Published online Mar 21, 2019. doi: 10.3748/wjg.v25.i11.1307
Peer-review started: January 18, 2019
First decision: January 30, 2019
Revised: February 20, 2019
Accepted: February 22, 2019
Article in press: February 22, 2019
Published online: March 21, 2019
With the increasing number of individuals with diabetes and obesity, nonalcoholic fatty liver disease (NAFLD) is becoming increasingly prevalent, affecting one-quarter of adults worldwide. The spectrum of NAFLD ranges from simple steatosis or nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH). NAFLD, especially NASH, may progress to fibrosis, leading to cirrhosis and hepatocellular carcinoma. NAFLD can impose a severe economic burden, and patients with NAFLD-related terminal or deteriorative liver diseases have become one of the main groups receiving liver transplantation. The increasing prevalence of NAFLD and the severe outcomes of NASH make it necessary to use effective methods to identify NAFLD. Although recognized as the gold standard, biopsy is limited by its sampling bias, poor acceptability, and severe complications, such as mortality, bleeding, and pain. Therefore, noninvasive methods are urgently needed to avoid biopsy for diagnosing NAFLD. This review discusses the current noninvasive methods for assessing NAFLD, including steatosis, NASH, and NAFLD-related fibrosis, and explores the advantages and disadvantages of measurement tools. In addition, we analyze potential noninvasive biomarkers for tracking disease processes and monitoring treatment effects, and explore effective algorithms consisting of imaging and nonimaging biomarkers for diagnosing advanced fibrosis and reducing unnecessary biopsies in clinical practice.
Core tip: Nonalcoholic fatty liver disease (NAFLD) is becoming a major public health issue worldwide. Currently, biopsy is the gold standard for the diagnosis of NAFLD, but it has well-known limitations including sampling errors and severe complications. Thus, noninvasive methods are best alterations to avoid the biopsy. Herein, the noninvasive methods currently available for the assessment of NAFLD in adults are discussed, and we further evaluate the advantages and disadvantages of different assessing tools. In addition, we also analyze the potential of noninvasive biomarkers and their application for tracking NAFLD progression and monitoring the treatment response.
- Citation: Zhou JH, Cai JJ, She ZG, Li HL. Noninvasive evaluation of nonalcoholic fatty liver disease: Current evidence and practice. World J Gastroenterol 2019; 25(11): 1307-1326
- URL: https://www.wjgnet.com/1007-9327/full/v25/i11/1307.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i11.1307
With the increasing number of individuals with diabetes and obesity, nonalcoholic fatty liver disease (NAFLD) is becoming increasingly prevalent, affecting more than one-quarter of adults in the world[1] and 60% of diabetic patients[2] and rising to 90% in the obese people[3,4]. In the United States, the prevalence of NAFLD in adults is 24.13%[1], and it is forecasted to be 33.5% in 2030, and NAFLD cases will reach 100.9 million in the general population[5]. In Asian, the prevalence of NAFLD has reached to 27.37%[1], with 20.09% in China[6]. In some developing countries, such as Sudan, Nigeria, and Iran, the prevalence of NAFLD is about 8.7%-20%[7-9]. The spectrum of NAFLD covers from simple steatosis or nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH). NAFLD, especially NASH, may progress to fibrosis, leading to cirrhosis and hepatocellular carcinoma (HCC)[10]. NAFLD can impose a severe economic burden[11-13], and patients with NAFLD-related terminal or deteriorative liver diseases have become one of the main groups receiving liver transplantation, overtaking hepatitis C patients[14,15]. Based on the double pressure of the increasing prevalence of NAFLD and severe outcomes of NASH, many effective treatments for NAFLD are under development. Lifestyle interventions combined with the loss of 10% of body weight may improve the state of steatosis, inflammation, and even fibrosis[16]. However, the majority of people poorly adhere to long-term, effective lifestyle interventions, which leads to the rapid development of pharmacological treatment. The current therapeutic targets of medicine in clinical trials cover metabolic targets, oxidative stress and inflammation, gut health, and antifibrotics[17-27]. During this period of clinical drug registration, histological biopsy is the key endpoint replacing the long-term main outcomes, such as mortality[28,29]. However, liver biopsy specimens have several limitations, such as representing only approximately 1/50000 of the organ and sampling bias. On the other hand, fibrosis is not uniformly distributed[30], and liver biopsy may cause severe complications, such as mortality, bleeding, and pain. Therefore, it is preferable to use effective noninvasive methods in clinical practice for identifying NAFLD, tracking disease processes, and monitoring treatment effects[31].
Normal hepatic fat content is commonly defined when steatosis in liver histology is less than 5% of hepatocytes[32-34]. NAFLD is diagnosed by a histological phenotype of steatosis with the exclusion of other chronic liver diseases in more than 5% of cases[35,36]. However, in clinical practice, noninvasive methods, including assessment of biomarker panels and imaging, are widely applied instead of biopsy for diagnosing NAFLD.
Fatty liver index (FLI): The FLI is a prevalent biomarker panel consisting of body mass index (BMI), waist circumference, triglycerides, and gamma-glutamyl transferase for identifying NAFLD, with a total score varying between 0 and 100[37]. The area under the receiver operating characteristic curve (AUROC) of FLI for identifying NAFLD is 0.84[37], a low cutoff of 30 is used to rule out NAFLD (the negative likelihood ratio 0.2), and a high cutoff of 60 rule is used with a positive likelihood ratio of 4.3. However, the FLI poorly distinguishes moderate-to-severe steatosis from mild steatosis[38].
Hepatic steatosis index (HSI): The HSI is a biomarker panel consisting of BMI, diabetes, and the alanine transaminase (ALT)/ aspartate transaminase (AST) ratio. It had an AUROC of 0.79 and 0.82 in the derivation and validation groups, and the two cutoffs, 30 and 36, achieved a > 90% sensitivity and specificity[39]. However, the HSI accuracy decreases in obese children, with an AUROC of 0.67, sensitivity of 67%, and specificity of 62%[40]. In addition, like the FLI, the HSI poorly distinguishes moderate-to-severe steatosis from mild steatosis[38].
SteatoTest: The SteatoTest is a biomarker panel consisting of 10 biochemical tests, age, gender, and BMI. SteatoTest exhibited an AUROC of 0.8 for identifying a > 5% liver fat content in patients with chronic liver diseases[41]. Further studies are needed to validate the SteatoTest for differentiating individuals with NAFLD from healthy people.
NAFL screening score: The NAFL screening score is an easy-to-calculate model for identifying NAFLD with age, fasting blood glucose, BMI, triglyceride, ALT/AST, and uric acid. In a study of 48,489 patients with the gold standard of ultrasound (US), the NAFL screening score had different cutoffs for males and females, with a cutoff of 32 yielding an AUROC of 0.83 for males and a cutoff of 29 yielding an AUROC of 0.86 for females[42]. In recent years, machine learning models based on laboratory parameters have been constructed. Yip et al[42] conducted a study in 922 patients involving 264 NAFLD patients diagnosed by proton-magnetic resonance spectroscopy (1H-MRS). Six biomarkers from 23 routine laboratory tests were included to construct the NAFLD ridge score, with an AUROC of 0.87-0.88. The low cutoff of 0.24 achieved a sensitivity of 92% and negative predictive value (NPV) of 95%, and the high cutoff of 0.44 achieved a 90% specificity with a corresponding positive predictive value (PPV) of 84%[42]. Other biomarker panels, such as the triglyceride and glucose index (TyG) and the FLD index, had a moderate AUROC of 0.78 (0.82-0.87) for identifying NAFLD in Chinese subjects[43-45]. In sum, most studies of biomarker panels for diagnosing NAFLD are based on suboptimal gold standards with US or 1H-MRS, and few panels are validated in an independent group. Thus, future studies should not only focus on the gold standard of biopsy but also include a large independent validation group.
US: US is the first-line imaging test used in clinical practice in individuals with suspected NAFLD[35], with a typical appearance of a hyperechogenic liver. One recent meta-analysis demonstrated that compared with histology, US had a pooled sensitivity of 85% and specificity of 94% for moderate-to-severe steatosis[46]. In contrast, US was incapable of detecting steatosis of less than 20%[36,47] or steatosis in individuals with morbid obesity[38]. In addition, the accuracy of US for hepatic steatosis assessment is affected by the presence of severe fibrosis[48] and intra- and inter-observer variability. To detect NAFLD at early stage, the computed-assisted US hepatic/renal ratio (H/R) and US hepatic attenuation rate are used to assess steatosis quantitatively[47,48]. Both measurements exhibit a slightly better performance than conventional US for assessing hepatic steatosis with an excellent performance with a sensitivity of 95% and specificity of 100%, but the NPV is still low (72% for US H/R ratio and 67% for US hepatic attenuation rate)[48,49]. In addition, this quantitative US model could improve the reliability and reproducibility in comparison with conventional US, when it is standardized by a tissue-mimicking phantom, while these findings are needed to verify in further studies[49]. Above all, US is still recommended for diagnosing moderate and severe steatosis in current guideline[44].
Computed tomography (CT): Nonenhanced CT has been used in clinics to evaluate the severity of fatty liver since 1970, based on the fact that hepatic attenuation is inversely associated with the hepatic fat content. Normal liver has an attenuation value of 50-65 HU, and 8-10 HU higher than that of the spleen. However, the attenuation value of the liver may decrease to less than 40 HU when fatty infiltration occurs. Nonenhanced CT outperforms US in evaluating the severity of fatty liver, achieving a specificity of 100% and sensitivity of 82% for diagnosing higher (>30%) degrees of hepatic steatosis[50]. Contrast-enhanced CT images are another CT model that can reduce the radiation exposure of nonenhanced CT[51]. However, contrast-enhanced CT may be more suitable for severe hepatic steatosis using paraspinal or intercostal muscle as the standard reference[52] because its sensitivity for mild-moderate hepatic steatosis is only 25%[53]. CT may also be used for hepatic fat quantification, such as dual-energy CT and hepatic attenuation measurement, but these methods for assessing fatty liver should be sufficiently validated in future clinical studies[54]. Although CT is more effective for evaluating hepatic steatosis, it is also limited by insufficient accuracy for mild-to-moderate hepatic steatosis and radiation exposure, especially in children[52].
Controlled attenuation parameter (CAP): CAP, a parameter based on ultrasonic signals, is measured by the FibroScan® with an M probe (3.5 MHz), with a result of 100-400 dB/m. CAP with an M probe is reported to have an AUROC of 0.82 for differentiating any degree of steatosis vs no steatosis[55]. In addition, the cutoff of 248 dB/m yields a sensitivity of 69% and specificity of 82%[55]. In addition, the study suggests deducting 10 dB/m from the optimal cutoff of the CAP value for individuals with NAFLD or NASH. However, the M probe is less accurate in differentiating hepatic steatosis in obese people[56]. Therefore, the XL probe was devised to overcome these limitations of the M probe with a lower failure rate and low reliability for measuring liver stiffness in patients with a BMI ≥ 28 kg/m2[57]. The XL probe has a higher AUROC than the M probe for distinguishing any degree of steatosis and no steatosis[58]. Even so, CAP is limited by a low sensitivity for mild steatosis and operator dependency. Few studies have compared CAP with 1H-MRS for measuring steatosis, and more studies in the future are required to further explore the role of CAP for steatosis assessment.
Magnetic resonance based techniques: Magnetic resonance imaging (MRI) determines steatosis by signal intensity differences on opposed-phase or fat saturation MRI[59]. MRI-derived proton density fat fraction (MRI-PDFF) is a robust, noninvasive MRI-based methods for assessing hepatic steatosis[60]. It uses MRI-visible protons that combine with fat in the liver to quantify steatosis by dividing all protons in the liver. Tang et al[60] found that MRI-PDFF was significantly associated with the histological steatosis grade according to the NASH-CRN grade (ρ = 0.69, P < 0.001), independent of age, sex, other NASH parameters, and NASH diagnosis. The robust correlation was confirmed in several studies[61-63]. Tang et al[60] also reported an AUROC value of 0.99 for any grade of steatosis vs grade 0, 0.83 for grade 2 or higher vs grade 1 or lower, and 0.89 for grade 3 vs grade 2 or lower. In addition, MRI-PDFF is superior to other imaging tools for the assessment of hepatic steatosis[64,65], and its performance is not affected by obesity. MRI-PDFF is also regarded as a robust noninvasive method to monitor the treatment effect[66]; this aspect will be described in detail below. 1H-MRS is another MR-based technique that directly measures the chemical compositions of the liver[67]. It is usually used in clinical studies of NAFLD representing biopsy for measurement of intrahepatocellular lipid (IHCL) through calculating PDFF[6,52]. 1H-MRS was reported to have a high correlation with biopsy in steatosis assessment[69] and a sensitivity of 80% for diagnosis of liver fat content ≥ 5%[70]. 1H-MRS was reported to have a good accuracy to detect small amounts of liver fat. Nasr et al[6] found that 1H-MRS had a specificity of 100% and sensitivity of 79% with a PDFF cut-off value of 3%, a specificity of 94% and sensitivity of 87% with a PDFF cut-off value of 2%. Although recognized as the most accurate noninvasive tool to assess PDFF quantitatively, MRS is limited to its device- and operator-dependency, complexity, and potentially errors[71]. Complex-based chemical shift imaging-based MRI (CSE‐MRI) is regarded as a promising method to quantify PDFF, which could quantitatively assess liver fat content with a refined pulse sequence[72-74]. It exhibits a high correction with MRS‐PDFF (r2 = 0.985 for 1.5 T MR systems, r2 = 0.991 for 3.0 T MR systems)[71]. MR diffusion weighted imaging (DWI) measures motion of water protons diffusing and tissue perfusing[75,76] and is regarded another promising tool for assessing liver fat content[77], while it exerts poor performance for detecting steatosis in comparison with MRS and dual echo in phase and out of phase imaging[78]. Therefore, more studies are needed to evaluate the performance of DWI in the future.
US is recommended as the first-line diagnostic method in assessing steatosis, while serum biomarkers and biomarker panels are alternative tools when imaging tools are not available in larger scale screening studies (Table 1)[35]. An increasing number of biomarker panels are used in clinical and research applications, while most are validated in studies with relatively small populations, in individuals at their health checkup, or in studies with suboptimal gold criteria. Therefore, future well-designed studies are needed to develop a more effective noninvasive biomarker panel for identifying NAFLD. MRI-PDFF not only exerts an excellent performance for diagnosing NAFLD but also accurately detects changes in fat content during disease progression[79]; however, MRI-PDFF is costly, time-consuming, and device dependent, which makes it difficult for wide application. More effective, feasible, and easily operated tools are needed for diagnosing NAFLD, especially for early steatosis.
| Test | Description | Accuracy | Advantages | Disadvantages | Guideline recommendation |
| Ultrasound | Hyperechoic texture or a bright liver | AUROC 0.93, Sn 85%, Sp 94% for diagnosis of steatosis[33] | Cheap; No radiation; Available; Easy to perform | Low sensitivity in individuals with steatosis < 20% or BMI > 40 kg/m2; Observer-dependency; Influenced by fibrosis or iron overload | The first-line diagnostic test for diagnosing moderate and severe steatosis[32] |
| Computed tomography | Measurement of liver steatosis with attenuation values of liver and spleen | AUROC 0.99, Sn 100%, Sp 82% for diagnosis of steatosis > 30%[29] | Visualize the whole liver; Higher applicability; Quantify moderate-severe steatosis | Low sensitivity for light-moderate steatosis; Radiation exposure | NA |
| CAP | Measurement of liver steatosis with ultrasound attenuation by Fibroscan | AUROC 0.82, Sn 69%, Sp 82% for diagnosis of any steatosis[44] | Immediate assessment; Can be used in ambulatory clinic setting; Measure LSM simultaneously | Operator-dependency; Limited sensitivity; High failure rates in obesity patient; Low accuracy for quantifying steatosis; Uncertain cut-off values | The role of CAP for steatosis assessment is inclusive, more future studies are needed to define the role of CAP[32] |
| Magnetic resonance based techniques | Quantitative measurement of steatosis over the entire liver by adding parameter to MRI scanners | MRI-PDFF: AUROC 0.99, Sn 96%, Sp 100% for diagnosis of any steatosis[49] MRS: Sn 80%, Sp 80% for diagnosing steatosis ≥ 5%[58] | Not affected by obesity; Quantify assess steatosis over the entire liver; Lower sampling variability | Expensive; Time consuming; Device- and operator-dependency; Not suitable for patients with implantable devices | It is excellent to quantify steatosis, but the high price limits its application[32] |
NASH is characterized by steatosis, ballooning, and inflammation, with/without fibrosis, which accelerates disease progression. Early detection of NASH is conducive to the prevention of NASH-related fibrosis. Noninvasive biomarkers for NASH include simple serum biomarkers, biomarker panels, and imaging.
Cytokeratin-18 (CK18): CK18, an intermediate filament protein, is one of the most studied biomarkers for the diagnosis of NASH. It is cleaved during the period of cell death, containing CK18 M30 and CK18 M65[80]. A meta-analysis of 25 studies reported that M30 and M65 had pooled AUROCs of 0.82 and 0.80, while the pooled sensitivity and specificity were 75% and 77%, and 71% and 77%, respectively[81]. Therefore, CK18 is commonly used with other serum biomarkers to diagnose NASH. Anty et al[82] found that combining metabolic syndrome, ALT, and CK18 in a morbidly obese population could achieve an AUROC of 0.88 compared with CK18 alone, with an AUROC of 0.74. Grigorescu et al[83] reported that the triple combination of adiponectin, CK18, and interleukin (IL)-6 achieved an AUROC of 0.90, a specificity of 85.7%, and a sensitivity of 84.5%. However, the results should be further verified in future studies. In addition, some studies have examined the difference in the accuracy of CK18 in assessing NASH with different stages of fibrosis. Huang et al[84] found an AUROC of 0.93 for NASH with fibrosis stages 3-4 and 0.63-0.78 for NASH with fibrosis stages 0-2, which may indicate that CK18 can predict the disease severity in NASH patients.
Inflammatory markers: CXCL10 is a proinflammatory cytokine involved in diabetes and obesity[85]. In a previous study, CXCL10 exhibited a moderate accuracy for differentiating NASH from simple steatosis (AUROC, 0.68) and non-NASH (AUROC, 0.77)[86]. Tumor necrosis factor-α (TNF-α) and IL-8 are common inflammatory markers, which also exhibit a moderate performance with a sensitivity and specificity of 72% and 76%, and 65% and 68%, respectively[87]. However, when combining these two markers with pyroglutamate, the panel could achieve a sensitivity of 91% and specificity of 87%[87].
Adipocytokines and hormones: Fibroblast growth factor 21 (FGF21) secreted by the liver is another potential biomarker for NASH. One study reported that FGF21 had an AUROC of 0.62, and the two cutoffs of 126 and 578 pg/mL had a > 90% sensitivity and specificity for diagnosing NASH, but the PPV and NPV of FGF21 were moderate (0.59-0.78) and low (0.49-0.60), respectively[88]. To improve the PPV and NPV, FGF21 was combined with CK18, which improved the PPV to 82% and the NPV to 74%. Adiponectin was reported to be decrease in NASH patients[89], which had an AUROC of 0.71 for diagnosing NASH[83]. However, the AUROC could reach to 0.90 when adiponectin was combined with CK18 M65 and IL-8[83]. Other adipocytokines, such as leptin and resistin, may be potentially markers for diagnosing NASH, while they are needed to be further validated in more groups[29].
Other serum biomarkers: Serum iron is a common protein associated with oxygen radicals, which contribute to necroinflammation and fibrosis, two important parameters of NAFLD[90,91]. Serum iron was higher in individuals with NASH than in those with simple steatosis[92,93]. In a Japanese study, serum ferritin exhibited a moderate performance for diagnosing NASH (AUROC, 0.73)[94]. Another study of 619 biopsy-proven NAFLD patients constructed a scoring system that combined serum ferritin with type IV collagen 7S and fasting insulin, which could be used to predict NASH with an AUROC of 0.78-0.85[95].
NASHTest: The NASHTest combines 13 parameters to diagnose NASH in three categories, namely, NASH, Borderline NASH, and No-NASH, according to Kleiner’s criteria[96,97]. A study with 257 people found that the NASHTest achieved an AUROC of 0.79 for NASH, 0.69 for borderline NASH, and 0.77-0.83 for no-NASH[98].
NASH ClinLipMet score: The NASH Clin score is a biomarker panel combining AST, fasting insulin, and the PNPLA3 genotype at rs738409, which achieved an AUROC of 0.78 for diagnosing NASH in 384 patients with a histological diagnosis[98,99]. To improve the accuracy, Zhou et al[98] added metabolic syndrome-based factors to the NASH Clin score, which was named the ‘NASH ClinLipMet score‘. This latter score can improve the AUROC to 0.87 and the sensitivity to 75%. However, it is more suitable for research because the measurement of fasting insulin and PNPLA3 genotype is costly and complex in clinical practice.
Other biomarker panels: Tai et al[100] constructed a simple biomarker panel with the parameters of BMI, ALT, and triglycerides. It achieved an AUROC of 0.80-0.82 in the training and validation cohorts and only included 180 morbidly obese patients after bariatric surgery. Li et al[101] developed a clinical score with ALT, gamma-glutamyl transpeptidase, C-reactive protein, and ApoB/ApoA1 ratios. The cutoff of 3.8 gave a sensitivity of 90% and a specificity of 87% for distinguishing NASH from NAFLD, but the panel is limited to a small sample and lacks validation in an independent group.
NASH consists of various parameters; thus, it is difficult to use routine imaging techniques (ultrasonography, CT, or MRI) to distinguish between NASH and simple steatosis. Elastography was investigated to distinguish NASH and simple steatosis. Chen et al[102] found that the cutoff of 2.74 kPa of magnetic resonance elastography (MRE) had an AUROC of 0.93, but the study had several limitations, such as a small sample and a clear histological definition. Vibration-controlled transient elastography (VCTE) was performed in South Korean patients with an AUROC of 0.75 and a sensitivity of 86% for diagnosing NASH, but the specificity was only 58%[103]. Another biomarker, liver iron accumulation (LIC), measured by the MR signal decay values, is reported to be significantly related to NAFLD disease severity or fibrosis progression. The MRI-based technology assessing LIC was found to have an AUROC of 0.91 for assessing NASH, with a sensitivity of 83% and specificity of 80%[103]. Multiparametric MRI technology was used to quantify hepatic steatosis, iron accumulation and fibrosis by 1H-MRS, a T2* map and a T1 relaxation time map, respectively[104-107]. The technology is regarded as a promising imaging biomarker in small studies[108] but awaits independent confirmation from larger trials.
Many potential biomarkers involving NASH are under study[109-114]. Circulating microRNAs are potentially regarded as attractive biomarkers for NAFLD disease severity due to their stability. A meta-analysis found that miR-34a was reported to have a moderate AUROC of 0.78[115]. MiR-122 had a pooled AUROC of 0.64-0.70 for differentiating NASH and simple steatosis[116,117]. The combination of miR-122, -192, and -21 with CK18-Asp396 achieved an AUROC of 0.83 for diagnosing NASH, while the optimal cutoff gave a moderate sensitivity and specificity[118]. Other new methods have been investigated, such as breath volatile organic compounds (VOCs). Breath VOCs are closely related to oxidative stress, inflammation, and liver diseases[119-121]. Froukje et al[122] found that a panel consisting of three exhaled compounds, 1-propanol, 3-methyl-butanonitrile, and n-tridecane, had an AUROC of 0.77, PPV of 81%, and NPV of 82% for differentiating NASH and non-NASH. In addition, some studies have focused on omic markers. The production of lipidomic, proteomic, metabolomics, and microbiome markers was elevated in NASH patients[123-131], but more studies with larger validation groups in the future are needed to confirm these findings.
Noninvasive biomarkers for NASH are an attractive field. CK18 is regarded as a popular biomarker for NASH, but the accuracy varies in current studies. Biomarker panels perform well in diagnosing NASH, but most of them are not validated in an independent group. Although other noninvasive biomarkers, such as imaging and gene biomarkers, are reported to be relatively high in accuracy, effective methods should be available, simple, inexpensive, and accurate in the clinic. In addition, serum biomarkers (e.g., CK18) are less accurate for diagnosing NASH with mild fibrosis, which could lead to higher rates of misdiagnosis. To improve the diagnosis of early NASH, biomarker panels or the combination of serum biomarkers with imaging may contribute to ruling in or ruling out NASH with early fibrosis, but this prospect should be verified in future studies.
According to the recommendation of the NASH-CRN, fibrosis is categorized into nonfibrosis or mild fibrosis (Metavir = F0-1), significant fibrosis (SF, Metavir ≥ F2), advanced fibrosis (AF, Metavir ≥ F3), and cirrhosis (Metavir = F4)[88]. The fibrosis stage is reported to increase the overall mortality in individuals with NAFLD, but not NASH[127]. Furthermore, SF, AF, and cirrhosis increased the hazard ratios by 1.6-, 3.04-, and 6.53-fold for overall mortality in comparison with that of F0-F1[127]. Therefore, it is urgent to identify early fibrosis through effective noninvasive methods.
The proprietary biomarkers of fibrosis include the procollagen of type III collagen (PIIINP), precursor C3-protein (PRO-C3), hyaluronic acid (HA), and TIMP1. Serum PIIINP is a common fibrosis marker during fibrogenesis. It has a good performance for diagnosing SF (AUROC, 0.81)[128]. Another PRO-C3 is a marker of the N-terminal propeptide of type III collagen. Several studies have demonstrated that PRO-C3 has an AUROC of 0.75-0.83 for diagnosing AF and 0.76 for cirrhosis[129,130]. HA is an important element of the extracellular matrix, and it has AUROCs of 0.87, 0.89, and 0.92 for SF, AF, and cirrhosis, respectively[131]. TIMP1 is a fibrosis biomarker reflecting tissue matrix remodeling, while TIMP1 shows a moderate performance for diagnosing SF (AUROC, 0.74)[128]. To improve the accuracy, some models were constructed by combining several specific fibrosis biomarkers or combinations of these fibrosis biomarkers with other variables. The enhanced liver fibrosis (ELF) test is a commercial tool that combines three circulating matrix turnover components, including HA, PIIINP, and TIMP-1, with age[128]. Using a cutoff of 9.8, the ELF test identified AF with a PPV of 72% and NPV of 97%[132]. Another model consisting of PRO-C3, age, platelets, and the presence of diabetes can achieve an AUROC of 0.86-0.87 and an NPV of 0.97 for identifying AF[129]. However, further studies validating these biomarkers in a large independent group are needed in the future.
AST-to-platelet ratio index (APRI): The APRI was originally a simpler calculation for diagnosing fibrosis severity in chronic hepatitis C[133]. A recent meta-analysis reported that the APRI had an AUROC of 0.70 for SF, 0.75 for AF, and 0.75 for cirrhosis[49]. Additionally, the pooled sensitivity of the APRI was relatively low, with a range of 0.33-0.73 for different cutoffs.
FIB-4: FIB-4 is a common biomarker panel used for assessing fibrosis severity and includes age, platelet count, AST, and ALT. FIB-4 was primarily devised to assess the liver fibrosis severity in hepatitis C patients who were also infected with human immunodeficiency virus[134]. An AUROC value of 0.75 for SF, 0.80 for AF, and 0.85 for cirrhosis was reported in NAFLD patients[49]. Two cutoffs were used for a higher PPV and NPV. For instance, using a cutoff of 1.3 for FIB-4, the panel predicted AF with an 85% sensitivity, 65% specificity, 36% PPV, and 95% NPV. On the other hand, using a cutoff of 3.25, FIB-4 predicted AF with a 26% sensitivity, 98% specificity, 75% PPV, and 85% NPV[135]. The two cutoffs may improve the PPV and NPV, avoiding unnecessary biopsy, while the specificity of FIB-4 was 0.35 for assessing AF in elderly individuals ≥ 65 years of age, which contributed to a high false positive rate[136]. Therefore, this study recommended a low cutoff of 2 for elderly patients > 65 years of age, with a 77% sensitivity and 70% specificity. In addition, a recent Japanese study of 1050 biopsy-confirmed NAFLD patients recommended cutoffs of 1.88 and 2.67 for 60-69 years of age and 1.95 and 2.67 for ≥ 70 years of age[137].
NAFLD fibrosis score (NFS): The NFS is the most common noninvasive biomarker panel for assessing fibrosis severity; the panel consists of age, BMI, hyperglycemia, AST/ALT ratio, platelets, and albumin. A multicenter study of 733 people reported a low cutoff of -1.455 for AF with a PPV of 51%-56% and NPV of 88%-93%, and a high cutoff of 0.676 yielded a PPV of 82%-90% and NPV of 80%-85%[138]. Using this model, 75% of biopsies could be spared with 90% correct prediction. In addition, Xiao et al[49] demonstrated that the NFS had an AUROC of 0.72 for SF, 0.73 for AF, and 0.83 for cirrhosis. The NFS was widely validated in different races, with a high AUROC and NPV[135,137,138]. However, a low cutoff of 0.12 for NFS assessing fibrosis is recommended for the elderly due to a high false positive rate[136]. The NFS and FIB-4 are recommended to identify those at low or high risk for AF or cirrhosis in clinical guidelines.
BARD score: The BARD score was an easily calculated score system for assessing fibrosis severity, containing the parameters of BMI, aldosterone renin activity ratio, and the presence of type 2 diabetes mellitus. A score of 2-4 increased the risk of AF by 17-fold, with an AUROC of 0.81 and NPV of 96%, but a low PPV of 43%[139]. However, a subsequent study validated that the tool in the Japanese group could not achieve a similar performance with an AUROC of 0.73 and NPV of 77% for AF[140]. In addition, a meta-analysis reported that the BARD score had a pooled AUROC of 0.64 for SF, 0.73 for AF, and 0.70 for cirrhosis in NAFLD patients[49]. Even so, the BARD score was a valuable model for predicting SF due to its ease and lack of indeterminate results in clinical application.
VCTE: VCTE is the first Food and Drug Administration (FDA)-approved elastographic modality performed by FibroScan employing US-based technology. This technology measures the velocity of a 50 MHz shear wave that is emitted by a probe in the intercostal space into the liver. The velocity is positively related to liver stiffness with a range of 1.5 to 75 kPa. A higher shear wave value indicates higher liver stiffness. However, technical failure was found to be a common phenomenon during the operation, ranging from 6.7% to 27.0%, and was primarily reported to be related to a high BMI[141,142]. The “M” probe was the most prevalent probe measuring shear wave velocity, with an AUROC of 0.83 for SF, 0.87 for AF, and 0.92 for cirrhosis[49]. Although the “XL” probe was usually used for fibrosis in obese people to reduce the failure rate, this rate was still 35% in patients with a BMI over 30 kg/m2[143]. Even so, the FibroScan XL probe yields an AUROC of 0.82 for SF, 0.86 for AF, and 0.94 for cirrhosis. One study investigating the suitable cutoffs indicated that 5.8 and 9.0, 7.9 and 9.6, and 10.3 and 11.5 had a > 90% sensitivity and specificity for SF, AF, and cirrhosis, respectively[141]. However, the PPV was low for diagnosing fibrosis, and transient elastography easily misclassifies AF as mild. One study comparing transient elastography with the NFS and FIB-4 found that transient elastography was better for AF and cirrhosis but less accurate for diagnosing fibrosis vs nonfibrosis and significant fibrosis[70]. Therefore, some studies have used VCTE along with a serum biomarker. Thomson et al[144] combined VCTE with a FibroMeter and achieved a PPV of 84% for SF and PPV of 89% for AF.
Shear wave elastography (SWE): SWE is a new method integrated into conventional US for assessing fibrosis. It can measure the shear wave velocity and provide a 2-D, real-time, color map of liver elasticity, but it should be conducted under apnea, and the region of the color map should be large vessel-free and at least 15 mm below the capsule. SWE reportedly has a high diagnostic performance for fibrosis assessment in chronic hepatitis patients[145,146]. In NAFLD patients, SWE yielded an AUROC value of 0.86 for SF, 0.89 for AF, and 0.88 for cirrhosis, respectively[147]. The results also demonstrated that SWE was better than FibroScan and acoustic radiation force impulse (ARFI). No specific regulations are recommended by the manufacturer for assessing the quality of measurement; thus, some studies assessed the failure rate of SWE with reliability criteria of FibroScan [147]. In addition, as with the ARFI, the accuracy of SWE is affected by interobserver variation and food intake[148]. Therefore, these measurements are recommended to be performed by very experienced radiologists in patients with fasting for at least 2 h[148].
ARFI: ARFI elastography is an alternative tool for fibrosis assessment integrated into conventional US. It uses short-term acoustic pulses to produce shear waves[149], with the results expressed in m/s. ARFI should be operated under apnea, and the region of interest should be a vessel-free region. ARFI had an AUROC of 0.77 for SF, 0.84 for AF, and 0.84 for cirrhosis[147]. Another meta-analysis reported that the pooled sensitivity and specificity were 80.2% and 85.2%, respectively, for detecting SF[150]. However, its accuracy was affected by the presence of severe steatosis[151,152]. Further studies are needed to explore the optimal cutoffs of ARFI at different levels of steatosis.
MRE: MRE is a noninvasive MRI-based method measuring liver stiffness by using a modified phase-contrast method[153-156]. MRE can assess the entire liver with a high success rate[157]. It is not affected by steatosis and may be applied in patients with obesity, ascites, or bowel interposition between the liver and anterior abdominal wall[158]. The available MRE model contains 2D-MRE (shear wave frequency 60 Hz) and 3D-MRE (shear wave frequency 40 Hz). 2D-MRE is more frequently used for assessing liver fibrosis in NAFLD patients. A meta-analysis reported that the pooled AUROCs of 2D MRE for diagnosing SF, AF, and cirrhosis were 0.87, 0.90 and 0.91, respectively[159]. 3D-MRE had a better performance (AUROC, 0.98) for detecting AF than 2D-MRE (AUROC, 0.92)[160], and the NPVs of 2D-MRE and 3D-MRE were 0.98 and 1.0, respectively[160]. Compared to other noninvasive fibrosis biomarkers, MRE was superior to FibroScan, ARFI, and common biomarker panels for discriminating dichotomized fibrosis stages in NAFLD patients[65,161]. Xiao et al[42] found that MRE had an AUROC of 0.96, sensitivity of 0.84, and specificity of 0.90 for detecting AF, which was better than BARD score, NFS, and FibroScan. Considering the higher accuracy of MRE in diagnosing fibrosis, it is increasingly regarded as a promising surrogate biomarker for monitoring fibrosis progression and endpoints of fibrosis therapy[60]. However, MRE has several limitations. It cannot be applied to individuals with hepatic iron overload due to the interfering signal intensity. On the other hand, the cost of MRE and its dependence on MRI facilities limit its wide application.
Serum DNA methylation has been investigated as a potential biomarker for assessing fibrosis. The plasma DNA methylation of PPARγ promoter was reported to have a good performance for diagnosing AF (AUROC, 0.91), and the cutoff of 0.81 gave a PPV of 91% and NPV of 87%[162]. In addition, the DNA methylation at the PPARγ promoter is superior to the NFS in diagnostic performance and avoids using two cutoffs, but it should be validated in more independent groups.
Biomarker panels are cheap, feasible, reproducible, and have a good NPV for fibrosis, but they are limited by its low PPV (Table 2). MRE shows excellent accuracy for fibrosis severity but may only be used in some drug studies due to its high cost and unavailability (Table 3). Transient elastography together with biomarker panels would be widely used for assessing fibrosis, but the efficiency should be evaluated in more independent groups. Above all, it is recommended to combine serum biomarkers or clinical rules with imaging tools to diagnose fibrosis, which could reduce unnecessary diagnostic liver biopsies.
| Test | Description | Accuracy | Advantages | Disadvantages | Guideline recommendation |
| APRI | AST/platelet ratio index | AUROC 0.70 for SF, 0.75 for AF, and 0.75 for cirrhosis[28] | High feasibility; Cheap; Reproducible | Low specificity to diagnose AF; The application of two cut-offs could not discriminate between intermediate stages of fibrosis | NA |
| Fibrosis-4 index | Age, AST, ALT, and platelet count | AUROC 0.75 for SF, 0.80 for AF, and 0.85 for cirrhosis[28] | High feasibility; Cheap; Reproducible | The application of two cut-offs could not discriminate between intermediate stages of fibrosis; Influenced by age | FIB-4 can be used to identify those at low or high risk for AF or cirrhosis [32,34] |
| NFS | Age, BMI, impaired fasting glucose and/or diabetes, AST, ALT, platelet, Count, and albumin | AUROC 0.72 for SF, 0.73 for AF, and 0.83 for cirrhosis [28] | High feasibility; Cheap; Reproducible | The application of two cut-offs could not discriminate between intermediate stages of fibrosis; Influenced by age; Influenced by interpretation of BMI across different ethnic groups | NFS can be used to identify those at low or high risk for AF or cirrhosis[32] |
| BARD score | AST, ALT, BMI, and diabetes | AUROC 0.64 for SF, 0.73 for AF, and 0.70 for cirrhosis[28] | High feasibility; Cheap; Reproducible; No intermediate stages of fibrosis | Low specificity to diagnose SF and cirrhosis; Influenced by interpretation of BMI across different ethnic groups | NA |
| Test | Description | Accuracy | Advantages | Disadvantages | Guideline recommendation |
| VCTE | Measuring the velocity of a 50 mHz shear wave, which is positively related to liver stiffness | AUROC 0.83, 0.87, and 0.92 , respectively, for AF, SF, and cirrhosis with M probe[28]; AUROC 0.82, 0.86, and 0.94, respectively, for AF, SF, and cirrhosis with XL probe[117] | Relatively low cost; Good reproducibility Short processing time; Can be used in ambulatory clinic setting | Fasting for 2 h; Device- and operator- dependency; Influenced by obesity, congestion, and inflammation; Uncertain cut-off values; Intermediate stages due to two cut-offs | FibroScan can be used to identify those at low or high risk for AF[32,34] |
| SWE | A method integrated into conventional ultrasound provides a 2-D, real-time, color map of liver elasticity | AUROC 0.86, 0.89, and 0.88, respectively, for AF, SF, and cirrhosis[123] | Good reproducibility; Not affected by obesity or ascites | Relatively high cost; Fasting for 2 h; Device- and operator- dependency; Quality criteria not well defined | NA |
| ARFI | A method integrated into a conventional ultrasound measures shear wave speed | AUROC 0.77, 0.84, and 0.84, respectively, for AF, SF, and cirrhosis[123] | Good reproducibility; Not affected by obesity or ascites ROI smaller than transient elastography | High cost; Fasting for 2 h; Device- and operator- dependency; Quality criteria not well defined; Intermediate stages due to two cut-offs | NA |
| MRE | A noninvasive MRI based method measures liver stiffness by a modified phase-contrast method | AUROC 0.87, 0.90, and 0.91, respectively, for AF, SF, and cirrhosis[131] | Good reproducibility; Not affected by obesity or ascites | High cost; Time consuming; Fasting for 2 h; Device- and operator- dependency; Intermediate stages due to two cut-offs | MRE is clinically useful tools for identifying advanced fibrosis in patients with nonalcoholic fatty liver disease[34] |
NAFLD significantly increases the risk of liver disease-related morbidity, mortality, and liver transplantation[163,164]. Fibrosis, but not simple steatosis and NASH, increased the risk of mortality in NAFLD patients in a retrospective study with a mean follow-up period of 20 years[119]. Singh et al[165] found that one stage of fibrosis progression takes 14.3 years and 7.1 years in individuals with simple steatosis and NASH patients, respectively. In addition, most NAFLD cases are asymptomatic until the disease has progressed to cirrhosis, and repeated biopsy is impractical. Therefore, there is a need to apply useful noninvasive biomarkers to monitor disease progression. A prospective study with a median of seven follow-ups found that the ELF test had an AUROC of 0.87 for predicting liver-related clinical outcomes, which was higher than that of biopsy (AUROC, 0.82)[166]. Sebastiani et al[167] found that baseline liver histology, APRI, FIB-4, and NFS for predicting clinical outcomes had AUROCs of 0.85, 0.89, 0.89 and 0.79, respectively. Another study reported that FibroScan had an accuracy of 0.73 for predicting all-course mortality[168]. Further studies are needed to determine more effective noninvasive biomarkers for the progression of NASH to NASH-related fibrosis and the progression of NASH-related fibrosis to adverse clinical outcomes.
In terms of NAFLD treatment, it is impractical to observe the primary endpoint of mortality due to long-term follow-up[28,169,170]. Therefore, the FDA recommends that histological improvement be confirmed when the resolution of NASH is obtained without the worsening of fibrosis or when fibrosis is improved without the worsening of NASH[171]. However, repeated biopsy hinders the development of drugs; thus, there is a need to investigate noninvasive surrogates replacing biopsy. MRI-PDFF was usually employed to evaluate the liver fat content change in clinical trials of NASH patients[66]. A study of 113 NASH patients treated with obeticholic acid found that MRI-PDFF had an AUROC of 0.81 for reduced histological steatosis grade[172]. In contrast, a recent phase II trial of selonsertib found that MRI-PDFF had an AUROC of 0.70 for reduced histological steatosis grade, and the optimal cutoff was 0% with a PPV of 39% and NPV of 92%[173]. Therefore, whether the change in MRI-PDFF could be regarded as an effective surrogate endpoint for NASH treatment should be further evaluated. Liver function has been regularly regarded as a noninvasive biomarker for assessing the monitoring treatment effect, while ALT concentrations in about two-thirds of patients is normal[174], and NASH patients usually exhibit spontaneous changes in liver function. Therefore, the ALT change is usually accompanied by a steatosis change, which is regarded as an effective noninvasive endpoint substituting the histological changes in NASH[171]. The change in liver stiffness measurement (LSM) measured by MRE was evaluated to investigate the antifibrosis effect in NAFLD. Jayakumar et al[173] showed that the MRE had an AUROC of 0.62, PPV of 39%, and NPV of 92% for fibrosis improvement. The biomarker panel has also been investigated for predicting fibrosis improvement in intervention studies of NASH patients. Vilar et al[175] constructed a model consisting of three variables, glycated hemoglobin, platelets, and ALT, which demonstrated an AUROC of 0.96 for fibrosis improvement, which is higher than the change in platelet count (AUROC, 0.80), APRI (AUROC, 0.50), FIB-4 index (AUROC, 0.63), and NFS (AUROC, 0.77). The biomarker panels may be the ideal noninvasive tools for assessing the response during the process of therapy, but they should be accurate, available, inexpensive, and simple.
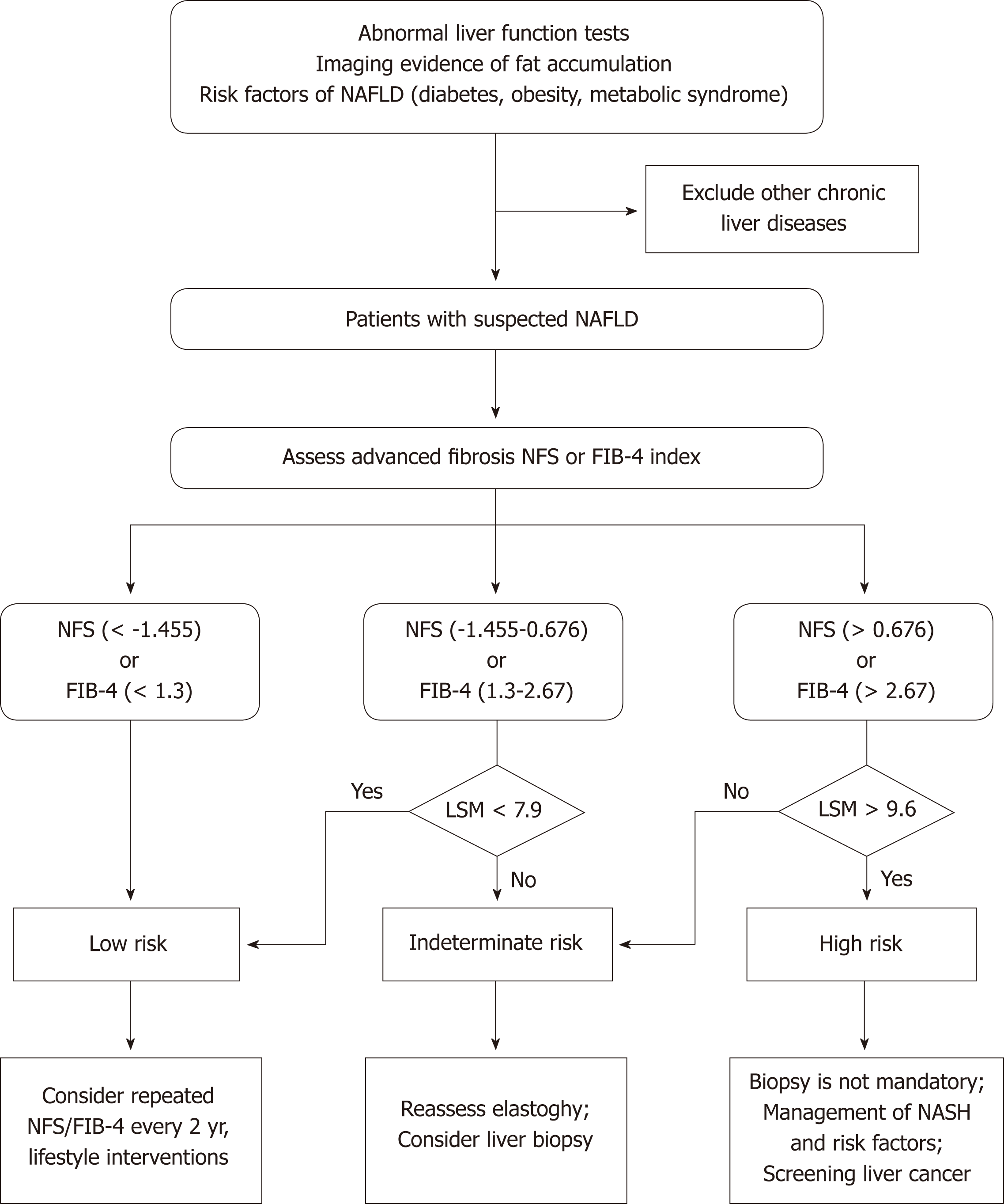
The past several years have witnessed the extensive development of noninvasive methods in the NAFLD field, from serum biomarkers and imaging to omics. US and H-MRI have a relatively high accuracy for diagnosing NAFLD, and US is prevalently used in clinical practice and research due to its availability and low cost. There are currently no effective noninvasive biomarkers recommended for diagnosing NASH. Future studies are needed to investigate more efficient noninvasive biomarkers for distinguishing NASH from simple steatosis. VCTE is the FDA-approved elastographic model for assessing fibrosis severity, and it could further improve the diagnostic performance when combined with biomarker panels. Furthermore, effective algorithms consisting of imaging and nonimaging biomarkers should be applied to clinical practice to reduce unnecessary biopsies (Figure 1). In addition, there is a need to investigate the cost-effectiveness of noninvasive evaluations in diagnosing NAFLD, tracking disease progression, and monitoring responses to the therapies.
The authors thank the staff at Institute of Model Animal of Wuhan University and Department of Cardiology, Renmin Hospital of Wuhan University.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Arslan N, Senturk H, Sijens PE, Tiribelli C, Toriguchi K, Trovato GM S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5322] [Cited by in F6Publishing: 6281] [Article Influence: 785.1] [Reference Citation Analysis (0)] |
| 2. | Dai W, Ye L, Liu A, Wen SW, Deng J, Wu X, Lai Z. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: A meta-analysis. Medicine (Baltimore). 2017;96:e8179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 3. | Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45:600-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 367] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 4. | Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330-9337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 198] [Reference Citation Analysis (1)] |
| 5. | Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1028] [Cited by in F6Publishing: 1303] [Article Influence: 217.2] [Reference Citation Analysis (0)] |
| 6. | Li Z, Xue J, Chen P, Chen L, Yan S, Liu L. Prevalence of nonalcoholic fatty liver disease in mainland of China: a meta-analysis of published studies. J Gastroenterol Hepatol. 2014;29:42-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 7. | Almobarak AO, Barakat S, Khalifa MH, Elhoweris MH, Elhassan TM, Ahmed MH. Non alcoholic fatty liver disease (NAFLD) in a Sudanese population: What is the prevalence and risk factors? Arab J Gastroenterol. 2014;15:12-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Onyekwere CA, Ogbera AO, Balogun BO. Non-alcoholic fatty liver disease and the metabolic syndrome in an urban hospital serving an African community. Ann Hepatol. 2011;10:119-124. [PubMed] [Cited in This Article: ] |
| 9. | Eshraghian A, Dabbaghmanesh MH, Eshraghian H, Fattahi MR, Omrani GR. Nonalcoholic fatty liver disease in a cluster of Iranian population: thyroid status and metabolic risk factors. Arch Iran Med. 2013;16:584-589. [PubMed] [Cited in This Article: ] |
| 10. | Ji YX, Huang Z, Yang X, Wang X, Zhao LP, Wang PX, Zhang XJ, Alves-Bezerra M, Cai L, Zhang P, Lu YX, Bai L, Gao MM, Zhao H, Tian S, Wang Y, Huang ZX, Zhu XY, Zhang Y, Gong J, She ZG, Li F, Cohen DE, Li H. The deubiquitinating enzyme cylindromatosis mitigates nonalcoholic steatohepatitis. Nat Med. 2018;24:213-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 11. | Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, Hunt S, Beckerman R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577-1586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 694] [Cited by in F6Publishing: 783] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 12. | Zhang XJ, She ZG, Li H. Time to step-up the fight against NAFLD. Hepatology. 2018;67:2068-2071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:8263-8276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 418] [Cited by in F6Publishing: 423] [Article Influence: 60.4] [Reference Citation Analysis (6)] |
| 14. | Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188-2195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 520] [Cited by in F6Publishing: 528] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 15. | Mikolasevic I, Filipec-Kanizaj T, Mijic M, Jakopcic I, Milic S, Hrstic I, Sobocan N, Stimac D, Burra P. Nonalcoholic fatty liver disease and liver transplantation - Where do we stand? World J Gastroenterol. 2018;24:1491-1506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 78] [Cited by in F6Publishing: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Lassailly G, Caiazzo R, Pattou F, Mathurin P. Perspectives on Treatment for Nonalcoholic Steatohepatitis. Gastroenterology. 2016;150:1835-1848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 305] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 18. | Xie L, Wang PX, Zhang P, Zhang XJ, Zhao GN, Wang A, Guo J, Zhu X, Zhang Q, Li H. DKK3 expression in hepatocytes defines susceptibility to liver steatosis and obesity. J Hepatol. 2016;65:113-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Yan FJ, Zhang XJ, Wang WX, Ji YX, Wang PX, Yang Y, Gong J, Shen LJ, Zhu XY, Huang Z, Li H. The E3 ligase tripartite motif 8 targets TAK1 to promote insulin resistance and steatohepatitis. Hepatology. 2017;65:1492-1511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | Gao L, Wang PX, Zhang Y, Yu CJ, Ji Y, Wang X, Zhang P, Jiang X, Jin H, Huang Z, Zhang ZR, Li H. Tumor necrosis factor receptor-associated factor 5 (Traf5) acts as an essential negative regulator of hepatic steatosis. J Hepatol. 2016;65:125-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Cai J, Xu M, Zhang X, Li H. Innate Immune Signaling in Nonalcoholic Fatty Liver Disease and Cardiovascular Diseases. Annu Rev Pathol. 2019;14:153-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 22. | Wang XA, Zhang R, Zhang S, Deng S, Jiang D, Zhong J, Yang L, Wang T, Hong S, Guo S, She ZG, Zhang XD, Li H. Interferon regulatory factor 7 deficiency prevents diet-induced obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2013;305:E485-E495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Wang PX, Zhang XJ, Luo P, Jiang X, Zhang P, Guo J, Zhao GN, Zhu X, Zhang Y, Yang S, Li H. Hepatocyte TRAF3 promotes liver steatosis and systemic insulin resistance through targeting TAK1-dependent signalling. Nat Commun. 2016;7:10592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Xu M, Liu PP, Li H. Innate Immune Signaling and Its Role in Metabolic and Cardiovascular Diseases. Physiol Rev. 2019;99:893-948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Xiang M, Wang PX, Wang AB, Zhang XJ, Zhang Y, Zhang P, Mei FH, Chen MH, Li H. Targeting hepatic TRAF1-ASK1 signaling to improve inflammation, insulin resistance, and hepatic steatosis. J Hepatol. 2016;64:1365-1377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 26. | Eshraghian A. Current and emerging pharmacological therapy for non-alcoholic fatty liver disease. World J Gastroenterol. 2017;23:7495-7504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 40] [Cited by in F6Publishing: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Kanda T, Matsuoka S, Yamazaki M, Shibata T, Nirei K, Takahashi H, Kaneko T, Fujisawa M, Higuchi T, Nakamura H, Matsumoto N, Yamagami H, Ogawa M, Imazu H, Kuroda K, Moriyama M. Apoptosis and non-alcoholic fatty liver diseases. World J Gastroenterol. 2018;24:2661-2672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 151] [Cited by in F6Publishing: 167] [Article Influence: 27.8] [Reference Citation Analysis (1)] |
| 28. | Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1647] [Cited by in F6Publishing: 1624] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 29. | Wong VW, Adams LA, de Lédinghen V, Wong GL, Sookoian S. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat Rev Gastroenterol Hepatol. 2018;15:461-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 354] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 30. | Merat S, Sotoudehmanesh R, Nouraie M, Peikan-Heirati M, Sepanlou SG, Malekzadeh R, Sotoudeh M. Sampling error in histopathology findings of nonalcoholic fatty liver disease: a post mortem liver histology study. Arch Iran Med. 2012;15:418-421. [PubMed] [Cited in This Article: ] |
| 31. | Cai J, Zhang XJ, Li H. Progress and challenges in the prevention and control of nonalcoholic fatty liver disease. Med Res Rev. 2019;39:328-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 32. | Petäjä EM, Yki-Järvinen H. Definitions of Normal Liver Fat and the Association of Insulin Sensitivity with Acquired and Genetic NAFLD-A Systematic Review. Int J Mol Sci. 2016;17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 33. | Roldan-Valadez E, Favila R, Martínez-López M, Uribe M, Ríos C, Méndez-Sánchez N. In vivo 3T spectroscopic quantification of liver fat content in nonalcoholic fatty liver disease: Correlation with biochemical method and morphometry. J Hepatol. 2010;53:732-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Kotronen A, Johansson LE, Johansson LM, Roos C, Westerbacka J, Hamsten A, Bergholm R, Arkkila P, Arola J, Kiviluoto T, Fisher RM, Ehrenborg E, Orho-Melander M, Ridderstråle M, Groop L, Yki-Järvinen H. A common variant in PNPLA3, which encodes adiponutrin, is associated with liver fat content in humans. Diabetologia. 2009;52:1056-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 238] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 35. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2290] [Cited by in F6Publishing: 2694] [Article Influence: 336.8] [Reference Citation Analysis (2)] |
| 36. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3544] [Cited by in F6Publishing: 4068] [Article Influence: 678.0] [Reference Citation Analysis (7)] |
| 37. | Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1238] [Cited by in F6Publishing: 1607] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 38. | Fedchuk L, Nascimbeni F, Pais R, Charlotte F, Housset C, Ratziu V; LIDO Study Group. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40:1209-1222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 290] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 39. | Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, Lundbom N, Rissanen A, Ridderstråle M, Groop L, Orho-Melander M, Yki-Järvinen H. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137:865-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 490] [Cited by in F6Publishing: 543] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 40. | Koot BG. van der Baan-Slootweg OH, Bohte AE, Nederveen AJ, van Werven JR, Tamminga-Smeulders CL, Merkus MP, Schaap FG, Jansen PL, Stoker J, Benninga MA. Accuracy of prediction scores and novel biomarkers for predicting nonalcoholic fatty liver disease in obese children. Obesity (Silver Spring). 2013;21:583-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Poynard T, Ratziu V, Naveau S, Thabut D, Charlotte F, Messous D, Capron D, Abella A, Massard J, Ngo Y, Munteanu M, Mercadier A, Manns M, Albrecht J. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol. 2005;4:10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 237] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 42. | Zhou YJ, Zhou YF, Zheng JN, Liu WY, Van Poucke S, Zou TT, Zhang DC, Shen S, Shi KQ, Wang XD, Zheng MH. NAFL screening score: A basic score identifying ultrasound-diagnosed non-alcoholic fatty liver. Clin Chim Acta. 2017;475:44-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Fuyan S, Jing L, Wenjun C, Zhijun T, Weijing M, Suzhen W, Yongyong X. Fatty liver disease index: a simple screening tool to facilitate diagnosis of nonalcoholic fatty liver disease in the Chinese population. Dig Dis Sci. 2013;58:3326-3334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Zhu J, He M, Zhang Y, Li T, Liu Y, Xu Z, Chen W. Validation of simple indexes for nonalcoholic fatty liver disease in western China: a retrospective cross-sectional study. Endocr J. 2018;65:373-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Zhang S, Du T, Zhang J, Lu H, Lin X, Xie J, Yang Y, Yu X. The triglyceride and glucose index (TyG) is an effective biomarker to identify nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16:15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 46. | Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, Clark JM. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 864] [Cited by in F6Publishing: 974] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 47. | Xia MF, Yan HM, He WY, Li XM, Li CL, Yao XZ, Li RK, Zeng MS, Gao X. Standardized ultrasound hepatic/renal ratio and hepatic attenuation rate to quantify liver fat content: an improvement method. Obesity (Silver Spring). 2012;20:444-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 48. | Zhang B, Ding F, Chen T, Xia LH, Qian J, Lv GY. Ultrasound hepatic/renal ratio and hepatic attenuation rate for quantifying liver fat content. World J Gastroenterol. 2014;20:17985-17992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. 2017;66:1486-1501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 521] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 50. | Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, Ha HK, Lee MG, Hwang S, Lee SG, Yu ES, Cho EY. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 378] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 51. | Kodama Y, Ng CS, Wu TT, Ayers GD, Curley SA, Abdalla EK, Vauthey JN, Charnsangavej C. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188:1307-1312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 354] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 52. | Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 530] [Cited by in F6Publishing: 520] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 53. | Panicek DM, Giess CS, Schwartz LH. Qualitative assessment of liver for fatty infiltration on contrast-enhanced CT: is muscle a better standard of reference than spleen? J Comput Assist Tomogr. 1997;21:699-705. [PubMed] [Cited in This Article: ] |
| 54. | Ma X, Holalkere NS, Kambadakone R A, Mino-Kenudson M, Hahn PF, Sahani DV. Imaging-based quantification of hepatic fat: methods and clinical applications. Radiographics. 2009;29:1253-1277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 226] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 55. | Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, Kumar M, Lupsor-Platon M, Han KH, Cardoso AC, Ferraioli G, Chan WK, Wong VW, Myers RP, Chayama K, Friedrich-Rust M, Beaugrand M, Shen F, Hiriart JB, Sarin SK, Badea R, Jung KS, Marcellin P, Filice C, Mahadeva S, Wong GL, Crotty P, Masaki K, Bojunga J, Bedossa P, Keim V, Wiegand J. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022-1030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 543] [Cited by in F6Publishing: 621] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 56. | Chan WK, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2014;29:1470-1476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 57. | Myers RP, Pomier-Layrargues G, Kirsch R, Pollett A, Duarte-Rojo A, Wong D, Beaton M, Levstik M, Crotty P, Elkashab M. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology. 2012;55:199-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 343] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 58. | Chan WK, Nik Mustapha NR, Wong GL, Wong VW, Mahadeva S. Controlled attenuation parameter using the FibroScan® XL probe for quantification of hepatic steatosis for non-alcoholic fatty liver disease in an Asian population. United European Gastroenterol J. 2017;5:76-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 59. | Springer F, Machann J, Claussen CD, Schick F, Schwenzer NF. Liver fat content determined by magnetic resonance imaging and spectroscopy. World J Gastroenterol. 2010;16:1560-1566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 88] [Cited by in F6Publishing: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 60. | Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: Clinical trials to clinical practice. J Hepatol. 2016;65:1006-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 236] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 61. | Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, Gamst AC, Middleton M, Brunt EM, Loomba R, Lavine JE, Schwimmer JB, Sirlin CB. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 366] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 62. | Idilman IS, Aniktar H, Idilman R, Kabacam G, Savas B, Elhan A, Celik A, Bahar K, Karcaaltincaba M. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology. 2013;267:767-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 272] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 63. | Bannas P, Kramer H, Hernando D, Agni R, Cunningham AM, Mandal R, Motosugi U, Sharma SD, Munoz del Rio A, Fernandez L, Reeder SB. Quantitative magnetic resonance imaging of hepatic steatosis: Validation in ex vivo human livers. Hepatology. 2015;62:1444-1455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 64. | Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 478] [Cited by in F6Publishing: 496] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 65. | Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, Hooker J, Sy E, Savides MT, Alquiraish MH, Valasek MA, Rizo E, Richards L, Brenner D, Sirlin CB, Loomba R. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:598-607.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 442] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 66. | Caussy C, Reeder SB, Sirlin CB, Loomba R. Noninvasive, Quantitative Assessment of Liver Fat by MRI-PDFF as an Endpoint in NASH Trials. Hepatology. 2018;68:763-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 67. | Cassidy FH, Yokoo T, Aganovic L, Hanna RF, Bydder M, Middleton MS, Hamilton G, Chavez AD, Schwimmer JB, Sirlin CB. Fatty liver disease: MR imaging techniques for the detection and quantification of liver steatosis. Radiographics. 2009;29:231-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 213] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 68. | Nasr P, Forsgren MF, Ignatova S, Dahlström N, Cedersund G, Leinhard OD, Norén B, Ekstedt M, Lundberg P, Kechagias S. Using a 3% Proton Density Fat Fraction as a Cut-Off Value Increases Sensitivity of Detection of Hepatic Steatosis, Based on Results From Histopathology Analysis. Gastroenterology. 2017;153:53-55.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 69. | Cowin GJ, Jonsson JR, Bauer JD, Ash S, Ali A, Osland EJ, Purdie DM, Clouston AD, Powell EE, Galloway GJ. Magnetic resonance imaging and spectroscopy for monitoring liver steatosis. J Magn Reson Imaging. 2008;28:937-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 70. | Lee SS, Park SH, Kim HJ, Kim SY, Kim MY, Kim DY, Suh DJ, Kim KM, Bae MH, Lee JY, Lee SG, Yu ES. Non-invasive assessment of hepatic steatosis: prospective comparison of the accuracy of imaging examinations. J Hepatol. 2010;52:579-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 265] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 71. | Kim HJ, Cho HJ, Kim B, You MW, Lee JH, Huh J, Kim JK. Accuracy and precision of proton density fat fraction measurement across field strengths and scan intervals: A phantom and human study. J Magn Reson Imaging. 2018;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 72. | Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007;58:354-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 376] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 73. | Yu H, McKenzie CA, Shimakawa A, Vu AT, Brau AC, Beatty PJ, Pineda AR, Brittain JH, Reeder SB. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging. 2007;26:1153-1161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 337] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 74. | Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008;60:1122-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 500] [Cited by in F6Publishing: 530] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 75. | Lewis S, Dyvorne H, Cui Y, Taouli B. Diffusion-weighted imaging of the liver: techniques and applications. Magn Reson Imaging Clin N Am. 2014;22:373-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 76. | Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254:47-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 623] [Cited by in F6Publishing: 594] [Article Influence: 42.4] [Reference Citation Analysis (2)] |
| 77. | Manning P, Murphy P, Wang K, Hooker J, Wolfson T, Middleton MS, Newton KP, Behling C, Awai HI, Durelle J, Paiz MN, Angeles JE, De La Pena D, McCutchan JA, Schwimmer JB, Sirlin CB. Liver histology and diffusion-weighted MRI in children with nonalcoholic fatty liver disease: A MAGNET study. J Magn Reson Imaging. 2017;46:1149-1158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 78. | d'Assignies G, Ruel M, Khiat A, Lepanto L, Chagnon M, Kauffmann C, Tang A, Gaboury L, Boulanger Y. Noninvasive quantitation of human liver steatosis using magnetic resonance and bioassay methods. Eur Radiol. 2009;19:2033-2040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 79. | Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, Fujita K, Yoneda M, Taguri M, Hyogo H, Sumida Y, Ono M, Eguchi Y, Inoue T, Yamanaka T, Wada K, Saito S, Nakajima A. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology. 2016;150:626-637.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 495] [Cited by in F6Publishing: 514] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 80. | Verma S, Jensen D, Hart J, Mohanty SR. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int. 2013;33:1398-1405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 81. | He L, Deng L, Zhang Q, Guo J, Zhou J, Song W, Yuan F. Diagnostic Value of CK-18, FGF-21, and Related Biomarker Panel in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Biomed Res Int. 2017;2017:9729107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 82. | Anty R, Iannelli A, Patouraux S, Bonnafous S, Lavallard VJ, Senni-Buratti M, Amor IB, Staccini-Myx A, Saint-Paul MC, Berthier F, Huet PM, Le Marchand-Brustel Y, Gugenheim J, Gual P, Tran A. A new composite model including metabolic syndrome, alanine aminotransferase and cytokeratin-18 for the diagnosis of non-alcoholic steatohepatitis in morbidly obese patients. Aliment Pharmacol Ther. 2010;32:1315-1322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 83. | Grigorescu M, Crisan D, Radu C, Grigorescu MD, Sparchez Z, Serban A. A novel pathophysiological-based panel of biomarkers for the diagnosis of nonalcoholic steatohepatitis. J Physiol Pharmacol. 2012;63:347-353. [PubMed] [Cited in This Article: ] |
| 84. | Huang JF, Yeh ML, Huang CF, Huang CI, Tsai PC, Tai CM, Yang HL, Dai CY, Hsieh MH, Chen SC, Yu ML, Chuang WL. Cytokeratin-18 and uric acid predicts disease severity in Taiwanese nonalcoholic steatohepatitis patients. PLoS One. 2017;12:e0174394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 85. | Schulthess FT, Paroni F, Sauter NS, Shu L, Ribaux P, Haataja L, Strieter RM, Oberholzer J, King CC, Maedler K. CXCL10 impairs beta cell function and viability in diabetes through TLR4 signaling. Cell Metab. 2009;9:125-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 86. | Zhang X, Shen J, Man K, Chu ES, Yau TO, Sung JC, Go MY, Deng J, Lu L, Wong VW, Sung JJ, Farrell G, Yu J. CXCL10 plays a key role as an inflammatory mediator and a non-invasive biomarker of non-alcoholic steatohepatitis. J Hepatol. 2014;61:1365-1375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 87. | Qi S, Xu D, Li Q, Xie N, Xia J, Huo Q, Li P, Chen Q, Huang S. Metabonomics screening of serum identifies pyroglutamate as a diagnostic biomarker for nonalcoholic steatohepatitis. Clin Chim Acta. 2017;473:89-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 88. | Shen J, Chan HL, Wong GL, Choi PC, Chan AW, Chan HY, Chim AM, Yeung DK, Chan FK, Woo J, Yu J, Chu WC, Wong VW. Non-invasive diagnosis of non-alcoholic steatohepatitis by combined serum biomarkers. J Hepatol. 2012;56:1363-1370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 89. | Tietge UJ, Schmidt HH, Schütz T, Dippe P, Lochs H, Pirlich M. Reduced plasma adiponectin in NASH: central obesity as an underestimated causative risk factor. Hepatology. 2005;41:401; author reply 401-401; author reply 402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 90. | George DK, Goldwurm S, MacDonald GA, Cowley LL, Walker NI, Ward PJ, Jazwinska EC, Powell LW. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114:311-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 470] [Cited by in F6Publishing: 483] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 91. | Kalantar-Zadeh K, Rodriguez RA, Humphreys MH. Association between serum ferritin and measures of inflammation, nutrition and iron in haemodialysis patients. Nephrol Dial Transplant. 2004;19:141-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 200] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 92. | Puljiz Z, Stimac D, Kovac D, Puljiz M, Bratanić A, Kovacić V, Kardum D, Bonacin D, Hozo I. Predictors of nonalcoholic steatohepatitis in patients with elevated alanine aminotransferase activity. Coll Antropol. 2010;34 Suppl 1:33-37. [PubMed] [Cited in This Article: ] |
| 93. | Canbakan B, Senturk H, Tahan V, Hatemi I, Balci H, Toptas T, Sonsuz A, Velet M, Aydin S, Dirican A, Ozgulle S, Ozbay G. Clinical, biochemical and histological correlations in a group of non-drinker subjects with non-alcoholic fatty liver disease. Acta Gastroenterol Belg. 2007;70:277-284. [PubMed] [Cited in This Article: ] |
| 94. | Yoneda M, Nozaki Y, Endo H, Mawatari H, Iida H, Fujita K, Yoneda K, Takahashi H, Kirikoshi H, Inamori M, Kobayashi N, Kubota K, Saito S, Maeyama S, Hotta K, Nakajima A. Serum ferritin is a clinical biomarker in Japanese patients with nonalcoholic steatohepatitis (NASH) independent of HFE gene mutation. Dig Dis Sci. 2010;55:808-814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 95. | Sumida Y, Yoneda M, Hyogo H, Yamaguchi K, Ono M, Fujii H, Eguchi Y, Suzuki Y, Imai S, Kanemasa K, Fujita K, Chayama K, Yasui K, Saibara T, Kawada N, Fujimoto K, Kohgo Y, Okanoue T; Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD). A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:257-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 96. | Poynard T, Ratziu V, Charlotte F, Messous D, Munteanu M, Imbert-Bismut F, Massard J, Bonyhay L, Tahiri M, Thabut D, Cadranel JF, Le Bail B, de Ledinghen V; LIDO Study Group; CYTOL study group. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 97. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6807] [Cited by in F6Publishing: 7396] [Article Influence: 389.3] [Reference Citation Analysis (5)] |
| 98. | Zhou Y, Orešič M, Leivonen M, Gopalacharyulu P, Hyysalo J, Arola J, Verrijken A, Francque S, Van Gaal L, Hyötyläinen T, Yki-Järvinen H. Noninvasive Detection of Nonalcoholic Steatohepatitis Using Clinical Markers and Circulating Levels of Lipids and Metabolites. Clin Gastroenterol Hepatol. 2016;14:1463-1472.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 99. | Hyysalo J, Männistö VT, Zhou Y, Arola J, Kärjä V, Leivonen M, Juuti A, Jaser N, Lallukka S, Käkelä P, Venesmaa S, Simonen M, Saltevo J, Moilanen L, Korpi-Hyövalti E, Keinänen-Kiukaanniemi S, Oksa H, Orho-Melander M, Valenti L, Fargion S, Pihlajamäki J, Peltonen M, Yki-Järvinen H. A population-based study on the prevalence of NASH using scores validated against liver histology. J Hepatol. 2014;60:839-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 100. | Tai CM, Yu ML, Tu HP, Huang CK, Hwang JC, Chuang WL. Derivation and validation of a scoring system for predicting nonalcoholic steatohepatitis in Taiwanese patients with severe obesity. Surg Obes Relat Dis. 2017;13:686-692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 101. | Chunming L, Jianhui S, Hongguang Z, Chunwu Q, Xiaoyun H, Lijun Y, Xuejun Y. The development of a clinical score for the prediction of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease using routine parameters. Turk J Gastroenterol. 2015;26:408-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 102. | Chen J, Talwalkar JA, Yin M, Glaser KJ, Sanderson SO, Ehman RL. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259:749-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 319] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 103. | Kim TH, Jeong CW, Jun HY, Kim YR, Kim JY, Lee YH, Yoon KH. Noninvasive Differential Diagnosis of Liver Iron Contents in Nonalcoholic Steatohepatitis and Simple Steatosis Using Multiecho Dixon Magnetic Resonance Imaging. Acad Radiol. 2018;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Ferreira VM, Piechnik SK, Dall'Armellina E, Karamitsos TD, Francis JM, Choudhury RP, Friedrich MG, Robson MD, Neubauer S. Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 330] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 105. | Piechnik SK, Ferreira VM, Dall'Armellina E, Cochlin LE, Greiser A, Neubauer S, Robson MD. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010;12:69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 511] [Cited by in F6Publishing: 490] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 106. | Rial B, Robson MD, Neubauer S, Schneider JE. Rapid quantification of myocardial lipid content in humans using single breath-hold 1H MRS at 3 Tesla. Magn Reson Med. 2011;66:619-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 107. | Banerjee R, Pavlides M, Tunnicliffe EM, Piechnik SK, Sarania N, Philips R, Collier JD, Booth JC, Schneider JE, Wang LM, Delaney DW, Fleming KA, Robson MD, Barnes E, Neubauer S. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol. 2014;60:69-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 301] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 108. | Eddowes PJ, McDonald N, Davies N, Semple SIK, Kendall TJ, Hodson J, Newsome PN, Flintham RB, Wesolowski R, Blake L, Duarte RV, Kelly CJ, Herlihy AH, Kelly MD, Olliff SP, Hübscher SG, Fallowfield JA, Hirschfield GM. Utility and cost evaluation of multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2018;47:631-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 109. | Zhang P, Wang PX, Zhao LP, Zhang X, Ji YX, Zhang XJ, Fang C, Lu YX, Yang X, Gao MM, Zhang Y, Tian S, Zhu XY, Gong J, Ma XL, Li F, Wang Z, Huang Z, She ZG, Li H. The deubiquitinating enzyme TNFAIP3 mediates inactivation of hepatic ASK1 and ameliorates nonalcoholic steatohepatitis. Nat Med. 2018;24:84-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 110. | Wang S, Yan ZZ, Yang X, An S, Zhang K, Qi Y, Zheng J, Ji YX, Wang PX, Fang C, Zhu XY, Shen LJ, Yan FJ, Bao R, Tian S, She ZG, Tang YD. Hepatocyte DUSP14 maintains metabolic homeostasis and suppresses inflammation in the liver. Hepatology. 2018;67:1320-1338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 111. | Zhao GN, Zhang P, Gong J, Zhang XJ, Wang PX, Yin M, Jiang Z, Shen LJ, Ji YX, Tong J, Wang Y, Wei QF, Wang Y, Zhu XY, Zhang X, Fang J, Xie Q, She ZG, Wang Z, Huang Z, Li H. Tmbim1 is a multivesicular body regulator that protects against non-alcoholic fatty liver disease in mice and monkeys by targeting the lysosomal degradation of Tlr4. Nat Med. 2017;23:742-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 112. | Wang PX, Ji YX, Zhang XJ, Zhao LP, Yan ZZ, Zhang P, Shen LJ, Yang X, Fang J, Tian S, Zhu XY, Gong J, Zhang X, Wei QF, Wang Y, Li J, Wan L, Xie Q, She ZG, Wang Z, Huang Z, Li H. Targeting CASP8 and FADD-like apoptosis regulator ameliorates nonalcoholic steatohepatitis in mice and nonhuman primates. Nat Med. 2017;23:439-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 113. | Zhu LH, Wang A, Luo P, Wang X, Jiang DS, Deng W, Zhang X, Wang T, Liu Y, Gao L, Zhang S, Zhang X, Zhang J, Li H. Mindin/Spondin 2 inhibits hepatic steatosis, insulin resistance, and obesity via interaction with peroxisome proliferator-activated receptor α in mice. J Hepatol. 2014;60:1046-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 114. | Liu XL, Pan Q, Zhang RN, Shen F, Yan SY, Sun C, Xu ZJ, Chen YW, Fan JG. Disease-specific miR-34a as diagnostic marker of non-alcoholic steatohepatitis in a Chinese population. World J Gastroenterol. 2016;22:9844-9852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 69] [Cited by in F6Publishing: 65] [Article Influence: 8.1] [Reference Citation Analysis (2)] |
| 115. | Liu CH. Ampuero J, Gil-Gómez A, Montero-Vallejo R, Rojas Á, Muñoz-Hernández R, Gallego-Durán R, Romero-Gómez M. miRNAs in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Hepatol. 2018;69:1335-1348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 116. | Cermelli S, Ruggieri A, Marrero JA, Ioannou GN, Beretta L. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS One. 2011;6:e23937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 423] [Cited by in F6Publishing: 436] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 117. | Pirola CJ, Fernández Gianotti T, Castaño GO, Mallardi P, San Martino J, Mora Gonzalez Lopez Ledesma M, Flichman D, Mirshahi F, Sanyal AJ, Sookoian S. Circulating microRNA signature in non-alcoholic fatty liver disease: from serum non-coding RNAs to liver histology and disease pathogenesis. Gut. 2015;64:800-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 403] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 118. | Becker PP, Rau M, Schmitt J, Malsch C, Hammer C, Bantel H, Müllhaupt B, Geier A. Performance of Serum microRNAs -122, -192 and -21 as Biomarkers in Patients with Non-Alcoholic Steatohepatitis. PLoS One. 2015;10:e0142661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 119. | Van Berkel JJ, Dallinga JW, Möller GM, Godschalk RW, Moonen EJ, Wouters EF, Van Schooten FJ. A profile of volatile organic compounds in breath discriminates COPD patients from controls. Respir Med. 2010;104:557-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 120. | Dallinga JW, Robroeks CM, van Berkel JJ, Moonen EJ, Godschalk RW, Jöbsis Q, Dompeling E, Wouters EF, van Schooten FJ. Volatile organic compounds in exhaled breath as a diagnostic tool for asthma in children. Clin Exp Allergy. 2010;40:68-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 121. | Lang AL, Beier JI. Interaction of volatile organic compounds and underlying liver disease: a new paradigm for risk. Biol Chem. 2018;399:1237-1248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 122. | Verdam FJ, Dallinga JW, Driessen A, de Jonge C, Moonen EJ, van Berkel JB, Luijk J, Bouvy ND, Buurman WA, Rensen SS, Greve JW, van Schooten FJ. Non-alcoholic steatohepatitis: a non-invasive diagnosis by analysis of exhaled breath. J Hepatol. 2013;58:543-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 123. | Duarte SMB, Stefano JT, Miele L, Ponziani FR, Souza-Basqueira M, Okada LSRR, de Barros Costa FG, Toda K, Mazo DFC, Sabino EC, Carrilho FJ, Gasbarrini A, Oliveira CP. Gut microbiome composition in lean patients with NASH is associated with liver damage independent of caloric intake: A prospective pilot study. Nutr Metab Cardiovasc Dis. 2018;28:369-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 124. | Alonso C, Fernández-Ramos D, Varela-Rey M, Martínez-Arranz I, Navasa N, Van Liempd SM, Lavín Trueba JL, Mayo R, Ilisso CP, de Juan VG, Iruarrizaga-Lejarreta M, delaCruz-Villar L, Mincholé I, Robinson A, Crespo J, Martín-Duce A, Romero-Gómez M, Sann H, Platon J, Van Eyk J, Aspichueta P, Noureddin M, Falcón-Pérez JM, Anguita J, Aransay AM, Martínez-Chantar ML, Lu SC, Mato JM. Metabolomic Identification of Subtypes of Nonalcoholic Steatohepatitis. Gastroenterology. 2017;152:1449-1461.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 180] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 125. | Soga T, Sugimoto M, Honma M, Mori M, Igarashi K, Kashikura K, Ikeda S, Hirayama A, Yamamoto T, Yoshida H, Otsuka M, Tsuji S, Yatomi Y, Sakuragawa T, Watanabe H, Nihei K, Saito T, Kawata S, Suzuki H, Tomita M, Suematsu M. Serum metabolomics reveals γ-glutamyl dipeptides as biomarkers for discrimination among different forms of liver disease. J Hepatol. 2011;55:896-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 126. | Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, Contos MJ, Sterling RK, Fuchs M, Zhou H, Watkins SM, Sanyal AJ. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827-1838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 470] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 127. | Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, Kechagias S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67:1265-1273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 540] [Cited by in F6Publishing: 626] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 128. | Polyzos SA, Slavakis A, Koumerkeridis G, Katsinelos P, Kountouras J. Noninvasive Liver Fibrosis Tests in Patients with Nonalcoholic Fatty Liver Disease: An External Validation Cohort. Horm Metab Res. 2019;51:134-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 129. | Hansen JF, Juul Nielsen M, Nyström K, Leeming DJ, Lagging M, Norkrans G, Brehm Christensen P, Karsdal M. PRO-C3: a new and more precise collagen marker for liver fibrosis in patients with chronic hepatitis C. Scand J Gastroenterol. 2018;53:83-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 130. | Daniels SJ, Leeming DJ, Eslam M, Hashem AM, Nielsen MJ, Krag A, Karsdal MA, Grove JI, Guha IN, Kawaguchi T, Torimura T, McLeod D, Akiba J, Kaye P, de Boer B, Aithal GP, Adams LA, George J. ADAPT: An algorithm incorporating PRO-C3 accurately identifies patients with NAFLD and advanced fibrosis. Hepatology. 2018;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 131. | Suzuki A, Angulo P, Lymp J, Li D, Satomura S, Lindor K. Hyaluronic acid, an accurate serum marker for severe hepatic fibrosis in patients with non-alcoholic fatty liver disease. Liver Int. 2005;25:779-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 132. | Miele L, De Michele T, Marrone G, Antonietta Isgrò M, Basile U, Cefalo C, Biolato M, Maria Vecchio F, Lodovico Rapaccini G, Gasbarrini A, Zuppi C, Grieco A. Enhanced liver fibrosis test as a reliable tool for assessing fibrosis in nonalcoholic fatty liver disease in a clinical setting. Int J Biol Markers. 2017;32:e397-e402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 133. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2762] [Cited by in F6Publishing: 2988] [Article Influence: 142.3] [Reference Citation Analysis (0)] |
| 134. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2633] [Cited by in F6Publishing: 2996] [Article Influence: 166.4] [Reference Citation Analysis (0)] |
| 135. | McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265-1269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 587] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 136. | McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, Oliveira CP, Francque S, Van Gaal L, Schattenberg JM, Tiniakos D, Burt A, Bugianesi E, Ratziu V, Day CP, Anstee QM. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol. 2017;112:740-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 482] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 137. | Ishiba H, Sumida Y, Tanaka S, Yoneda M, Hyogo H, Ono M, Fujii H, Eguchi Y, Suzuki Y, Yoneda M, Takahashi H, Nakahara T, Seko Y, Mori K, Kanemasa K, Shimada K, Imai S, Imajo K, Kawaguchi T, Nakajima A, Chayama K, Saibara T, Shima T, Fujimoto K, Okanoue T, Itoh Y; Japan Study Group of Non-Alcoholic Fatty Liver Disease (JSG-NAFLD). The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: a multi-center study. J Gastroenterol. 2018;53:1216-1224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 138. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1917] [Cited by in F6Publishing: 2020] [Article Influence: 118.8] [Reference Citation Analysis (1)] |
| 139. | Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441-1447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 545] [Cited by in F6Publishing: 553] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 140. | Fujii H, Enomoto M, Fukushima W, Tamori A, Sakaguchi H, Kawada N. Applicability of BARD score to Japanese patients with NAFLD. Gut. 2009;58:1566-7; author reply 1567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 141. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W, Sung JJ, de Lédinghen V. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 876] [Cited by in F6Publishing: 876] [Article Influence: 62.6] [Reference Citation Analysis (1)] |
| 142. | de Lédinghen V, Vergniol J. Transient elastography (FibroScan). Gastroenterol Clin Biol. 2008;32:58-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 143. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan AW, Chermak F, Choi PC, Merrouche W, Chu SH, Pesque S, Chan HL, de Lédinghen V. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012;107:1862-1871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 244] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 144. | Loong TC, Wei JL, Leung JC, Wong GL, Shu SS, Chim AM, Chan AW, Choi PC, Tse YK, Chan HL, Wong VW. Application of the combined FibroMeter vibration-controlled transient elastography algorithm in Chinese patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2017;32:1363-1369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 145. | Leung VY, Shen J, Wong VW, Abrigo J, Wong GL, Chim AM, Chu SH, Chan AW, Choi PC, Ahuja AT, Chan HL, Chu WC. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology. 2013;269:910-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 216] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 146. | Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C; Liver Fibrosis Study Group. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125-2133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 468] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 147. | Cassinotto C, Boursier J, de Lédinghen V, Lebigot J, Lapuyade B, Cales P, Hiriart JB, Michalak S, Bail BL, Cartier V, Mouries A, Oberti F, Fouchard-Hubert I, Vergniol J, Aubé C. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817-1827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 328] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 148. | Karagoz E, Ozturker C, Sonmez G. Noninvasive Evaluation of Liver Fibrosis: Supersonic Shear Imaging or Acoustic Radiation Force Impulse Imaging? Radiology. 2016;279:979-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 149. | Friedrich-Rust M, Romen D, Vermehren J, Kriener S, Sadet D, Herrmann E, Zeuzem S, Bojunga J. Acoustic radiation force impulse-imaging and transient elastography for non-invasive assessment of liver fibrosis and steatosis in NAFLD. Eur J Radiol. 2012;81:e325-e331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 150. | Liu H, Fu J, Hong R, Liu L, Li F. Acoustic Radiation Force Impulse Elastography for the Non-Invasive Evaluation of Hepatic Fibrosis in Non-Alcoholic Fatty Liver Disease Patients: A Systematic Review & Meta-Analysis. PLoS One. 2015;10:e0127782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 151. | Joo SK, Kim W, Kim D, Kim JH, Oh S, Lee KL, Chang MS, Jung YJ, So YH, Lee MS, Bae JM, Kim BG. Steatosis severity affects the diagnostic performances of noninvasive fibrosis tests in nonalcoholic fatty liver disease. Liver Int. 2018;38:331-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 152. | Yanrong Guo. Haoming Lin, Xinyu Zhang, Huiying Wen, Siping Chen, Xin Chen. The influence of hepatic steatosis on the evaluation of fibrosis with non-alcoholic fatty liver disease by acoustic radiation force impulse. Conf Proc IEEE Eng Med Biol Soc. 2017;2017:2988-2991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 153. | Wang QB, Zhu H, Liu HL, Zhang B. Performance of magnetic resonance elastography and diffusion-weighted imaging for the staging of hepatic fibrosis: A meta-analysis. Hepatology. 2012;56:239-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 154. | Loomba R. Role of imaging-based biomarkers in NAFLD: Recent advances in clinical application and future research directions. J Hepatol. 2018;68:296-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 155. | Doycheva I, Cui J, Nguyen P, Costa EA, Hooker J, Hofflich H, Bettencourt R, Brouha S, Sirlin CB, Loomba R. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther. 2016;43:83-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 156. | Cui J, Ang B, Haufe W, Hernandez C, Verna EC, Sirlin CB, Loomba R. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther. 2015;41:1271-1280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 157. | Yin M, Talwalkar JA, Glaser KJ, Manduca A, Grimm RC, Rossman PJ, Fidler JL, Ehman RL. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5:1207-1213.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 703] [Cited by in F6Publishing: 668] [Article Influence: 39.3] [Reference Citation Analysis (1)] |
| 158. | Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013;37:544-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 441] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 159. | Singh S, Venkatesh SK, Loomba R, Wang Z, Sirlin C, Chen J, Yin M, Miller FH, Low RN, Hassanein T, Godfrey EM, Asbach P, Murad MH, Lomas DJ, Talwalkar JA, Ehman RL. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol. 2016;26:1431-1440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 160. | Loomba R, Cui J, Wolfson T, Haufe W, Hooker J, Szeverenyi N, Ang B, Bhatt A, Wang K, Aryafar H, Behling C, Valasek MA, Lin GY, Gamst A, Brenner DA, Yin M, Glaser KJ, Ehman RL, Sirlin CB. Novel 3D Magnetic Resonance Elastography for the Noninvasive Diagnosis of Advanced Fibrosis in NAFLD: A Prospective Study. Am J Gastroenterol. 2016;111:986-994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 161. | Cui J, Heba E, Hernandez C, Haufe W, Hooker J, Andre MP, Valasek MA, Aryafar H, Sirlin CB, Loomba R. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: A prospective study. Hepatology. 2016;63:453-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 150] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 162. | Hardy T, Zeybel M, Day CP, Dipper C, Masson S, McPherson S, Henderson E, Tiniakos D, White S, French J, Mann DA, Anstee QM, Mann J. Plasma DNA methylation: a potential biomarker for stratification of liver fibrosis in non-alcoholic fatty liver disease. Gut. 2017;66:1321-1328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 163. | Pais R. Barritt AS 4th, Calmus Y, Scatton O, Runge T, Lebray P, Poynard T, Ratziu V, Conti F. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016;65:1245-1257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 274] [Article Influence: 34.3] [Reference Citation Analysis (1)] |
| 164. | Ray K. NAFLD-the next global epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 165. | Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643-654.e1-9; quiz e39-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 932] [Cited by in F6Publishing: 1017] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 166. | Parkes J, Roderick P, Harris S, Day C, Mutimer D, Collier J, Lombard M, Alexander G, Ramage J, Dusheiko G, Wheatley M, Gough C, Burt A, Rosenberg W. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut. 2010;59:1245-1251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 167. | Sebastiani G, Alshaalan R, Wong P, Rubino M, Salman A, Metrakos P, Deschenes M, Ghali P. Prognostic Value of Non-Invasive Fibrosis and Steatosis Tools, Hepatic Venous Pressure Gradient (HVPG) and Histology in Nonalcoholic Steatohepatitis. PLoS One. 2015;10:e0128774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 168. | Boursier J, Vergniol J, Guillet A, Hiriart JB, Lannes A, Le Bail B, Michalak S, Chermak F, Bertrais S, Foucher J, Oberti F, Charbonnier M, Fouchard-Hubert I, Rousselet MC, Calès P, de Lédinghen V. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol. 2016;65:570-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 248] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 169. | Buckley AJ, Thomas EL, Lessan N, Trovato FM, Trovato GM, Taylor-Robinson SD. Non-alcoholic fatty liver disease: Relationship with cardiovascular risk markers and clinical endpoints. Diabetes Res Clin Pract. 2018;144:144-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 170. | Trovato FM, Tognarelli JM, Crossey MM, Catalano D, Taylor-Robinson SD, Trovato GM. Challenges of liver cancer: Future emerging tools in imaging and urinary biomarkers. World J Hepatol. 2015;7:2664-2675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 171. | Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 537] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 172. | Middleton MS, Heba ER, Hooker CA, Bashir MR, Fowler KJ, Sandrasegaran K, Brunt EM, Kleiner DE, Doo E, Van Natta ML, Lavine JE, Neuschwander-Tetri BA, Sanyal A, Loomba R, Sirlin CB; NASH Clinical Research Network. Agreement Between Magnetic Resonance Imaging Proton Density Fat Fraction Measurements and Pathologist-Assigned Steatosis Grades of Liver Biopsies From Adults With Nonalcoholic Steatohepatitis. Gastroenterology. 2017;153:753-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 188] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 173. | Jayakumar S, Middleton MS, Lawitz EJ, Mantry PS, Caldwell SH, Arnold H, Mae Diehl A, Ghalib R, Elkhashab M, Abdelmalek MF, Kowdley KV, Stephen Djedjos C, Xu R, Han L, Mani Subramanian G, Myers RP, Goodman ZD, Afdhal NH, Charlton MR, Sirlin CB, Loomba R. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: Analysis of data from a phase II trial of selonsertib. J Hepatol. 2019;70:133-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 174. | Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis. 2008;28:386-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 175. | Vilar-Gomez E, Calzadilla-Bertot L, Friedman SL, Gra-Oramas B, Gonzalez-Fabian L, Lazo-Del Vallin S, Diago M, Adams LA. Serum biomarkers can predict a change in liver fibrosis 1 year after lifestyle intervention for biopsy-proven NASH. Liver Int. 2017;37:1887-1896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |