inhibition of NO synthesis | [47,75,78,84,99,104] |
| NFκB | Increase of ADMA | |
| TLR | Increase of Caveolin-1 | |
| Ang II | Reduction of eNOS activity | |
| Inhibition of DDAH enzyme | ||
| Upregulation of iNOS | ||
| Increase of superoxide production | ||
| Reduction of antioxidant capacity | ||
| Oxidative marker | ||
| 4-HNE | Reduction of DDAH enzyme activity | [42,78,104] |
| NADPH | Decrease of NO bioavailability | |
| Increase of ADMA levels | ||
| Increase of ROS generation | ||
| Cyclooxygenase -derived prostanoids | ||
| TXA2 | Reduction of intrahepatic nitrate/nitrite | [110-112] |
| PGI2 | Upregulation of iNOS expression | |
| Increase of intrahepatic resistance | ||
| Other marker | ||
| ET-1 | Increase of inflammation | [122,130,138,139, 145] |
| LOX-1 | Stimulation of ROS generation | |
| PARs | ||
| Adiponectin | ||
| Palmitic acid | ||
TNF-α
TNF-α, a proinflammatory cytokine with the broad spectrum of deleterious effects is believed to exert vascular effects by increasing vascular permeability and causing vasodilatation, which mediated through NO dependent pathways[68]. In cirrhosis, increased systemic and hepatic TNF-α concentration has shown to associate with overwhelming NO production[47]. Interestingly, in cultured endothelial cells, TNF-α reduces NO bioavailability[48], and in vivo TNF-α may also directly alter endothelial vasomotor function[69]. Moreover, Plasma TNF-α concentration was shown to be significantly higher in alcoholic hepatitis (AH) patients who subsequently died than those who survived[70]. Patients with AH had significantly higher plasma TNF-α concentration than did patients with inactive cirrhosis or alcoholics having no liver disease[71,72]. It has also shown that lipopolysaccharide (LPS), a component of the gram-negative bacterial cell wall, induces a marked TNF-α production in vivo in cirrhotic rats[73] and ex vivo in monocytes from cirrhotic patients[74]. Infliximab (anti-TNF antibody) treatment has been shown to reduce systemic TNF-α and a concomitant drop in portal pressure in alcoholic hepatitis patients with severe ED[75]. Our previous study also supported the above notion that treatment with anti-TNF improved hepatic DDAH enzyme function by decreasing ADMA level and a concomitant increase of hepatic eNOS activity and NO bioavailability in a bile duct-ligated cirrhotic rat[42]. Infliximab treatment also shown to beneficial in CCl4, and high fat diet induced liver disease models[76,77]. Furthermore, anti-TNF treatment to the portal vein ligated rats significantly blunts the development of the hyperdynamic circulation and reduces portal pressure[75].
Nuclear factor-κB
Nuclear factor-κB (NFκB) is a ubiquitous transcription factor that activated by a variety of cytokines which including TNF α and is thought to be a key regulator of genes involved in inflammation[47,78]. In BDL cirrhotic rats, elevated plasma F2-isoprostanes correlated with an increase of TNF-α and constitutive activation of NFκB[79]. Osanai et al[80] showed in HUVECs that synthesised ADMA by PRMT-1 was further stimulated by shear stress via activation of the NFκB pathway. Recently ADMA induces TNF-α production via ROS/NFκB dependent pathway also reported in human monocytes[81]. Activation of NFκB also increased in the liver of chronically HCV-infected patients compared with controls[82], which is in line with our previous observation that increased NFκB protein expression in BDL cirrhotic rats was downregulated following Infliximab treatment[47,78]. TNF-α facilitates the translocation of free NFκB from cytosol to the nucleus and the induction of iNOS gene expression. The overproduction of NO by iNOS is important in inflammation and causes ED and may associate with hyperdynamic circulation in cirrhosis[27,83,84]. In this context, recently Jalan et al[84] observed that an incubation of cirrhotic patient plasma or LPS with HUVECS showing increased iNOS activity. Thus, transjugular intrahepatic stent-shunt insertion (TIPSS) induced endotoxemia results in upregulation of the iNOS pathway in the endothelium of critically ill cirrhotic patients[84]. Hence, increased iNOS driven NO is marker for the treatment of inflammatory disorders, and its prevention is a target for the design of new drugs acting on iNOS[85,86]. In fact, inhibition of NFκB activation was initially considered important for designing NOS inhibitors, since NFκB mainly involved in iNOS expression during inflammatory conditions[47,85,86]. Therefore, the regulation of iNOS via the NFκB pathway is an important mechanism in inflammatory processes and potential site for intervention in inflammatory diseases.
Toll-like receptors (TLRs) belong to the family of transmembrane pattern-recognition receptors that recognize pathogen-associated molecular patterns, which including LPS[87]. TLR4 expressed on both parenchymal and non-parenchymal cell types in the liver and its activation trigger hepatic innate immune signaling, and may contribute to endothelial dysfunction and intrahepatic vascular tone in patients with cirrhosis[8,87,88]. In addition, the potential role of TLR4 in mediating renal injury in patients with cirrhosis was described recently[89]. The seminal observations made in this study were that the renal expression of TLR4 and the excretion of TLR4 protein was significantly higher in patients with cirrhosis who presented with acute deterioration and had renal dysfunction compared with those that did not[89]. Furthermore, urinary TLR4 was associated with significantly greater risk of death in patients with renal dysfunction and in those with superimposed inflammation[89]. TLR4 expressed on the surface of several cell types, including endothelial cells and its activation shown to reduce NO concentration, resulting ED[90]. Accordingly, anti-TLR4 treatment improved endothelium-dependent relaxation, and improved NO[91]. TLR4 also contributes to the increased ROS production and ED in hypertension, diabetes and obesity[92]. Recently, Benhamou et al[93] revealed that both TLR2 and TLR4 in mediating endothelial dysfunction and vascular remodeling in primary arterial antiphospholipid syndrome. TRL4 signalling leads to activation of NF-κB[94], a pathway associated with endothelial injury[95], and increased TLR4 expression has also shown in advanced liver disease[89,96]. LPS induced TLR4 mediated proinflammatory signalling has also showed in human hepatic stellate cells (HSC)[97], and functional expression of TLR9 has detected in sinusoidal hepatic endothelial cells and hepatocytes[98]. Furthermore, several animal studies support the importance of TLR4 in hepatic fibrosis, and TLR4 knockout mice showing less fibrosis induced by BDL or carbon tetrachloride (CCl4) compared to wild type[99]. These results suggested an important function of TLRs on the development of inflammatory pathology in hepatic cirrhosis. Stadlbauer et al[96] observed that in AH patients, the increased TLR 2, 4 and 9 expressions correlated with neutrophil dysfunction and endotoxemia, albumin an endotoxin scavenger attenuated these complaints by decreasing TLRs expression. Several lines of evidence exist implicating gut derived endotoxemia in the pathogenesis of portal hypertension[100]. Administration of norfloxacin, a selective gut decontaminant prophylactic reduced endotoxin levels, TLR4 expression and decreased NO-mediated forearm vasodilatation and improved survival in cirrhosis[94,101].
Angiotensin II
The renin-angiotensin system (RAS) plays a key physiological role in regulating vascular function. In the pathophysiology, RAS has also shown to promote vascular injury by triggering ED, vascular remodelling and vascular inflammation[102,103]. Angiotensin (Ang) II the core composition of the RAS involved in many chronic diseases, which including hepatic cirrhosis[104]. Increased Ang II causes endothelial dysfunction, vasoconstriction, sodium water retention, elevated blood pressure, ROS generation, inflammatory mediators and pro-fibrotic cytokines[105]. The adverse effects of Ang II induced ED is mediated by interaction with the plasma membrane AT 1 receptors (Ang II type 1 receptors) and causes NO reduction by inducing eNOS enzyme dysfunction and promoting NOS uncoupling. Thus, pharmacological inhibition of the production or actions of Ang II receptor blockers now represents an effective strategy to delay the progression of endothelial dysfunction in experimental models and humans[105,106]. In this context, previous clinical studies have shown evidence that RAS play an important role in the elevation of the ADMA concentration in hypertensive patients, and blockade of Ang II by ACEI or Ang II receptor blocker (ARB) significantly attenuates the elevated level of ADMA, resulting in endothelial protection[107,108]. Pharmacological blockade of angiotensin II receptors using the drug Candesartan cilextil (CC) may attenuate the progression of liver cirrhosis and endothelial dysfunction. In HUVECS, CC increased the eNOS protein level, inhibited the expression of nicotinamide adenine dinucleotide phosphate oxidase (Nox) subunits and Ang II induced intracellular ROS and nitric oxide, and promoted the extracellular release of nitric oxide[109] suggesting that it augmented the bioavailability of nitric oxide. Thus, CC administration may attenuate ED and for the future therapeutic approach in portal hypertensive patients with cirrhosis. Ang II can also activate NADPH oxidase (Nox), leading to increased ROS generation and commencing ED in cirrhosis[104].
TXA2, a vasoactive prostanoid and COX metabolite, increased intrahepatic vascular tone in cirrhosis, more specifically in the phenomenon of hyper responsiveness to vasoconstrictors and caused intrahepatic ED[110,111]. Administration of COX inhibitors such as flurbiprofen and nitroflurbiprofen (NO releasing COX inhibitor) to cirrhotic rats result in decreased hepatic TXA2 production and an increased intrahepatic nitrate/nitrite (an index of NO synthesis) concentration thereby by attenuating intrahepatic vascular resistance, endothelial dysfunction, and hepatic hyper reactivity to vasoconstrictors[111]. TXA2 and PGI2, the other COX 2 derivatives may act simultaneously, producing a compensatory effect that reduces NO release and may limit the hyperdynamic circulation in cirrhosis[112]. The use of indomethacin, a COX inhibitor has shown to prevent liver fibrosis, and rise in portal hypertension in liver cirrhosis[113]. Indeed, study also shows that COX inhibitors could worsen the hyperdynamic circulation associated with liver cirrhosis[112]. TXA2 induces vasoconstriction by activating the TXA2/prostaglandin-endoperoxide (TP) receptor[114]. TP receptor ligands include TXA2, PGH2, and isoprostanes[115,116]. TXA2 acts through its G-protein-coupled receptor leading to vasoconstriction by activating the RhoA/Rho-kinase pathway, and by increasing calcium levels in HSC[117]. Oral administration of terutroban, a specific antagonist of the TP-receptor[118] has shown to attenuates inflammation and oxidative stress and reduce RhoA/Rho-kinase-dependent signaling and restore NO bioavailability in endothelial cells[119] may represent a useful agent in the treatment of endothelial dysfunction in cirrhosis with portal hypertension.
A most potent vasoconstrictor endothelin (ET) -1 regarded as a key player in ED, primarily binding to G-protein coupled receptors such as ETA and ETB and acts in a paracrine fashion[120]. Previous study has shown that ETB receptors present in endothelial cell can elicit endothelium-dependent relaxation by improving NO release by contrast, ETA and ETB receptors present on the fibroblasts and smooth muscle cells trigger vasoconstriction and inflammation[121]. Cirrhotic rats with diabetes showed higher intrahepatic ET-1 vasoresponsiveness than normoglycemic cirrhotic rats[122]. ED also found in patients with insulin resistance (IR)[123,124]. In this context, IR can be triggered by ET-1 infusion in rats by activating phosphatidylinositol (PI) 3-kinase activity in smooth muscle cells in an ETA dependent manner and treatment with ETA receptor antagonists results in improvement of insulin sensitivity and associated endothelial function via an inhibition of PI 3-kinase activity[125]. In this context, a very recent study pointed out that LPS stimulation to portal hypertensive rats showed enhanced renal vascular response to ET-1 through ETA overexpression[126].
Lectin-like oxidised LDL receptor-1 (LOX1) is a key receptor for oxidised low-density lipoprotein (Ox-LDL) and identified in endothelial cells, and considered as a marker of ED in various pathological setting[127,128]. LOX-1 promotes ROS generation augments endothelial adhesiveness to monocytes and inhibits NO synthesis[129]. The recent review by Lubrano et al[130] described in detail about the relationship between LOX1 and ROS. Furthermore, increased expression of LOX1 was found in the placenta of women with intrahepatic cholestasis during pregnancy[131]. LOX-1 polymorphism also associated with liver disease severity in non-alcoholic steatohepatitis[132] and could be a biomarker for patients with endothelial dysfunction in liver cirrhosis.
Protease activated receptors (PARs) are G protein-coupled receptors which, mediating cellular effects of some proteases of the activated coagulation system such as thrombin, trypsin or metalloproteinase[133]. ECs express PAR1, PAR2 and PAR4[134]. Endothelial PARs play important roles in the crosstalk between coagulation and inflammation in sepsis[133]. Acute PAR1 activation causes an increase in vascular permeability, presumably due to direct endothelial contractile responses[133]. PAR1 deficiency and blockade has shown to reduce inflammation in a mouse model of colitis[135]. Moreover, activation of protease through its receptor following thrombus formation, hemorrhage and inflammation led to the conversion of ECs to a proinflammatory phenotype and may result in vascular lesion development[133]. Garcia et al[136] have shown in cultured ECs, PAR1 activation stimulates the production of prostacyclin and NO, consisting with other reports shown in in vivo that PAR1 activators cause hypotension when injected intravenously and cause NO mediated vasodilation[137]. In this context, Knight et al[138] and Sakata et al[139] reported that PAR2 promotes experimental liver fibrosis by increase of TGF β production in mice and to induce a profibrogenic phenotype in human HSCs. Thus, targeting PARs and using its antagonists in endothelial dysfunction with cirrhosis may represent a novel therapeutic approach in preventing portal hypertension in cirrhosis.
Adiponectin is a protein hormone synthesized by adipose tissue. Plasma physiological concentration of adiponectin represents 0.05% of all plasma proteins and is a key component in the relationship between adiposity, inflammation and insulin resistance[140]. Previous studies have shown evidence that the association between hypoadiponectinemia and ED[141,142]. Wang et al[142] showed in ECs that adiponectin stimulating NO synthesis by activating AMPK mediated pathway. Furthermore, adiponectin knockout mice exhibited impaired endothelium dependent vasodilation and NO production[143]. Earlier published studies have pointed out that adiponectin administration significantly increases NO production by regulating eNOS enzyme activity and its phosphorylation and maintain endothelial function[141]. However, despite the hepatoprotective effect of adiponectin have shown in NAFLD and other chronic liver ailments, the plasma concentration of which increased in patients with cirrhosis of different aetiologies. Indeed, the factors related to elevated levels of adiponectin in cirrhosis are not yet completely understood. In this context, Tietge et al[144] showed an evidence that increased adiponectin levels in cirrhotic patients correlate exclusively with reduced liver function and altered hepatic haemodynamics. Furthermore, Salman et al[145] and Tacke et al[146] reported that an elevated adiponectin correlated with inflammation and liver damage and high levels were found in human cholestasis as well as in an animal model of cirrhosis[145,146]. Thus, adiponectin may serve as a novel biomarker for cholestasis in liver cirrhosis and agents that modifying adiponectin concentration in liver cirrhosis may use as a potential diagnostic tool but also as therapeutic target for ED in cirrhosis.
Liver plays a significant role in lipid homeostasis including several stages of lipid synthesis and transportation. Thus, it is reasonable to anticipate an abnormal lipid profile associated with the progression of hepatic dysfunction. Furthermore, hyperlipidaemia is main risk factor for ED, which is a common indicator in patients with hepatic cirrhosis[147]. Accumulated evidence indicates that increased hepatic and plasma free fatty acid (FFA) concentration led to hyperlipidemia and may cause ED in cirrhosis. Previously it has been shown that FFA trigger HUVECs apoptosis and inhibit cell cycle progression[148]. In this regard, palmitic acid a key FFA in the bloodstream, exposure to ECs causes cell necrosis and the release of proinflammatory cytokines[149,150] consistent with other report showing in cultured bovine retinal pericytes, in which palmitate can induce apoptosis by promoting oxidative stress[151]. Recently Ristic-Medic et al[152] observed in cirrhotic patients, increased levels of palmitic acid and total saturated fatty acids when compared to healthy controls. Thus, FFA play an important role in cirrhosis with ED and agents that reduce palmitic acid concentration would be used as a possible future target for therapy. One such agent, eicosapentaenoic acid (EPA), ω-3 polyunsaturated fatty acid (PUFA) is abundant in fish oil has shown to enhance the production of NO, via activating eNOS and improve normal vascular endothelium[153]. Very recently Lee et al[154] have demonstrated that treatment with EPA protects against palmitic acid induced ED through activation of the AMPK-eNOS mediated pathway. Highly purified EPA also shown to prevent the development of inflammation and hepatic fibrosis in rats[155,156]. In addition, peroxisome proliferator-activated receptor (PPAR)-α, a member of the nuclear receptor superfamily and a key regulator of fatty acid homeostasis[157], has been shown to improve endothelial dysfunction and portal pressure in cirrhotic rats[158]. The above indices, therefore, provide a rationale for novel insights into the pathophysiology of ED and the potential for the development of novel biomarkers and therapeutic approaches in patients with endothelial dysfunction and advanced liver disease.
ED is an early event in the pathogenesis of cirrhosis with PHT and can be reversible with certain therapies (Table 2). Restoration of ED appears to be a crucial therapeutic target, since ED predicts most of the liver related problems in alcoholic liver disease (ALD), hepatorenal syndrome (HRS), hepatic encephalopathy (HE) and sepsis.
| Therapeuticagent | Endothelial function | Ref. |
| Anti- inflammatory agents | Increase of NO bioavailability | [25,42,75,111] |
| Reduction of ADMA | ||
| Upregulation of eNOS activity | ||
| Decrease of Inflammation | ||
| Vitamins | Improvement of eNOS activity | [159,160,162] |
| Increase of NO bioavailability | ||
| Scavenging of ROS generation | ||
| Antioxidant function | ||
| Flavonoids | Increase of NO bioavailability | [15,166,168] |
| Prevention of oxidative stress | ||
| Improvement of antioxidant enzymes | ||
| Nuclear receptors | Increase of NO bioavailability | [33,50,187] |
| Improvement of DDAH | ||
| Reduction of ADMA | ||
| Amelioration of hepatic vascular tone | ||
| Ammonia lowering agents | Detoxification of ammonia levels | [27,189,190,192,201] |
| Increase of NO bioavailability | ||
| Reduction of ADMA | ||
| Upregulation DDAH expression | ||
| Statins | Decrease of total cholesterol | [147,170,172,202,203] |
| Improvement of Akt-dependent eNOS phosphorylation | ||
| Promoting NO biosynthesis | ||
| Reduction of Ox-LDL | ||
| Attenuation of inflammatory indices | ||
| Beta blockers | Amelioration of oxidative stress | [194,196] |
| Attenuation of Inflammation | ||
| Restoration of antioxidant enzymes | ||
| Angiotensin- receptor antagonists | Increase of NO | [200,204] |
| Decrease of Ang-II mediated inflammation | ||
| Decrease of TIMP-1, MMP-2 mediated fibrosis |
Ascorbic acid (vitamin C) has been shown to improve the NO-dependent vasodilatation in vascular beds of patients with conditions characterized by marked ED in cirrhosis[159]. The other antioxidant α-tocopherol (vitamin E) has also shown to improvement of hepatic ED by suppressed hepatic ADMA and oxidative stress and improved hepatic NO in cirrhotic rats[160]. In addition, folic acid, a superoxide scavenging vitamin B9 and its active metabolite 5-methyltetrahydrofolate (5-MTHF) has been shown to restore ED in patients with many cardiovascular diseases[161]. Folic acid mainly involved in downregulating eNOS derived superoxide and eNOS uncoupling thereby improving regeneration of BH4 from BH2, by preventing BH4 oxidation, which results in increased NO[161]. The beneficial effects of 5-MTHF have shown in decompensated cirrhotic patients recently[162]. Superoxide dismutase (SOD) gene transfer also has shown to reduce portal pressure in CCl4 cirrhotic rats with portal hypertension through reducing oxidative stress and increased NO bioavailability[61]. Tempol, a SOD mimetic reduces superoxide, increases nitric oxide, and reduces portal pressure in sinusoidal endothelial cells of cirrhotic livers[163]. Furthermore, flavonoids, an integral part of the human diet have been shown to confer protective effects on vascular endothelial function in humans[164], and its protective effects are chiefly ascribed to their antioxidant and vasodilatory actions[165]. Very recently, Hsu et al[15] found that green tea polyphenol decreases the severity of portosystemic collaterals and mesenteric angiogenesis in rats with liver cirrhosis. Furthermore, De Gottardi et al[166] observed in cirrhotic patients with portal hypertension that dark chocolate blunted the postprandial increase in hepatic venous pressure gradient (HVPG) by improving flow-mediated hepatic vasorelaxation and ameliorated systemic hypotension. In addition, Lin et al[167] showed quercetin supplementation has associated with multifactorial potential as well as down-regulation of NF-κB and TGF-β/Smad signalling, probably via interference with TLR signalling. Resveratrol, a natural polyphenolic flavonoid present higher amount in grapes has shown to reduce portal pressure by attenuating ED. Moreover, resveratrol supplementation also results in reducing oxidative stress and upregulating eNOS expression without affecting systemic hemodynamics in cirrhotic rats[168].
Statins (HMG-CoA reductase inhibitors) lower serum cholesterol concentrations and exhibits beneficial therapeutic effects in cirrhotic patients, as evidenced by various clinical trials[169-171]. Abraldes et al[169] demonstrated that simvastatin improve hepatic NO generation and endothelial function and lowers portal pressure in patients with cirrhosis. Moreover, atorvastatin has been shown to prevent liver inflammation and HSC activation induced by Ang-II infusion[172]. Schwabl et al[172] found that pioglitazone, an insulin sensitiser decreases portosystemic shunting by modulating inflammation and angiogenesis in cirrhotic and non-cirrhotic portal hypertensive rats. Additionally, Sorafenib, a tyrosine kinase inhibitor approved in the treatment of hepatocellular carcinoma, has shown to have beneficial effects in reducing portal pressure in cirrhosis[173]. The other multitarget receptor tyrosine kinase inhibitors such as Sunitinib and Imatinib were also shown to use for the treatment of portal hypertension[174] and may have a potential role in regulating ED in cirrhosis through NO mediated mechanisms. However, further encouraging clinical studies of statins strategy to ED in cirrhosis needs to be explored.
Human serum albumin (HSA) is one of the most frequent treatments in patients with decompensated cirrhosis[175]. It also reduced the severity of other chronic liver diseases such as HE and HRS and improved survival of patients with spontaneous bacterial peritonitis (SBP)[175-177]. In addition, albumin has been demonstrated to have a clinically significant beneficial effect on ED and survival during experimental endotoxemia[178]. It also reduced sequential organ failure assessment (SOFA) score in critically ill patients with hypoalbuminemia[179]. Furthermore, pentoxifylline and N-acetylcysteine, the other known anti-inflammatory agents have shown to associated with reduced the risk of inflammation and ED in cirrhosis[180-182]. Antibiotics such as quinolones and rifaximin and high-density lipoprotein (HDL) treatment have shown to associate with the reduction of inflammation and portal pressure by neutralising portal bacterial endotoxin load[20,101,183]. Thus, anti-inflammatory agents would improve NO bioavailability and reduce ED, considered a potential therapeutic approach for the management of portal hypertension in cirrhosis.
Obeticholic acid, a synthetic farnesoid X receptor (FXR) ligand belongs to a nuclear receptor superfamily of transcription factor, which plays an important role in bile acid and lipid metabolism[184,185] has also been the subject of considerable attention over recent years[50]. FXR agonists have numerous target genes including DDAH1[186]. Our previous study has shown evidence that obeticholic acid significantly increases hepatic DDAH-1 and eNOS activity and improved NO bioavailability in cirrhotic rats, leading to improvement in endothelial function and associated drop in portal pressure[33]. Similarly, a multi-centre phase 2a trial of obeticholic acid in decompensated cirrhotic patients show a trend towards a drop in portal pressure[187]. Another promising approach of transfection of cirrhotic liver with DDAH-1 decreased ED and portal hypertension in BDL rats[188]. Furthermore, other member of the nuclear receptor superfamily, PPAR- α also has shown to improve endothelial dysfunction and portal pressure in cirrhosis[158].
AST-120, an oral adsorbent carbon microspheres and ammonia-lowering agent[189] has shown to reduce ED in adenine-induced uremic rats[190]. AST-120 treatment was also shown to prevent the progression of HE in cirrhotic rats[189] and chronic kidney disease (CKD) in a clinical setting[191], which may be used for the treatment of ED in cirrhosis. Indeed, further studies would be needed for describing the potential role of AST-120 on NO mediated ED in cirrhosis. Furthermore, we established a new promising therapy such as OCR- 002 (ornithine-phenylacetate) for the treatment of HE[27,192]. OCR-002 is currently being advanced in the clinic and also effective in reducing PHT by lowering ammonia mediated inflammation and improving NO bioavailability[193] and may consider for the future therapeutic approach for the management of ED and associated PHT in patient with cirrhosis.
Non-selectiveβblockers
Carvedilol is a non-selective β blocker with alpha-1 adrenergic blocker activity has been shown to amelioration of oxidative stress and restoration of antioxidant enzyme activities, and attenuation of NF-κB mediated inflammation in chronic liver disease[194]. Interestingly, Reiberger et al[195] observed in BDL cirrhotic rat that nebivolol, a third generation beta-blocker increased splanchnic blood flow and portal pressure via NO mediated signalling. In this context, Ma et al[196] demonstrated in myocardial infraction that targeting NO with a nebivolol treatment improves diastolic dysfunction through reducing myocardial oxidative stress by enhancing 5’-AMP-activated protein kinase and Akt activation of NO biosynthesis. Moreover, long-term nebivolol administration reduces renal fibrosis and prevents endothelial dysfunction in a rat hypertensive model[197]. In this context, Mookerjee et al[11] have pointed out that specific controlled studies are addressing the use of β-blockers in patients with severe decompensation of cirrhosis with high risk of sepsis and renal dysfunction are inadequate. Hence, further clinical studies on the effect of β-blockers through NO mediated pathway are challenging in liver cirrhosis.
Additionally, Candesartan cilextil (CC), a selective angiotensin II type I (AT1) receptor antagonist widely used as an antihypertensive in clinical practice has shown to improve ED[198,199]. In addition, recent clinical study has shown evidence that CC administration was safe and well tolerated to compensated cirrhotic patients, without an underlying cause of renal failure or hepatic decompensation[200]. Given the substantial experimental evidence that CC has the potential beneficial effect in distinct human diseases by blocking Ang-II mediated AT1 receptor and may improve ED and NO bioavailability and associated mechanism in cirrhosis.
In conclusion, this review has been discussed the involvement of various inflammatory and oxidative stress markers on the regulation of NO biosynthesis and associated ED. The therapeutic interventions, which including antioxidants, anti-inflammatory and ammonia lowering agents, bile acid receptors, statins, insulin sensitisers, beta blockers and ARBs have been shown to increasingly recognised to attenuate liver cirrhosis by decreasing inflammation, oxidative stress and promoting NO biosynthesis. Subsequently, the development of an ideal therapy based on the increase of hepatic NO synthesis through ADMA-DDAH pathway may improve endothelial function and reduce inflammation, subsequent portal pressure reduction and without compromising systemic arterial pressure in patients with advanced liver disease. Remarkably, DDAH-1 gene strategy to cirrhotic liver would advance future therapeutic attention in the context of improvement of NO synthesis and reduce inflammation and associated ED and portal pressure reduction in-patient with cirrhosis.
P- Reviewer: Pan WS, Rajeshwari K S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 864] [Cited by in F6Publishing: 904] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 2. | Inagami T, Naruse M, Hoover R. Endothelium as an endocrine organ. Annu Rev Physiol. 1995;57:171-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 115] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 795] [Cited by in F6Publishing: 798] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 4. | Ganz P, Vita JA. Testing endothelial vasomotor function: nitric oxide, a multipotent molecule. Circulation. 2003;108:2049-2053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Galle J, Quaschning T, Seibold S, Wanner C. Endothelial dysfunction and inflammation: what is the link? Kidney Int Suppl. 2003;S45-S49. [PubMed] [Cited in This Article: ] |
| 6. | Gupta TK, Toruner M, Chung MK, Groszmann RJ. Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats. Hepatology. 1998;28:926-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 250] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Bosch J, Abraldes JG, Fernández M, García-Pagán JC. Hepatic endothelial dysfunction and abnormal angiogenesis: new targets in the treatment of portal hypertension. J Hepatol. 2010;53:558-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Mehta G, Gustot T, Mookerjee RP, Garcia-Pagan JC, Fallon MB, Shah VH, Moreau R, Jalan R. Inflammation and portal hypertension - the undiscovered country. J Hepatol. 2014;61:155-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Goveia J, Stapor P, Carmeliet P. Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease. EMBO Mol Med. 2014;6:1105-1120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 10. | Strisciuglio T, De Luca S, Capuano E, Luciano R, Niglio T, Trimarco B, Galasso G. Endothelial dysfunction: its clinical value and methods of assessment. Curr Atheroscler Rep. 2014;16:417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Mookerjee RP. Acute-on-chronic liver failure: the liver and portal haemodynamics. Curr Opin Crit Care. 2011;17:170-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Mookerjee RP, Lackner C, Stauber R, Stadlbauer V, Deheragoda M, Aigelsreiter A, Jalan R. The role of liver biopsy in the diagnosis and prognosis of patients with acute deterioration of alcoholic cirrhosis. J Hepatol. 2011;55:1103-1111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Henderson NC, Iredale JP. Liver fibrosis: cellular mechanisms of progression and resolution. Clin Sci (Lond). 2007;112:265-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 14. | Mookerjee RP, Vairappan B, Jalan R. The puzzle of endothelial nitric oxide synthase dysfunction in portal hypertension: The missing piece? Hepatology. 2007;46:943-946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Hsu SJ, Wang SS, Hsin IF, Lee FY, Huang HC, Huo TI, Lee WS, Lin HC, Lee SD. Green tea polyphenol decreases the severity of portosystemic collaterals and mesenteric angiogenesis in rats with liver cirrhosis. Clin Sci (Lond). 2014;126:633-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157. [PubMed] [Cited in This Article: ] |
| 17. | Rockey D. The cellular pathogenesis of portal hypertension: stellate cell contractility, endothelin, and nitric oxide. Hepatology. 1997;25:2-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Pechánová O, Simko F. The role of nitric oxide in the maintenance of vasoactive balance. Physiol Res. 2007;56 Suppl 2:S7-S16. [PubMed] [Cited in This Article: ] |
| 19. | Mookerjee RP, Balasubramaniyan V, Mehta G. ADMA and hepatic endothelial dysfunction in cirrhosis--the DDAH isoform is the key. Liver Int. 2012;32:1186; author reply 1187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Thabut D, Massard J, Gangloff A, Carbonell N, Francoz C, Nguyen-Khac E, Duhamel C, Lebrec D, Poynard T, Moreau R. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology. 2007;46:1872-1882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 21. | Laleman W, Landeghem L, Wilmer A, Fevery J, Nevens F. Portal hypertension: from pathophysiology to clinical practice. Liver Int. 2005;25:1079-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Clapp BR, Hingorani AD, Kharbanda RK, Mohamed-Ali V, Stephens JW, Vallance P, MacAllister RJ. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res. 2004;64:172-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 193] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 23. | Gracia-Sancho J, Maeso-Díaz R, Fernández-Iglesias A, Navarro-Zornoza M, Bosch J. New cellular and molecular targets for the treatment of portal hypertension. Hepatol Int. 2015;Mar 5; Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 24. | Bosch-Marcé M, Morales-Ruiz M, Jiménez W, Bordas N, Solé M, Ros J, Deulofeu R, Arroyo V, Rivera F, Rodés J. Increased renal expression of nitric oxide synthase type III in cirrhotic rats with ascites. Hepatology. 1998;27:1191-1199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Kakumitsu S, Shijo H, Yokoyama M, Kim T, Akiyoshi N, Ota K, Kubara K, Okumura M, Inoue K. Effects of L-arginine on the systemic, mesenteric, and hepatic circulation in patients with cirrhosis. Hepatology. 1998;27:377-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Balasubramaniyan V, Davies N, Daltan RN, Turner C, Williams R, Jalan R, Mook-erjee RP. The role of DDAH-ADMA in the regulation of hepatic eNOS activity in acute and chronic liver failure. Hepatology. 2008;48:1045A. [Cited in This Article: ] |
| 27. | Balasubramaniyan V, Wright G, Sharma V, Davies NA, Sharifi Y, Habtesion A, Mookerjee RP, Jalan R. Ammonia reduction with ornithine phenylacetate restores brain eNOS activity via the DDAH-ADMA pathway in bile duct-ligated cirrhotic rats. Am J Physiol Gastrointest Liver Physiol. 2012;302:G145-G152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Shah V, Toruner M, Haddad F, Cadelina G, Papapetropoulos A, Choo K, Sessa WC, Groszmann RJ. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology. 1999;117:1222-1228. [PubMed] [Cited in This Article: ] |
| 29. | Zimmermann K, Opitz N, Dedio J, Renne C, Muller-Esterl W, Oess S. NOSTRIN: a protein modulating nitric oxide release and subcellular distribution of endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2002;99:17167-17172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Icking A, Matt S, Opitz N, Wiesenthal A, Müller-Esterl W, Schilling K. NOSTRIN functions as a homotrimeric adaptor protein facilitating internalization of eNOS. J Cell Sci. 2005;118:5059-5069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Icking A, Schilling K, Wiesenthal A, Opitz N, Müller-Esterl W. FCH/Cdc15 domain determines distinct subcellular localization of NOSTRIN. FEBS Lett. 2006;580:223-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Mookerjee RP, Malaki M, Davies NA, Hodges SJ, Dalton RN, Turner C, Sen S, Williams R, Leiper J, Vallance P. Increasing dimethylarginine levels are associated with adverse clinical outcome in severe alcoholic hepatitis. Hepatology. 2007;45:62-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Vairappan B, Sharma V, Winstanley A, Davies N, Shah N, Jalan R, Mookerjee RP. Modulation of the DDAH-ADMA pathway with the Farnesoid receptor (FXR) agonist INT-747 restores hepatic eNOS activity and lowers portal pressure in cirrhotic rats. Hepatology. 2009;50:336A-337A. [Cited in This Article: ] |
| 34. | Nijveldt RJ, Teerlink T, Van Der Hoven B, Siroen MP, Kuik DJ, Rauwerda JA, van Leeuwen PA. Asymmetrical dimethylarginine (ADMA) in critically ill patients: high plasma ADMA concentration is an independent risk factor of ICU mortality. Clin Nutr. 2003;22:23-30. [PubMed] [Cited in This Article: ] |
| 35. | Kasumov T, Edmison JM, Dasarathy S, Bennett C, Lopez R, Kalhan SC. Plasma levels of asymmetric dimethylarginine in patients with biopsy-proven nonalcoholic fatty liver disease. Metabolism. 2011;60:776-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Vizzutti F, Romanelli RG, Arena U, Rega L, Brogi M, Calabresi C, Masini E, Tarquini R, Zipoli M, Boddi V. ADMA correlates with portal pressure in patients with compensated cirrhosis. Eur J Clin Invest. 2007;37:509-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Mookerjee RP, Dalton RN, Davies NA, Hodges SJ, Turner C, Williams R, Jalan R. Inflammation is an important determinant of levels of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine (ADMA) in acute liver failure. Liver Transpl. 2007;13:400-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Leiper J, Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res. 1999;43:542-548. [PubMed] [Cited in This Article: ] |
| 39. | Antoniades C, Shirodaria C, Leeson P, Antonopoulos A, Warrick N, Van-Assche T, Cunnington C, Tousoulis D, Pillai R, Ratnatunga C. Association of plasma asymmetrical dimethylarginine (ADMA) with elevated vascular superoxide production and endothelial nitric oxide synthase uncoupling: implications for endothelial function in human atherosclerosis. Eur Heart J. 2009;30:1142-1150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 40. | Abedini S, Meinitzer A, Holme I, März W, Weihrauch G, Fellstrøm B, Jardine A, Holdaas H. Asymmetrical dimethylarginine is associated with renal and cardiovascular outcomes and all-cause mortality in renal transplant recipients. Kidney Int. 2010;77:44-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O’Hara B, Rossiter S, Anthony S, Madhani M, Selwood D. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med. 2007;13:198-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 42. | Balasubramaniyan V, Sharma V, Metha G, Habtesion A, Turner C, Dalton RN, Davies N, Jalan R, Mookerjee RP. Acute lowering of portal pressure in cirrhotic rats by Anti-TNF therapy is associated with reduced NFkB-driven inflammation and improved eNOS function through the asymmetric dimethylarginine-dimethylarginine-diaminohydrolase axis. Gut. 2010;59 Suppl:A35. [DOI] [Cited in This Article: ] |
| 43. | Chen MF, Xie XM, Yang TL, Wang YJ, Zhang XH, Luo BL, Li YJ. Role of asymmetric dimethylarginine in inflammatory reactions by angiotensin II. J Vasc Res. 2007;44:391-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Laleman W, Omasta A, Van de Casteele M, Zeegers M, Vander Elst I, Van Landeghem L, Severi T, van Pelt J, Roskams T, Fevery J. A role for asymmetric dimethylarginine in the pathophysiology of portal hypertension in rats with biliary cirrhosis. Hepatology. 2005;42:1382-1390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Leiper JM, Santa Maria J, Chubb A, MacAllister RJ, Charles IG, Whitley GS, Vallance P. Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem J. 1999;343 Pt 1:209-214. [PubMed] [Cited in This Article: ] |
| 46. | Leiper JM. The DDAH-ADMA-NOS pathway. Ther Drug Monit. 2005;27:744-746. [PubMed] [Cited in This Article: ] |
| 47. | Balasubramaniyan V, Davies NA, Wright G, Dalton RN, Turner C, Williams R, Jalan R, Mookerjee RP. Treatment with Infliximab increases eNOS activity in cirrhosis through modulation of the DDAH-ADMA pathway. J Hepatol. 2009;50:S270. [Cited in This Article: ] |
| 48. | Ito A, Tsao PS, Adimoolam S, Kimoto M, Ogawa T, Cooke JP. Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrolase. Circulation. 1999;99:3092-3095. [PubMed] [Cited in This Article: ] |
| 49. | Forbes SP, Druhan LJ, Guzman JE, Parinandi N, Zhang L, Green-Church KB, Cardounel AJ. Mechanism of 4-HNE mediated inhibition of hDDAH-1: implications in no regulation. Biochemistry. 2008;47:1819-1826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 50. | Verbeke L, Farre R, Trebicka J, Komuta M, Roskams T, Klein S, Elst IV, Windmolders P, Vanuytsel T, Nevens F. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59:2286-2298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 193] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 51. | Laviña B, Gracia-Sancho J, Rodríguez-Vilarrupla A, Chu Y, Heistad DD, Bosch J, García-Pagán JC. Superoxide dismutase gene transfer reduces portal pressure in CCl4 cirrhotic rats with portal hypertension. Gut. 2009;58:118-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Lluch P, Mauricio MD, Vila JM, Segarra G, Medina P, Del Olmo JA, Rodrigo JM, Serra MA. Accumulation of symmetric dimethylarginine in hepatorenal syndrome. Exp Biol Med (Maywood). 2006;231:70-75. [PubMed] [Cited in This Article: ] |
| 53. | Siroen MP, Wiest R, Richir MC, Teerlink T, Rauwerda JA, Drescher FT, Zorger N, van Leeuwen PA. Transjugular intrahepatic portosystemic shunt-placement increases arginine/asymmetric dimethylarginine ratio in cirrhotic patients. World J Gastroenterol. 2008;14:7214-7219. [PubMed] [Cited in This Article: ] |
| 54. | Lluch P, Torondel B, Medina P, Segarra G, Del Olmo JA, Serra MA, Rodrigo JM. Plasma concentrations of nitric oxide and asymmetric dimethylarginine in human alcoholic cirrhosis. J Hepatol. 2004;41:55-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Gracia-Sancho J, Laviña B, Rodríguez-Vilarrupla A, García-Calderó H, Fernández M, Bosch J, García-Pagán JC. Increased oxidative stress in cirrhotic rat livers: A potential mechanism contributing to reduced nitric oxide bioavailability. Hepatology. 2008;47:1248-1256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Squadrito GL, Pryor WA. Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic Biol Med. 1998;25:392-403. [PubMed] [Cited in This Article: ] |
| 57. | Lee JH, Yang ES, Park JW. Inactivation of NADP+-dependent isocitrate dehydrogenase by peroxynitrite. Implications for cytotoxicity and alcohol-induced liver injury. J Biol Chem. 2003;278:51360-51371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 58. | Ridnour LA, Thomas DD, Mancardi D, Espey MG, Miranda KM, Paolocci N, Feelisch M, Fukuto J, Wink DA. The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxide species. Putting perspective on stressful biological situations. Biol Chem. 2004;385:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 217] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 59. | Bec N, Gorren AFC B, Schmidt PP, Andersson KK, Lange R. The role of tetrahydrobiopterin in the activation of oxygen by nitric-oxide synthase. J Inorg Biochem. 2000;81:207-211. [PubMed] [Cited in This Article: ] |
| 60. | Vásquez-Vivar J, Whitsett J, Martásek P, Hogg N, Kalyanaraman B. Reaction of tetrahydrobiopterin with superoxide: EPR-kinetic analysis and characterization of the pteridine radical. Free Radic Biol Med. 2001;31:975-985. [PubMed] [Cited in This Article: ] |
| 61. | Guillaume M, Rodriguez-Vilarrupla A, Gracia-Sancho J, Rosado E, Mancini A, Bosch J, Garcia-Pagán JC. Recombinant human manganese superoxide dismutase reduces liver fibrosis and portal pressure in CCl4-cirrhotic rats. J Hepatol. 2013;58:240-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 62. | Matei V, Rodríguez-Vilarrupla A, Deulofeu R, Colomer D, Fernández M, Bosch J, Garcia-Pagán JC. The eNOS cofactor tetrahydrobiopterin improves endothelial dysfunction in livers of rats with CCl4 cirrhosis. Hepatology. 2006;44:44-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 63. | Matei V, Rodríguez-Vilarrupla A, Deulofeu R, García-Calderó H, Fernández M, Bosch J, Garcia-Pagán JC. Three-day tetrahydrobiopterin therapy increases in vivo hepatic NOS activity and reduces portal pressure in CCl4 cirrhotic rats. J Hepatol. 2008;49:192-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 64. | MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641-650. [PubMed] [Cited in This Article: ] |
| 65. | Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 434] [Cited by in F6Publishing: 421] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 66. | Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829-837, 837a-837d. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2678] [Cited by in F6Publishing: 2500] [Article Influence: 208.3] [Reference Citation Analysis (0)] |
| 67. | Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1169] [Cited by in F6Publishing: 1283] [Article Influence: 91.6] [Reference Citation Analysis (1)] |
| 68. | Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002-2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4289] [Cited by in F6Publishing: 4089] [Article Influence: 131.9] [Reference Citation Analysis (0)] |
| 69. | Chia S, Qadan M, Newton R, Ludlam CA, Fox KA, Newby DE. Intra-arterial tumor necrosis factor-alpha impairs endothelium-dependent vasodilatation and stimulates local tissue plasminogen activator release in humans. Arterioscler Thromb Vasc Biol. 2003;23:695-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 70. | McClain CJ, Barve S, Deaciuc I, Kugelmas M, Hill D. Cytokines in alcoholic liver disease. Semin Liver Dis. 1999;19:205-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 315] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 71. | Bird GL, Sheron N, Goka AK, Alexander GJ, Williams RS. Increased plasma tumor necrosis factor in severe alcoholic hepatitis. Ann Intern Med. 1990;112:917-920. [PubMed] [Cited in This Article: ] |
| 72. | Schwabl P, Payer BA, Grahovac J, Klein S, Horvatits T, Mitterhauser M, Stift J, Boucher Y, Trebicka J, Trauner M. Pioglitazone decreases portosystemic shunting by modulating inflammation and angiogenesis in cirrhotic and non-cirrhotic portal hypertensive rats. J Hepatol. 2014;60:1135-1142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 73. | Heller J, Sogni P, Barrière E, Tazi KA, Chauvelot-Moachon L, Guimont MC, Bories PN, Poirel O, Moreau R, Lebrec D. Effects of lipopolysaccharide on TNF-alpha production, hepatic NOS2 activity, and hepatic toxicity in rats with cirrhosis. J Hepatol. 2000;33:376-381. [PubMed] [Cited in This Article: ] |
| 74. | Riordan SM, Skinner N, Nagree A, McCallum H, McIver CJ, Kurtovic J, Hamilton JA, Bengmark S, Williams R, Visvanathan K. Peripheral blood mononuclear cell expression of toll-like receptors and relation to cytokine levels in cirrhosis. Hepatology. 2003;37:1154-1164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Mookerjee RP, Sen S, Davies NA, Hodges SJ, Williams R, Jalan R. Tumour necrosis factor alpha is an important mediator of portal and systemic haemodynamic derangements in alcoholic hepatitis. Gut. 2003;52:1182-1187. [PubMed] [Cited in This Article: ] |
| 76. | Bahcecioglu IH, Koca SS, Poyrazoglu OK, Yalniz M, Ozercan IH, Ustundag B, Sahin K, Dagli AF, Isik A. Hepatoprotective effect of infliximab, an anti-TNF-alpha agent, on carbon tetrachloride-induced hepatic fibrosis. Inflammation. 2008;31:215-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 77. | Barbuio R, Milanski M, Bertolo MB, Saad MJ, Velloso LA. Infliximab reverses steatosis and improves insulin signal transduction in liver of rats fed a high-fat diet. J Endocrinol. 2007;194:539-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 78. | Balasubramaniyan V, Dhar DK, Warner AE, Vivien Li WY, Amiri AF, Bright B, Mookerjee RP, Davies NA, Becker DL, Jalan R. Importance of Connexin-43 based gap junction in cirrhosis and acute-on-chronic liver failure. J Hepatol. 2013;58:1194-1200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 79. | Harry D, Anand R, Holt S, Davies S, Marley R, Fernando B, Goodier D, Moore K. Increased sensitivity to endotoxemia in the bile duct-ligated cirrhotic Rat. Hepatology. 1999;30:1198-1205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 80. | Osanai T, Saitoh M, Sasaki S, Tomita H, Matsunaga T, Okumura K. Effect of shear stress on asymmetric dimethylarginine release from vascular endothelial cells. Hypertension. 2003;42:985-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 81. | Zhang GG, Bai YP, Chen MF, Shi RZ, Jiang DJ, Fu QM, Tan GS, Li YJ. Asymmetric dimethylarginine induces TNF-alpha production via ROS/NF-kappaB dependent pathway in human monocytic cells and the inhibitory effect of reinioside C. Vascul Pharmacol. 2008;48:115-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 82. | Boya P, Larrea E, Sola I, Majano PL, Jiménez C, Civeira MP, Prieto J. Nuclear factor-kappa B in the liver of patients with chronic hepatitis C: decreased RelA expression is associated with enhanced fibrosis progression. Hepatology. 2001;34:1041-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Ferguson JW, Dover AR, Chia S, Cruden NL, Hayes PC, Newby DE. Inducible nitric oxide synthase activity contributes to the regulation of peripheral vascular tone in patients with cirrhosis and ascites. Gut. 2006;55:542-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | Jalan R, Olde Damink SW, Ter Steege JC, Redhead DN, Lee A, Hayes PC, Deutz NE. Acute endotoxemia following transjugular intrahepatic stent-shunt insertion is associated with systemic and cerebral vasodilatation with increased whole body nitric oxide production in critically ill cirrhotic patients. J Hepatol. 2011;54:265-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 85. | Balasubramaniyan V, Davies N, Mookerjee R, Becker DL, Warner A, Jalan R. Inflammation upregulates hepatic connexin-43 expression in cirrhosis which defines susceptibility to development of acute-on chronic liver failure. J Hepatol. 2012;56:S236. [DOI] [Cited in This Article: ] |
| 86. | Wright G, Vairappan B, Stadlbauer V, Mookerjee RP, Davies NA, Jalan R. Reduction in hyperammonaemia by ornithine phenylacetate prevents lipopolysaccharide-induced brain edema and coma in cirrhotic rats. Liver Int. 2012;32:410-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258-1266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 349] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 88. | Hayashi T, Suzuki K. LPS-Toll-Like Receptor-Mediated Signaling on Expression of Protein S and C4b-Binding Protein in the Liver. Gastroenterol Res Pract. 2010;2010:pii: 189561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 89. | Shah N, Mohamed FE, Jover-Cobos M, Macnaughtan J, Davies N, Moreau R, Paradis V, Moore K, Mookerjee R, Jalan R. Increased renal expression and urinary excretion of TLR4 in acute kidney injury associated with cirrhosis. Liver Int. 2013;33:398-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 90. | Otsui K, Inoue N, Kobayashi S, Shiraki R, Honjo T, Takahashi M, Hirata K, Kawashima S, Yokoyama M. Enhanced expression of TLR4 in smooth muscle cells in human atherosclerotic coronary arteries. Heart Vessels. 2007;22:416-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 91. | Freitas MR, Schott C, Corriu C, Sassard J, Stoclet JC, Andriantsitohaina R. Heterogeneity of endothelium-dependent vasorelaxation in conductance and resistance arteries from Lyon normotensive and hypertensive rats. J Hypertens. 2003;21:1505-1512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 92. | Liang CF, Liu JT, Wang Y, Xu A, Vanhoutte PM. Toll-like receptor 4 mutation protects obese mice against endothelial dysfunction by decreasing NADPH oxidase isoforms 1 and 4. Arterioscler Thromb Vasc Biol. 2013;33:777-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 93. | Benhamou Y, Bellien J, Armengol G, Brakenhielm E, Adriouch S, Iacob M, Remy-Jouet I, Le Cam-Duchez V, Monteil C, Renet S. Role of Toll-like receptors 2 and 4 in mediating endothelial dysfunction and arterial remodeling in primary arterial antiphospholipid syndrome. Arthritis Rheumatol. 2014;66:3210-3220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 94. | Shah N, Dhar D, El Zahraa Mohammed F, Habtesion A, Davies NA, Jover-Cobos M, Macnaughtan J, Sharma V, Olde Damink SW, Mookerjee RP. Prevention of acute kidney injury in a rodent model of cirrhosis following selective gut decontamination is associated with reduced renal TLR4 expression. J Hepatol. 2012;56:1047-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 95. | Sun R, Zhu Z, Su Q, Li T, Song Q. Toll-like receptor 4 is involved in bacterial endotoxin-induced endothelial cell injury and SOC-mediated calcium regulation. Cell Biol Int. 2012;36:475-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 96. | Stadlbauer V, Mookerjee RP, Wright GA, Davies NA, Jürgens G, Hallström S, Jalan R. Role of Toll-like receptors 2, 4, and 9 in mediating neutrophil dysfunction in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G15-G22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 97. | Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043-1055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 484] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 98. | Martin-Armas M, Simon-Santamaria J, Pettersen I, Moens U, Smedsrød B, Sveinbjørnsson B. Toll-like receptor 9 (TLR9) is present in murine liver sinusoidal endothelial cells (LSECs) and mediates the effect of CpG-oligonucleotides. J Hepatol. 2006;44:939-946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 99. | Jagavelu K, Routray C, Shergill U, O’Hara SP, Faubion W, Shah VH. Endothelial cell toll-like receptor 4 regulates fibrosis-associated angiogenesis in the liver. Hepatology. 2010;52:590-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 100. | Benten D, Wiest R. Gut microbiome and intestinal barrier failure--the “Achilles heel” in hepatology? J Hepatol. 2012;56:1221-1223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 101. | Rasaratnam B, Kaye D, Jennings G, Dudley F, Chin-Dusting J. The effect of selective intestinal decontamination on the hyperdynamic circulatory state in cirrhosis. A randomized trial. Ann Intern Med. 2003;139:186-193. [PubMed] [Cited in This Article: ] |
| 102. | Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916-1923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1856] [Cited by in F6Publishing: 1804] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 103. | Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Fukui H. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology. 2001;34:745-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 302] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 104. | Grace JA, Klein S, Herath CB, Granzow M, Schierwagen R, Masing N, Walther T, Sauerbruch T, Burrell LM, Angus PW. Activation of the MAS receptor by angiotensin-(1-7) in the renin-angiotensin system mediates mesenteric vasodilatation in cirrhosis. Gastroenterology. 2013;145:874-884.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 105. | Pugsley MK. The angiotensin-II (AT-II) receptor blocker olmesartan reduces renal damage in animal models of hypertension and diabetes. Proc West Pharmacol Soc. 2005;48:35-38. [PubMed] [Cited in This Article: ] |
| 106. | Locatelli F, Del Vecchio L, Cavalli A. Inhibition of the renin-angiotensin system in chronic kidney disease: a critical look to single and dual blockade. Nephron Clin Pract. 2009;113:c286-c293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 107. | Ito A, Egashira K, Narishige T, Muramatsu K, Takeshita A. Renin-angiotensin system is involved in the mechanism of increased serum asymmetric dimethylarginine in essential hypertension. Jpn Circ J. 2001;65:775-778. [PubMed] [Cited in This Article: ] |
| 108. | Napoli C, Sica V, de Nigris F, Pignalosa O, Condorelli M, Ignarro LJ, Liguori A. Sulfhydryl angiotensin-converting enzyme inhibition induces sustained reduction of systemic oxidative stress and improves the nitric oxide pathway in patients with essential hypertension. Am Heart J. 2004;148:e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 109. | Liu H, Kitazato KT, Uno M, Yagi K, Kanematsu Y, Tamura T, Tada Y, Kinouchi T, Nagahiro S. Protective mechanisms of the angiotensin II type 1 receptor blocker candesartan against cerebral ischemia: in-vivo and in-vitro studies. J Hypertens. 2008;26:1435-1445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 110. | Graupera M, March S, Engel P, Rodés J, Bosch J, García-Pagán JC. Sinusoidal endothelial COX-1-derived prostanoids modulate the hepatic vascular tone of cirrhotic rat livers. Am J Physiol Gastrointest Liver Physiol. 2005;288:G763-G770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 111. | Laleman W, Van Landeghem L, Van der Elst I, Zeegers M, Fevery J, Nevens F. Nitroflurbiprofen, a nitric oxide-releasing cyclooxygenase inhibitor, improves cirrhotic portal hypertension in rats. Gastroenterology. 2007;132:709-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 112. | Xavier FE, Blanco-Rivero J, Sastre E, Badimón L, Balfagón G. Simultaneous inhibition of TXA(2) and PGI(2) synthesis increases NO release in mesenteric resistance arteries from cirrhotic rats. Clin Sci (Lond). 2010;119:283-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 113. | Birney Y, Redmond EM, Sitzmann JV, Cahill PA. Eicosanoids in cirrhosis and portal hypertension. Prostaglandins Other Lipid Mediat. 2003;72:3-18. [PubMed] [Cited in This Article: ] |
| 114. | Rosado E, Rodríguez-Vilarrupla A, Gracia-Sancho J, Monclús M, Bosch J, García-Pagán JC. Interaction between NO and COX pathways modulating hepatic endothelial cells from control and cirrhotic rats. J Cell Mol Med. 2012;16:2461-2470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 115. | Dogné JM, Hanson J, de Leval X, Pratico D, Pace-Asciak CR, Drion P, Pirotte B, Ruan KH. From the design to the clinical application of thromboxane modulators. Curr Pharm Des. 2006;12:903-923. [PubMed] [Cited in This Article: ] |
| 116. | Gardi C, Arezzini B, Monaco B, De Montis MG, Vecchio D, Comporti M. F2-isoprostane receptors on hepatic stellate cells. Lab Invest. 2008;88:124-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 117. | Nakahata N. Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacol Ther. 2008;118:18-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 292] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 118. | Cimetière B, Dubuffet T, Muller O, Descombes JJ, Simonet S, Laubie M, Verbeuren TJ, Lavielle G. Synthesis and biological evaluation of new tetrahydronaphthalene derivatives as thromboxane receptor antagonists. Bioorg Med Chem Lett. 1998;8:1375-1380. [PubMed] [Cited in This Article: ] |
| 119. | Liu CQ, Leung FP, Wong SL, Wong WT, Lau CW, Lu L, Yao X, Yao T, Huang Y. Thromboxane prostanoid receptor activation impairs endothelial nitric oxide-dependent vasorelaxations: the role of Rho kinase. Biochem Pharmacol. 2009;78:374-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 120. | Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8220] [Cited by in F6Publishing: 7891] [Article Influence: 219.2] [Reference Citation Analysis (0)] |
| 121. | Iglarz M, Clozel M. Mechanisms of ET-1-induced endothelial dysfunction. J Cardiovasc Pharmacol. 2007;50:621-628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 122. | Lee JY, Lee FY, Huo TI, Wang SS, Huang HC, Lin HC, Chuang CL, Lee SD. Diabetes enhances the intrahepatic vascular response to endothelin-1 in cirrhotic rats: association with the ETA receptor and pERK up-regulation. Liver Int. 2015;35:704-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 123. | Jiang ZY, Zhou QL, Chatterjee A, Feener EP, Myers MG, White MF, King GL. Endothelin-1 modulates insulin signaling through phosphatidylinositol 3-kinase pathway in vascular smooth muscle cells. Diabetes. 1999;48:1120-1130. [PubMed] [Cited in This Article: ] |
| 124. | Juan CC, Fang VS, Huang YJ, Kwok CF, Hsu YP, Ho LT. Endothelin-1 induces insulin resistance in conscious rats. Biochem Biophys Res Commun. 1996;227:694-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 125. | Berthiaume N, Carlson CJ, Rondinone CM, Zinker BA. Endothelin antagonism improves hepatic insulin sensitivity associated with insulin signaling in Zucker fatty rats. Metabolism. 2005;54:1515-1523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 126. | Chuang CL, Huang HC, Chang CC, Lee FY, Wu JC, Lee JY, Hsieh HG, Lee SD. Lipopolysaccharide enhanced renal vascular response to endothelin-1 through ETA overexpression in portal hypertensive rats. J Gastroenterol Hepatol. 2015;30:199-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 127. | Mehta JL, Chen J, Hermonat PL, Romeo F, Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res. 2006;69:36-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 350] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 128. | Xu S, Ogura S, Chen J, Little PJ, Moss J, Liu P. LOX-1 in atherosclerosis: biological functions and pharmacological modifiers. Cell Mol Life Sci. 2013;70:2859-2872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 129. | Kita T, Kume N, Minami M, Hayashida K, Murayama T, Sano H, Moriwaki H, Kataoka H, Nishi E, Horiuchi H. Role of oxidized LDL in atherosclerosis. Ann N Y Acad Sci. 2001;947:199-205; discussion 205-206. [PubMed] [Cited in This Article: ] |
| 130. | Lubrano V, Balzan S. LOX-1 and ROS, inseparable factors in the process of endothelial damage. Free Radic Res. 2014;48:841-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 131. | Yin Y, Zhu QY, Ren SJ, Wang DM. Increased lectin-like oxidized LDL receptor-1 expression in the placentas of women with intrahepatic cholestasis during pregnancy. Clin Exp Obstet Gynecol. 2012;39:149-152. [PubMed] [Cited in This Article: ] |
| 132. | Musso G, Cassader M, De Michieli F, Saba F, Bo S, Gambino R. Effect of lectin-like oxidized LDL receptor-1 polymorphism on liver disease, glucose homeostasis, and postprandial lipoprotein metabolism in nonalcoholic steatohepatitis. Am J Clin Nutr. 2011;94:1033-1042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 133. | Alberelli MA, De Candia E. Functional role of protease activated receptors in vascular biology. Vascul Pharmacol. 2014;62:72-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 134. | Kataoka H, Hamilton JR, McKemy DD, Camerer E, Zheng YW, Cheng A, Griffin C, Coughlin SR. Protease-activated receptors 1 and 4 mediate thrombin signaling in endothelial cells. Blood. 2003;102:3224-3231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 135. | Vergnolle N, Cellars L, Mencarelli A, Rizzo G, Swaminathan S, Beck P, Steinhoff M, Andrade-Gordon P, Bunnett NW, Hollenberg MD. A role for proteinase-activated receptor-1 in inflammatory bowel diseases. J Clin Invest. 2004;114:1444-1456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 136. | Garcia JG, Patterson C, Bahler C, Aschner J, Hart CM, English D. Thrombin receptor activating peptides induce Ca2+ mobilization, barrier dysfunction, prostaglandin synthesis, and platelet-derived growth factor mRNA expression in cultured endothelium. J Cell Physiol. 1993;156:541-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 92] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 137. | Cheung WM, D’Andrea MR, Andrade-Gordon P, Damiano BP. Altered vascular injury responses in mice deficient in protease-activated receptor-1. Arterioscler Thromb Vasc Biol. 1999;19:3014-3024. [PubMed] [Cited in This Article: ] |
| 138. | Knight V, Tchongue J, Lourensz D, Tipping P, Sievert W. Protease-activated receptor 2 promotes experimental liver fibrosis in mice and activates human hepatic stellate cells. Hepatology. 2012;55:879-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 139. | Sakata K, Eda S, Lee ES, Hara M, Imoto M, Kojima S. Neovessel formation promotes liver fibrosis via providing latent transforming growth factor-β. Biochem Biophys Res Commun. 2014;443:950-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 140. | Silva TE, Colombo G, Schiavon LL. Adiponectin: A multitasking player in the field of liver diseases. Diabetes Metab. 2014;40:95-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 141. | Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021-45026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 757] [Cited by in F6Publishing: 734] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 142. | Wang ZV, Scherer PE. Adiponectin, cardiovascular function, and hypertension. Hypertension. 2008;51:8-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 143. | Cao Y, Tao L, Yuan Y, Jiao X, Lau WB, Wang Y, Christopher T, Lopez B, Chan L, Goldstein B. Endothelial dysfunction in adiponectin deficiency and its mechanisms involved. J Mol Cell Cardiol. 2009;46:413-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 144. | Tietge UJ, Böker KH, Manns MP, Bahr MJ. Elevated circulating adiponectin levels in liver cirrhosis are associated with reduced liver function and altered hepatic hemodynamics. Am J Physiol Endocrinol Metab. 2004;287:E82-E89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 145. | Salman TA, Allam N, Azab GI, Shaarawy AA, Hassouna MM, El-Haddad OM. Study of adiponectin in chronic liver disease and cholestasis. Hepatol Int. 2010;4:767-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 146. | Tacke F, Wüstefeld T, Horn R, Luedde T, Srinivas Rao A, Manns MP, Trautwein C, Brabant G. High adiponectin in chronic liver disease and cholestasis suggests biliary route of adiponectin excretion in vivo. J Hepatol. 2005;42:666-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 147. | Cash WJ, O’Neill S, O’Donnell ME, McCance DR, Young IS, McEneny J, McDougall NI, Callender ME. Randomized controlled trial assessing the effect of simvastatin in primary biliary cirrhosis. Liver Int. 2013;33:1166-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 148. | Artwohl M, Roden M, Waldhäusl W, Freudenthaler A, Baumgartner-Parzer SM. Free fatty acids trigger apoptosis and inhibit cell cycle progression in human vascular endothelial cells. FASEB J. 2004;18:146-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 149. | Khan MJ, Rizwan Alam M, Waldeck-Weiermair M, Karsten F, Groschner L, Riederer M, Hallström S, Rockenfeller P, Konya V, Heinemann A. Inhibition of autophagy rescues palmitic acid-induced necroptosis of endothelial cells. J Biol Chem. 2012;287:21110-21120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 150. | Staiger H, Staiger K, Stefan N, Wahl HG, Machicao F, Kellerer M, Häring HU. Palmitate-induced interleukin-6 expression in human coronary artery endothelial cells. Diabetes. 2004;53:3209-3216. [PubMed] [Cited in This Article: ] |
| 151. | Cacicedo JM, Benjachareowong S, Chou E, Ruderman NB, Ido Y. Palmitate-induced apoptosis in cultured bovine retinal pericytes: roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes. 2005;54:1838-1845. [PubMed] [Cited in This Article: ] |
| 152. | Ristić-Medić D, Takić M, Vučić V, Kandić D, Kostić N, Glibetić M. Abnormalities in the serum phospholipids fatty acid profile in patients with alcoholic liver cirrhosis - a pilot study. J Clin Biochem Nutr. 2013;53:49-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 153. | Okuda Y, Kawashima K, Sawada T, Tsurumaru K, Asano M, Suzuki S, Soma M, Nakajima T, Yamashita K. Eicosapentaenoic acid enhances nitric oxide production by cultured human endothelial cells. Biochem Biophys Res Commun. 1997;232:487-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 109] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 154. | Lee CH, Lee SD, Ou HC, Lai SC, Cheng YJ. Eicosapentaenoic acid protects against palmitic acid-induced endothelial dysfunction via activation of the AMPK/eNOS pathway. Int J Mol Sci. 2014;15:10334-10349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 155. | Kajikawa S, Harada T, Kawashima A, Imada K, Mizuguchi K. Highly purified eicosapentaenoic acid ethyl ester prevents development of steatosis and hepatic fibrosis in rats. Dig Dis Sci. 2010;55:631-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 156. | Kajikawa S, Imada K, Takeuchi T, Shimizu Y, Kawashima A, Harada T, Mizuguchi K. Eicosapentaenoic acid attenuates progression of hepatic fibrosis with inhibition of reactive oxygen species production in rats fed methionine- and choline-deficient diet. Dig Dis Sci. 2011;56:1065-1074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 157. | Mandard S, Müller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci. 2004;61:393-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 740] [Cited by in F6Publishing: 737] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 158. | Rodríguez-Vilarrupla A, Laviña B, García-Calderó H, Russo L, Rosado E, Roglans N, Bosch J, García-Pagán JC. PPARα activation improves endothelial dysfunction and reduces fibrosis and portal pressure in cirrhotic rats. J Hepatol. 2012;56:1033-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 159. | Hernández-Guerra M, García-Pagán JC, Turnes J, Bellot P, Deulofeu R, Abraldes JG, Bosch J. Ascorbic acid improves the intrahepatic endothelial dysfunction of patients with cirrhosis and portal hypertension. Hepatology. 2006;43:485-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 160. | Yang YY, Lee TY, Huang YT, Chan CC, Yeh YC, Lee FY, Lee SD, Lin HC. Asymmetric dimethylarginine (ADMA) determines the improvement of hepatic endothelial dysfunction by vitamin E in cirrhotic rats. Liver Int. 2012;32:48-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 161. | Kietadisorn R, Juni RP, Moens AL. Tackling endothelial dysfunction by modulating NOS uncoupling: new insights into its pathogenesis and therapeutic possibilities. Am J Physiol Endocrinol Metab. 2012;302:E481-E495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 162. | Patanwala I, King MJ, Barrett DA, Rose J, Jackson R, Hudson M, Philo M, Dainty JR, Wright AJ, Finglas PM. Folic acid handling by the human gut: implications for food fortification and supplementation. Am J Clin Nutr. 2014;100:593-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 163. | García-Calderó H, Rodríguez-Vilarrupla A, Gracia-Sancho J, Diví M, Laviña B, Bosch J, García-Pagán JC. Tempol administration, a superoxide dismutase mimetic, reduces hepatic vascular resistance and portal pressure in cirrhotic rats. J Hepatol. 2011;54:660-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 164. | Stein JH, Keevil JG, Wiebe DA, Aeschlimann S, Folts JD. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation. 1999;100:1050-1055. [PubMed] [Cited in This Article: ] |
| 165. | Zenebe W, Pechánová O, Bernátová I. Protective effects of red wine polyphenolic compounds on the cardiovascular system. Exp Clin Cardiol. 2001;6:153-158. [PubMed] [Cited in This Article: ] |
| 166. | De Gottardi A, Berzigotti A, Seijo S, D’Amico M, Thormann W, Abraldes JG, García-Pagán JC, Bosch J. Postprandial effects of dark chocolate on portal hypertension in patients with cirrhosis: results of a phase 2, double-blind, randomized controlled trial. Am J Clin Nutr. 2012;96:584-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 167. | Lin SY, Wang YY, Chen WY, Chuang YH, Pan PH, Chen CJ. Beneficial effect of quercetin on cholestatic liver injury. J Nutr Biochem. 2014;25:1183-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 168. | Di Pascoli M, Diví M, Rodríguez-Vilarrupla A, Rosado E, Gracia-Sancho J, Vilaseca M, Bosch J, García-Pagán JC. Resveratrol improves intrahepatic endothelial dysfunction and reduces hepatic fibrosis and portal pressure in cirrhotic rats. J Hepatol. 2013;58:904-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 169. | Abraldes JG, Albillos A, Bañares R, Turnes J, González R, García-Pagán JC, Bosch J. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136:1651-1658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 293] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 170. | Stojakovic T, Claudel T, Putz-Bankuti C, Fauler G, Scharnagl H, Wagner M, Sourij H, Stauber RE, Winkler K, März W. Low-dose atorvastatin improves dyslipidemia and vascular function in patients with primary biliary cirrhosis after one year of treatment. Atherosclerosis. 2010;209:178-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 171. | Zafra C, Abraldes JG, Turnes J, Berzigotti A, Fernández M, Garca-Pagán JC, Rodés J, Bosch J. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology. 2004;126:749-755. [PubMed] [Cited in This Article: ] |
| 172. | Moreno M, Ramalho LN, Sancho-Bru P, Ruiz-Ortega M, Ramalho F, Abraldes JG, Colmenero J, Dominguez M, Egido J, Arroyo V. Atorvastatin attenuates angiotensin II-induced inflammatory actions in the liver. Am J Physiol Gastrointest Liver Physiol. 2009;296:G147-G156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 173. | Mejias M, Garcia-Pras E, Tiani C, Miquel R, Bosch J, Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49:1245-1256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 249] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 174. | Tugues S, Fernandez-Varo G, Muñoz-Luque J, Ros J, Arroyo V, Rodés J, Friedman SL, Carmeliet P, Jiménez W, Morales-Ruiz M. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology. 2007;46:1919-1926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 175. | Arroyo V, García-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61:396-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 329] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 176. | Jalan R, Kapoor D. Reversal of diuretic-induced hepatic encephalopathy with infusion of albumin but not colloid. Clin Sci (Lond). 2004;106:467-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 177. | Ortega R, Ginès P, Uriz J, Cárdenas A, Calahorra B, De Las Heras D, Guevara M, Bataller R, Jiménez W, Arroyo V. Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology. 2002;36:941-948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 308] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 178. | Kremer H, Baron-Menguy C, Tesse A, Gallois Y, Mercat A, Henrion D, Andriantsitohaina R, Asfar P, Meziani F. Human serum albumin improves endothelial dysfunction and survival during experimental endotoxemia: concentration-dependent properties. Crit Care Med. 2011;39:1414-1422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 179. | Dubois MJ, Orellana-Jimenez C, Melot C, De Backer D, Berre J, Leeman M, Brimioulle S, Appoloni O, Creteur J, Vincent JL. Albumin administration improves organ function in critically ill hypoalbuminemic patients: A prospective, randomized, controlled, pilot study. Crit Care Med. 2006;34:2536-2540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 180. | Corradi F, Brusasco C, Fernández J, Vila J, Ramirez MJ, Seva-Pereira T, Fernández-Varo G, Mosbah IB, Acevedo J, Silva A. Effects of pentoxifylline on intestinal bacterial overgrowth, bacterial translocation and spontaneous bacterial peritonitis in cirrhotic rats with ascites. Dig Liver Dis. 2012;44:239-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 181. | Lee PC, Yang YY, Huang CS, Hsieh SL, Lee KC, Hsieh YC, Lee TY, Lin HC. Concomitant inhibition of oxidative stress and angiogenesis by chronic hydrogen-rich saline and N-acetylcysteine treatments improves systemic, splanchnic and hepatic hemodynamics of cirrhotic rats. Hepatol Res. 2014;Jun 24; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 182. | Yang YY, Lee KC, Huang YT, Wang YW, Hou MC, Lee FY, Lin HC, Lee SD. Effects of N-acetylcysteine administration in hepatic microcirculation of rats with biliary cirrhosis. J Hepatol. 2008;49:25-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 183. | Vlachogiannakos J, Saveriadis AS, Viazis N, Theodoropoulos I, Foudoulis K, Manolakopoulos S, Raptis S, Karamanolis DG. Intestinal decontamination improves liver haemodynamics in patients with alcohol-related decompensated cirrhosis. Aliment Pharmacol Ther. 2009;29:992-999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 184. | Fiorucci S, Clerici C, Antonelli E, Orlandi S, Goodwin B, Sadeghpour BM, Sabatino G, Russo G, Castellani D, Willson TM. Protective effects of 6-ethyl chenodeoxycholic acid, a farnesoid X receptor ligand, in estrogen-induced cholestasis. J Pharmacol Exp Ther. 2005;313:604-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 185. | Fiorucci S, Rizzo G, Antonelli E, Renga B, Mencarelli A, Riccardi L, Morelli A, Pruzanski M, Pellicciari R. Cross-talk between farnesoid-X-receptor (FXR) and peroxisome proliferator-activated receptor gamma contributes to the antifibrotic activity of FXR ligands in rodent models of liver cirrhosis. J Pharmacol Exp Ther. 2005;315:58-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 186. | Hu T, Chouinard M, Cox AL, Sipes P, Marcelo M, Ficorilli J, Li S, Gao H, Ryan TP, Michael MD. Farnesoid X receptor agonist reduces serum asymmetric dimethylarginine levels through hepatic dimethylarginine dimethylaminohydrolase-1 gene regulation. J Biol Chem. 2006;281:39831-39838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 187. | Mookerjee R, Rosselli M, Pieri G, Beecher-Jones T, Hooshmand-Rad R, Chouhan M, Mehta G, Jalan R, Shapiro D. Effect of the FXR Agonist Obeticholic acid on portal pressure in alcoholic cirrhosis: a proof of concept Phase 2a study. Hepatology. 2012;56:1529-1530. [DOI] [Cited in This Article: ] |
| 188. | Mehta G, Sharma V, Habtesion A, Balasubramaniyan V, Davies N, Jalan R, Budhram-Mahadeo V, Mookerjee RP. Gene transfer of dimethylarginine dimethylaminohydrolase-1 reduces portal pressure in a rodent model of cirrhosis. Gut. 2012;61 Suppl 2:A123. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 189. | Bosoi CR, Parent-Robitaille C, Anderson K, Tremblay M, Rose CF. AST-120 (spherical carbon adsorbent) lowers ammonia levels and attenuates brain edema in bile duct-ligated rats. Hepatology. 2011;53:1995-2002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 190. | Inami Y, Hamada C, Seto T, Hotta Y, Aruga S, Inuma J, Azuma K, Io H, Kaneko K, Watada H. Effect of AST-120 on Endothelial Dysfunction in Adenine-Induced Uremic Rats. Int J Nephrol. 2014;2014:164125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 191. | Lee CT, Hsu CY, Tain YL, Ng HY, Cheng BC, Yang CC, Wu CH, Chiou TT, Lee YT, Liao SC. Effects of AST-120 on blood concentrations of protein-bound uremic toxins and biomarkers of cardiovascular risk in chronic dialysis patients. Blood Purif. 2014;37:76-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 192. | Davies NA, Wright G, Ytrebø LM, Stadlbauer V, Fuskevåg OM, Zwingmann C, Davies DC, Habtesion A, Hodges SJ, Jalan R. L-ornithine and phenylacetate synergistically produce sustained reduction in ammonia and brain water in cirrhotic rats. Hepatology. 2009;50:155-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 193. | Balasubramaniyan V, Davies N, Gavin W, Vikram S, Raj M, Rajiv J. L-ornithine phenylacetate (OCR-002) reduces portal pressure by modulating hepatic NFkB and hepatic eNOS activity in cirrhotic rats. Hepatology. 2009;50:338A-340A. [Cited in This Article: ] |
| 194. | Hamdy N, El-Demerdash E. New therapeutic aspect for carvedilol: antifibrotic effects of carvedilol in chronic carbon tetrachloride-induced liver damage. Toxicol Appl Pharmacol. 2012;261:292-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 195. | Reiberger T, Payer BA, Schwabl P, Hayden H, Horvatits T, Jäger B, Hummel T, Mitterhauser M, Trauner M, Fuhrmann V. Nebivolol treatment increases splanchnic blood flow and portal pressure in cirrhotic rats via modulation of nitric oxide signalling. Liver Int. 2013;33:561-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 196. | Ma L, Gul R, Habibi J, Yang M, Pulakat L, Whaley-Connell A, Ferrario CM, Sowers JR. Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the transgenic (mRen2) rat. Am J Physiol Heart Circ Physiol. 2012;302:H2341-H2351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 197. | Pires MJ, Rodríguez-Peña AB, Arévalo M, Cenador B, Evangelista S, Esteller A, Sánchez-Rodríguez A, Colaço A, López-Novoa JM. Long-term nebivolol administration reduces renal fibrosis and prevents endothelial dysfunction in rats with hypertension induced by renal mass reduction. J Hypertens. 2007;25:2486-2496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 198. | Rakugi H, Enya K, Sugiura K, Ikeda Y. Comparison of the efficacy and safety of azilsartan with that of candesartan cilexetil in Japanese patients with grade I-II essential hypertension: a randomized, double-blind clinical study. Hypertens Res. 2012;35:552-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 199. | Yasuno S, Fujimoto A, Nakagawa Y, Kuwahara K, Ueshima K. Fixed-dose combination therapy of candesartan cilexetil and amlodipine besilate for the treatment of hypertension in Japan. Expert Rev Cardiovasc Ther. 2012;10:577-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 200. | Debernardi-Venon W, Martini S, Biasi F, Vizio B, Termine A, Poli G, Brunello F, Alessandria C, Bonardi R, Saracco G. AT1 receptor antagonist Candesartan in selected cirrhotic patients: effect on portal pressure and liver fibrosis markers. J Hepatol. 2007;46:1026-1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 201. | Vairappan B, Davies N, Sharma V, Shah N, Mookerjee RP, Jalan R. Reduction in hyperammonemia with L-ornithine phenylacetate (OCR-002) in bile-duct-ligated (BDL) cirrhoticrats restores brain eNOS activity by modulating the DDAH-ADMA pathway. Hepatology. 2009;476A. [Cited in This Article: ] |
| 202. | Abraldes JG, Rodríguez-Vilarrupla A, Graupera M, Zafra C, García-Calderó H, García-Pagán JC, Bosch J. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats. J Hepatol. 2007;46:1040-1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 203. | La Mura V, Pasarín M, Meireles CZ, Miquel R, Rodríguez-Vilarrupla A, Hide D, Gracia-Sancho J, García-Pagán JC, Bosch J, Abraldes JG. Effects of simvastatin administration on rodents with lipopolysaccharide-induced liver microvascular dysfunction. Hepatology. 2013;57:1172-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 204. | Kim MY, Cho MY, Baik SK, Jeong PH, Suk KT, Jang YO, Yea CJ, Kim JW, Kim HS, Kwon SO. Beneficial effects of candesartan, an angiotensin-blocking agent, on compensated alcoholic liver fibrosis - a randomized open-label controlled study. Liver Int. 2012;32:977-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |