INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer, representing about 80% of all cases. It is a devastating disease with a 5-year survival rate of only approximately 4%-7% (http://seer.cancer.gov/statfacts/html/pancreas.html), and this figure has not been improved in recent decades. Although surgical resection is the only curative treatment for PDAC, only 15% to 20% of patients present with resectable disease and the majority are diagnosed with locally advanced or metastatic cancer[1]. This situation is mainly a consequence of the aggressive nature of this disease and the lack of an efficient method for detection of early-stage lesions. In addition, very early stage organ metastasis is often observed in patients which are treated with potentially curative surgery. This suggests that occult tumor cells may be present in blood even with small lesions
Currently the detection and diagnosis of pancreatic cancer largely rely on imaging modalities, including ultrasonography, computed tomography, positron emission tomography, magnetic resonance imaging and endoscopic ultrasonography[1]. However, early-stage pancreatic cancers and very small metastases are difficult to detect even if combinations of these modalities are employed. In addition, these modalities require expensive equipment and specialist technicians. Although blood-based tumor biomarkers, such as carcinoembryonic antigen and carbohydrate antigen (CA) 19-9, are much cheaper, simple and minimally invasive alternatives, the sensitivity and specificity of these currently used tumor biomarkers are not sufficient for effective early detection of pancreatic cancer. Despite recent progress in understanding of the disease at the molecular level, no reliable blood-based biomarker for screening of pancreatic cancer has yet become clinically available.
The problem is compounded by the few viable therapeutic options for patients with advanced pancreatic cancer who are not eligible for resection. Chemotherapy for pancreatic cancer patients is limited, and cytotoxic drugs, such as gemcitabine, which have been the standard chemotherapeutic drugs for patients with advanced disease for many years, provide limited survival advantage[1]. Personalized therapies based on cancer-specific alterations are currently not conducted in clinical practice for pancreatic cancer.
In this context, new effective biomarkers are required to improve diagnosis, disease monitoring and process of therapeutic choice of PDAC. As tumor-derived somatic gene alterations can be detected in circulating tumor DNA (ctDNA) from cancer patients, ctDNA could provide a less-invasive diagnostic tool based on the concept of “liquid biopsy”. In this review, we summarize the current status of ctDNA analysis, and discuss the potential clinical utility of liquid biopsy for pancreatic cancer.
OVERVIEW OF LIQUID BIOPSY IN CANCER
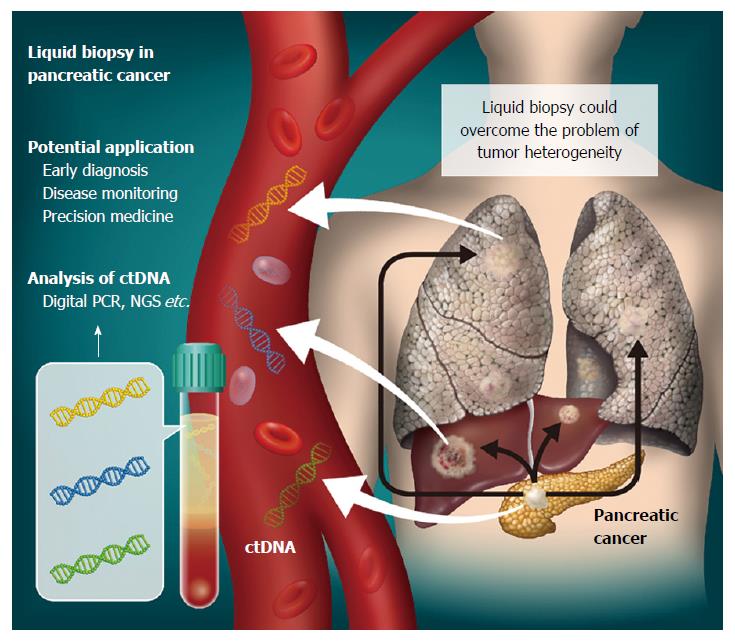
The concept of detecting tumor-specific molecular alterations by analysis of bodily fluids, including peripheral blood, of cancer patients is termed “liquid biopsy” (Figure 1). Cell-free DNA (cfDNA) consists of small double-stranded DNA fragments found in blood. In 1948, Mandel and Metais firstly reported the presence of cfDNA in the circulation[2]. Tumor-derived cfDNA, now commonly known as ctDNA, was described in 1989[3]. The clinical utility of cfDNA in plasma and serum has been an active area of research in a variety of clinical settings. Indeed, evaluation of fetal cfDNA in the circulation of pregnant women is becoming a routine diagnostic test for high-risk patients in the clinic. To date, cfDNA has been the main target of liquid biopsy for cancer detection, together with exosomes, micro-RNA and circulating tumor cells. In the oncology setting, ctDNA is expected to provide a minimally-invasive approach for cancer diagnosis, monitoring of chemotherapy-resistant mutations, and overcoming the problem of tumor heterogeneity[4-6].
Figure 1 Liquid biopsy for pancreatic cancer.
Circulating tumor DNA (ctDNA) can be isolated from plasma as a liquid biopsy approach. Genomic alterations detected could then have various clinical applications in pancreatic cancer cases. As ctDNA is released not only from primary tumors, but also metastases, its analysis might overcome the problem of tumor heterogeneity.
It has been suggested that cancer patients have higher levels of cfDNA than healthy individuals[7], although an increase is also observed with a variety of other physiological and pathological conditions, including exercise, inflammation, exposure to smoking, sepsis and trauma[8]. cfDNA is shed into the bloodstream via apoptosis, necrosis, direct release from viable cells, and lysis of circulating cells, but the major sources are now thought to be apoptotic and necrotic cells. In fact, the length of cfDNA fragments in the circulation often shows a characteristic laddering pattern with multiples of 170-180 base pairs, which is a well-known feature of apoptosis[7]. Apoptosis is programmed even for many normal cells on a daily basis and it has been suggested that a large fraction of cfDNA is derived from bone marrow and liver in healthy individuals[9]. However, in a tumor mass, hyperproliferation and rapid cellular turnover of cancer cells can lead to very greatly increased programmed cell death. Features of intratumoral microenvironments such as hypoxia may also lead to necrosis. Cellular debris from apoptotic or necrotic cells is normally phagocytozed by infiltrating macrophages and the cellular components are cleared. However, this clearance mechanism does not proceed effectively in a tumor mass, leading to accumulation of cellular debris, including DNA, and its release into the circulation[5,7,10].
Although total cfDNA may be generally increased in patients with cancer, the sensitivity and specificity for cancer detection are low, and the utility as a cancer biomarker is questionable. On the other hand, tumor DNA can be discriminated from normal cfDNA by detecting tumor-specific somatic mutations that exist only in the genomes of cancer or precancerous cells, but not in the genomes of their normal counterparts. This assures the specificity of ctDNA as a cancer biomarker.
However, detection of ctDNA has in practice proven challenging, since the percentage of ctDNA may be very low (< 1.0% in many cases) in total cfDNA[5,11]. Traditional methods such as Sanger sequencing or pyrosequencing can detect mutated tumor-derived DNA fragments only in patients with a high tumor burden and a large amount of ctDNA. However, recent advances in sequencing technologies, including the digital polymerase chain reaction (dPCR) and next-generation sequencing (NGS), have made it possible to detect ctDNA present at relatively low frequencies in blood, and there has been an explosive increase of studies of clinical utility of ctDNA[12-14].
METHODS FOR DETECTION OF CTDNA
The dPCR is now one of the major methods to sensitively detect genomic alterations in cfDNA. In 2003, a PCR-based digital approach, named BEAMing (Beads, Emulsion, Amplification, and Magnetics) was first described[15]. Using emulsion PCR and flow cytometry, BEAMing can efficiently identify rare mutations with allele fractions as low as 0.01%[16].
Nowadays several dPCR systems, including droplet-based platforms, are commercially available. Generally, the sensitivity of a droplet dPCR system depends on the number of droplets. One of the most widely used droplet dPCR devices, the QX200 Droplet Digital PCR System (Bio-Rad Laboratories) generates 20000 nanoliter-sized droplets. The RainDrop Digital PCR System (RainDance Technologies) can perform “single-molecule” PCR for up to 10 million picoliter-sized droplets, and therefore possesses very high sensitivity. In addition, multiplex assays are possible in the RainDrop system by using combinations of two color probes (up to 10 targets)[17].
NGS is also widely applied to analyze genomic alterations in cfDNA. Unlike dPCR, NGS techniques can analyze multiple, broad regions of interest. Even whole-genome sequencing or whole-exome sequencing of cfDNA from advanced cancer patients has been reported, and various alterations, including single nucleotide variants (SNV), copy number alterations (CNA) and structural alterations of DNA, were detected[13,18,19]. However, only genomic alterations with high allele frequencies may be appropriate with these platforms, since deep genome-wide analysis, especially whole-genome sequencing, is quite costly and not feasible in the routine clinical context. Therefore, global genomic analyses can be applied for only cfDNA samples from advanced cancer patients with high tumor burden. On the other hand, targeted sequencing can be performed at relatively low cost. By focusing on clinically important genes, mutations can be detected with higher sensitivity compared to genome-wide analyses.
Amplicon sequencing is one of the major techniques for analyzing mutations in specific genomic regions. Ion AmpliSeq Technology (Thermo Fisher Scientific) is a widely used targeted sequencing platform. Highly multiplex PCR followed by NGS, such as Ion Personal Genome Machine (Ion PGM), allows deep sequencing of target regions with as little as 10 ng input DNA at low cost and with a short turnaround time. However, the Ion Ampliseq system has issues such as a relatively high error rate in detection of small insertions and deletions (indels)[20].
Target enrichment techniques, namely target capture-based platforms, are widely used for analyzing gene alterations of cancer. In principle, fragmented genomic DNA is hybridized with DNA/RNA probes designed for capturing targeted regions, and the enriched DNA libraries are analyzed by NGS. The SureSelect Target Enrichment System (Agilent Technologies) is widely employed for targeted sequencing in combination with the Illumina paired-end sequencing platform, which has a relatively low error rate amongst high-throughput sequencing instruments[21]. Although the manufacturer’s protocol for the SureSelect Target Enrichment System requires at least 200 ng of input DNA, the amount can be reduced by using particular library preparation kits, such as the KAPA Hyper Prep Kit (KAPA Biosystems)[22].
In addition to these commercially available technologies, various highly sensitive sequencing methods have been developed for detecting ctDNA. In an amplicon-based system, Safe-Sequencing System, individual DNA molecules are tagged with a unique identifier, then amplified and sequenced. According to the original paper, the error rate could be lowered to 9 × 10-6 by taking into account unique identifiers[23]. Forshew et al[24] reported a method termed Tagged-Amplicon deep Sequencing in 2012. They detected somatic mutations in cfDNA at a 2% allele frequency. In the case of non-small cell lung cancer, another method for profiling ctDNA, Cancer Personalized Profiling by deep Sequencing, has been described[25]. In addition to methods for detecting SNVs, Personalized Analysis of Rearranged Ends identifies cancer-specific genome rearrangements, and it has been shown that such alterations can be used as personalized cancer biomarkers[26].
CTDNA AS A BIOMARKER FOR PANCREATIC CANCER
Clinical utility of ctDNA has been investigated in various types of cancer. For diagnosis, the most commonly mutated genes are considered to be best suited for analysis as blood-based biomarkers. However, even within a single tumor type, the mutation profile generally varies from patient to patient. Even if a single gene is commonly mutated in a particular cancer type, the altered locus can vary, especially in tumor suppressor genes such as TP53. For this reason, among others, it is not simple to utilize tumor-derived DNA in plasma for the diagnosis of many cancer types without information about actual mutations in the tumor tissues themselves.
The molecular genetics landscape of PDAC has been studied by whole-genome or exome sequencing and somatic alterations associated with this disease have been identified[28-31]. Four genes, KRAS, CDKN2A, TP53 and SMAD4, are commonly mutated or modified epigenetically in PDAC, and dozens of candidate driver genes are altered at low frequency (< 5%)[27-30]. Clonal evolution of pancreatic cancer has also been investigated and it has been exhibited that genetic heterogeneity arise during subclonal evolution[4]. Since point mutations of KRAS are particularly commonly observed in PDAC and 90% of all KRAS mutations occur in codon 12 or 13, these have been a focus of attention. To date, many studies have confirmed that mutant KRAS can be detected in plasma or serum from patients with PDAC, although detection methods applied were diverse[22,31-36].
In the early 21st century, several research groups investigated the potential use of KRAS mutation in cfDNA as a biomarker of pancreatic cancer and demonstrated that such mutations were more frequently detected in the blood of PDAC patients than in individuals suffering from chronic pancreatitis[31,32]. It has also been suggested that sensitivity and specificity for detection of PDAC can be improved by combining KRAS mutations in blood with increase in the serum CA19-9 level[32,33]. Maire et al[31] reported that the sensitivity and specificity of serum KRAS mutations for the diagnosis of pancreatic cancer were 47 and 87%, respectively, whereas the combination of serum KRAS mutations and CA19-9 had a sensitivity and specificity of 98 and 77%, respectively. Analysis by Däbritz et al[32] also suggested that detectable KRAS mutations in the plasma were associated with progressive disease (75%), whereas the association was more evident when combining plasma KRAS mutations and elevated CA19-9 (92%). Furthermore, it has been reported that the presence of mutant KRAS in the circulation is associated with poor prognosis of patients with pancreatic cancer[23,33-36]. Multivariate analysis also showed that KRAS mutations in plasma DNA were stronger prognostic factor for survival (HR = 7.39, P < 0.001) than elevated CA19-9 (HR = 2.49, P = 0.087)[33]. Thus, KRAS mutant cfDNA could be useful as a predictive biomarker for treatment decisions.
One of the major potential applications of ctDNA is disease monitoring. Tjensvoll et al[36] reported that changes in mutant KRAS levels in the circulation correlated with radiological imaging data and CA19-9 levels during the course of chemotherapy. They suggested the utility of KRAS mutant cfDNA for monitoring treatment efficacy and tumor progression in pancreatic cancer patients. Our own experiments further suggested that the detectability of KRAS mutant cfDNA is associated with the presence of distant organ metastasis, and thus ctDNA might be also useful to monitor tiny distant metastases that are hard to detect by routine imaging tests[22].
As mentioned above, currently available tumor biomarkers, such as CA19-9, are insufficient to detect PDAC due to low sensitivity and low specificity. Somatic mutations, on the other hand, are highly specific to DNA derived from cancer or precancerous cells. Especially, KRAS is the most frequently mutated gene in PDAC and the mutations occur at the very early stage of carcinogenesis. As technology advances, ctDNA discriminated by KRAS mutation may have great potential as a blood-based biomarker for PDAC.
DETECTING TARGETABLE GENOMIC ALTERATIONS IN CTDNA
It is noteworthy that various other cancer-related genes are mutated at relatively low frequencies in PDAC. Importantly, it has been indicated that 20% of patients with pancreatic cancer have somatic alterations in genes that are potential targets of therapies approved by the U.S. Food and Drug Administration for oncologic indications or therapies in published prospective clinical studies[37]. This suggests that genomic profiling in pancreatic cancer could be useful to design precision treatment strategies. Due to improvements of sequencing technologies, global or highly multiplexed genomic analysis of ctDNA is becoming feasible using NGS. Analyzing ctDNA has also been proposed as an alternative method to tissue biopsy in the setting of precision medicine, which relies on the presence of specific targets. Although tumor tissue biopsy is the gold standard for molecular screening of cancer, some patients are precluded from molecular screening because of difficulty in obtaining a tissue biopsy or insufficient tumor content in the available specimens[38]. Indeed, adequate biopsy tissues for molecular diagnosis are often difficult to acquire in pancreatic cancer patients. Very importantly, taking tissue biopsies is invasive and therefore not without clinical complications. Zill et al[39] analyzed 54 genes in tumor tissues and cfDNA samples using a commercially available gene panel, and demonstrated that a large proportion of mutations in pancreatic and biliary cancer could be detected in both. Although 35% of patients had an insufficient quantity or quality of tissue biopsy samples for sequencing analysis in their cohort, sequencing of cfDNA identified somatic mutations in many of these cases. We also have reported targeted deep sequencing analysis of cfDNA using a modified SureSelect-Illumina platform and an original gene panel for pancreatic cancer[22]. Our gene panel consisted of 60 genes, including 17 potentially actionable examples. In order to apply the SureSelect Target Enrichment System for the small amounts of cfDNA samples, we modified the library preparation conditions by combination with a KAPA Hyper Prep Kit. In our protocol, input cfDNA could be reduced to as little as 5 ng. As prescreening for sequencing analysis, dPCR assays were first performed to determine the mutational status of KRAS in plasma cfDNA of 259 patients with PDAC. We then carried out targeted deep sequencing in 48 patients, including 43 cases that were considered to have ≥ 1% tumor DNA in total cfDNA based on dPCR KRAS assay and 5 patients with obvious distant organ metastasis, even though they were negative for KRAS mutation in plasma on dPCR assay. We found somatic mutations in potentially targetable genes in 14 of 48 patients (29.2%). In addition, we analyzed somatic CNA using targeted sequencing data for cfDNA, and potentially targetable gene amplifications, such as in CCND1 and ERBB2, were also detected. At present, as NGS assays are still costly and the sensitivities of standard sequencing technologies are limited, targeted deep sequencing of cfDNA may not be practical in clinical settings for all patients. Since KRAS mutation is a good cancer biomarker in pancreatic cancer patients, our two-step approach combining dPCR and NGS could be cost-effective and applicable in the clinic. It may be possible to apply such ctDNA assays to broader range of patients by using a larger volume of plasma because the sensitivities of these assays should depend on the amount of input cfDNA. In addition, the use of novel techniques, including molecular barcoding, and error reduction methods by bioinformatics approaches could improve the sensitivities of sequencing analysis[40,41]. Thus, the available data indicate that liquid biopsy has great potential for diagnosis and treatment design in pancreatic cancer in diverse clinical settings.
EARLY DETECTION OF PANCREATIC CANCER BY LIQUID BIOPSY
Although PDAC is a highly aggressive disease, the investigation of clonal evolution of this disease and mathematical modeling of the rate of mutation acquisition suggests that there is an 11.7-year period from acquisition of the initiating mutation to full transformation in a pancreatic cell, and another 6.8 years are needed to develop the first metastatic subclone[4]. This model implies that there is a substantial time window for early detection of PDAC. Early diagnosis could have a major impact on patient survival, and therefore new effective biomarkers are urgently needed to improve prognosis.
At present, clinical screening for early detection of PDAC has only limited effectiveness, and liquid biopsy appears to be a promising approach to overcome this problem. In general, however, detection of ctDNA is still challenging in early-stage cancer patients because of the high background levels of normal cfDNA. KRAS mutation has been proposed as a biomarker in cfDNA for detection of PDAC, but in early-stage malignant disease (and also in some metastatic cancers), ctDNA may be extremely rare in total cfDNA (0.01% or less)[23,25,39]. Although many analyses of ctDNA have been reported in various cancer types, the vast majority of those studies were analyses of advanced-stage cancer patients, with metastasis or high tumor burden, and the utility of detecting ctDNA in patients with early-stage lesions has been poorly investigated[14,25,42].
A multicenter study of liquid biopsies in 846 patients with 15 cancer types (including PDAC), using digital technologies and approximately 5 mL plasma, reported a detection rate of ctDNA of 80% in patients with advanced cancer, but only 47% in cases of localized cancer[12]. This finding implies that current technologies for ctDNA analysis are still insufficiently sensitive for reliable detection of early-stage cancers. Novel detection methods with much higher sensitivity are required. The same study also demonstrated that detection rates for ctDNA differ depending on the type of cancer[12]. The factors determining ctDNA levels are still not completely understood, but may include tumor burden and spatial proximity to the vasculature, in addition to type. Detailed analyses and accumulation of larger numbers of experimental data for patients with pancreatic cancer in various clinical situations are needed to develop ctDNA analysis that would be practical for early diagnosis.
In addition to peripheral blood, other body fluids such as pancreatic juice may be a secondary source of tumor DNA for liquid biopsy. While collection of pancreatic juice is invasive, as it requires endoscopic techniques which are much more intricate than simple drawing of blood, pancreatic juice would be expected to contain a much higher concentration of tumor DNA. Indeed, mutant KRAS has been detected in pancreatic juice from pancreatic cancer patients[43,44].
Not only genetic alterations, but also epigenetic aberrations, such as DNA hypermethylation, occur during pancreatic carcinogenesis. Aberrant DNA methylation seems to occur in early-stage tumors, resulting in inactivation of tumor suppressor genes or gain-of-function of oncogenic signaling pathways[45,46]. Genes that are aberrantly methylated in a high proportion of pancreatic cancer patients could thus be biomarkers for cancer screening. Methylation of several genes (including NPTX2, SFRP1 and SPARK) has been detected in pancreatic juice samples, and allow distinction of patients with chronic pancreatitis or normal individuals from cancer cases[47]. Detecting tumor-specific epigenetic alterations in cfDNA could be an attractive option for diagnosis of pancreatic cancer by means of a liquid biopsy approach, since epigenetic markers, including aberrant DNA methylation, can be also found in ctDNA. Indeed, Yi et al[48] demonstrated this possibility with promoter methylation of BNC1 and ADAMTS1. In the future, it may be worth investigating the feasibility of utilizing combinatorial approaches with multiple blood-based biomarkers, including genomic mutations in ctDNA and epigenetic alterations in ctDNA, as a strategy to improve sensitivity and specificity in the diagnosis of early-stage pancreatic cancer.
CONCLUSIONS AND FUTURE DIRECTIONS
Although pancreatic cancer is a highly lethal disease with limited treatment options, a novel diagnostic test able to accurately detect the disease at an early stage, when curative surgery may be feasible, should greatly improve the prognosis. Minimally-invasive blood tests might also be useful for cancer screening. A number of studies have already detected genomic alterations in blood from patients with pancreatic cancer, confirming the potential value of liquid biopsy approaches. In addition, detecting actionable genomic alterations in ctDNA might provide a less-invasive approach for precision medicine even in PDAC, which is often inaccessible for tumor tissue biopsy.
However, at present there is still insufficient concrete evidence of the utility of ctDNA analysis regarding treatment of pancreatic cancer, and several issues need to be addressed. One of the most urgent is improvement of sensitivity. While the prospects for technological development and analytical advances seem promising, implementation of new ctDNA analyses for pancreatic cancer screening will depend on demonstration of clinical validity in large prospective studies. Especially for investigating the feasibility of utilizing ctDNA for early diagnosis, it is particularly important to analyze samples from patients with early-stage disease, although this will presumably only be possible with a generalized screening approach. Prospective follow-up and sequential blood sampling of individuals at high risk of pancreatic cancer (e.g., those with a family history of pancreatic cancer or chronic pancreatitis) might thus be essential. Another issue is the diverse range of methods used so far for processing of blood samples and extraction of cfDNA. It will be important to standardize preanalytical processes for cfDNA analysis, such as blood sample acquisition, plasma separation, sample storage, cfDNA extraction and quantification. This issue has only just begun to be discussed. Recently, there are an increasing number of new products for cfDNA processing including blood collection tubes [e.g., Cell-Free DNA BCT® (Streck) and Cell-Free DNA Collection Tube (Roche)] and cfDNA extraction kits [e.g., Quick-cfDNA™ Serum & Plasma Kit (Zymo Research), Maxwell® RSC ccfDNA Plasma Kit (Promega), and MagMAX™ Cell-Free DNA Isolation Kit (Thermo Fisher Scientific)]. For sequencing of cfDNA, new library preparation kits optimized for small amounts of fragmented DNA, such as Accel-NGS® DNA Library Kits (Swift Biosciences) and ThruPLEX® Plasma-seq Kit (Rubicon Genomics), have also been available. It is worth evaluating the new products to establish standardized methods of ctDNA analysis. In view of the potential benefit to patients of a liquid biopsy approach using ctDNA for early detection of pancreatic cancer, we believe work to address these issues should be a high priority.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Baron B, Jiang BJ, Tang ZG S- Editor: Yu J L- Editor: A E- Editor: Zhang FF