INTRODUCTION
The Global Health Observatory of the World Health Organization reported that 13% of all deaths originate from cancer. Colorectal cancer is the third most common cancer in the world and is one of the most common causes of female cancer mortality followed by lung and/or breast cancer[1]. Statistics from Korea and Japan indicated that colorectal cancer ranks number one cause of cancer morbidity in women aged more than 65 years old[2,3]. Also, the incidence and mortality of colorectal cancer in populations over 65 years old are higher in women than those in men implying that colorectal cancer is a major health threat among older women[1]. Considering the longer life expectancy of women compared to that of men, gender-targeted strategies to prevent and treat colorectal cancer should be properly delivered to improve the quality of life especially in older women.
Colorectal cancer screening guideline in general does not apply gender-specific recommendations. However, scientists have suggested that right-sided (proximal) colon cancer is more aggressive type tumor compared to left-sided (distal) colon cancer[4], and patients with proximal colon cancer are more often females than males[5]. In advanced colonic neoplasia, proximal colonic tumors are more often flat, while distal colonic tumors are polypoid-type which is more distinguishable by colonoscopy[6] implying an alternative screening suggestion needs to be discussed. Also, women possess a longer transverse colon compared to men posing lower detection rate in colonoscopy[7]. The sensitivity of fecal occult blood test (iFOBT), a most commonly used colorectal cancer screening test, is found to differ by sex[8]. The decreased gender-specificity of screening tools therefore may explain a higher mortality and shorter 5-year survival rate of women in many regions of the world.
Another crucial point that requires gender-specificity is dietary recommendations for cancer prevention. Dietary factors have been suggested to account for 30% of cancer deaths, indicating that dietary guidelines for cancer prevention represent an essential strategy to lower the burden of cancer[9]. Dietary guidelines for cancer prevention are mostly derived from the summary findings of prospective cohort studies, which are less prone to selection or recall bias than case-control studies. Despite gender-specific differences in dietary risk factors associated with cancer risk, the evidences to generate sex-specific summary estimates are limited.
Also, cancer treatment plan needs to consider sex-specific responses towards anti-cancer drugs based on their biological and genetic characteristics. Possible association between socioeconomic circumstances of women and cancer treatment also requires attention. Therefore, in this paper, we present the biological and socio-cultural differences between genders to provide strategies for gender-targeted colorectal cancer screening, treatment, and prevention.
SEX-RELATED BIOLOGICAL DIFFERENCES IN COLORECTAL CANCER RISK
The estimates of colorectal cancer morbidity greatly increase with older ages. The incidence and mortality of colorectal cancer in populations over 65 years old are higher in women than those in men[1]. Also, the 5-year survival rate of colorectal cancer among women is lower than among men, which is particularly noteworthy in women over 70 years old[10]. These evidences imply that colorectal cancer is a major health threat among older women. However, the need for scientific researches on sex- and gender-associated differences in colorectal cancer development has not been properly emphasized.
A recent systemic review reported that a higher proportion of women presents with right-sided colon cancer than men[4]. Right-sided colon cancer is often at a more advanced stage at diagnosis[4]. Therefore, the lower 5-year survival rate in women may be due to their increased incidence of right-sided cancer. A major cohort study involving 17641 patients compared left-sided colon cancer to right-sided colon cancer for clinical and histological characteristics, progress after the operation, and survival[11]. The results revealed a higher incidence of right-sided colon cancer in women and in older subjects. Also, the effect of age was more significant in women[11]. In the same study, patients with right-sided colon cancer exhibited vague symptoms and suffered from more associated diseases. Right-sided colon cancer was more advanced and less differentiated compared to left-sided colon cancer[11]. A recent cohort study has also indicated that the risk of proximal large polyps increased with age, female sex, and black race[12]. Although the effect of tumor location on survival remains uncertain, more information on pathophysiological differences in relation to gender is needed to plan strategies for screening and treatment of colorectal cancer.
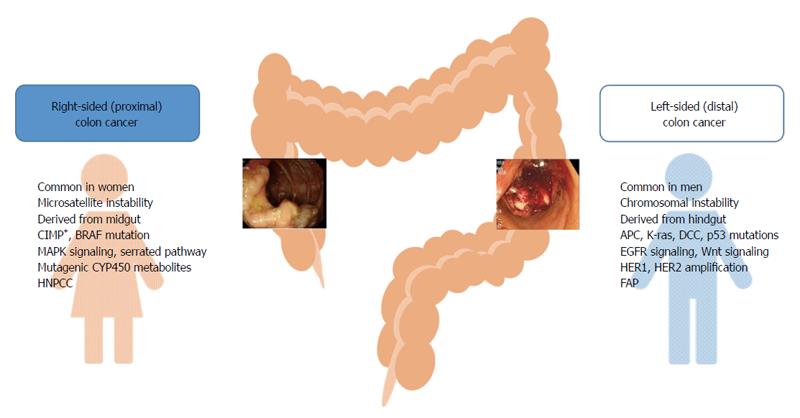
Colorectal cancer exhibits different molecular and pathological characteristics depending on tumor location. Differences between right- and left-sided colon cancers are possibly due to differences in genetic makeup, life style, and/or dietary habits (Figure 1). Chromosomal instability, which is associated with 60%-70% of colorectal cancer, is more often observed in left-sided colon cancer, and defective genes include adenomatous polyposis coli, Kirsten-ras, deleted in colorectal cancer, and p53[13-15]. On the other hand, microsatellite instability (MSI)-high, CpG island methylator phenotype (CIMP)-high, and BRAF mutation are often observed in right-sided colon cancer[14,15]. Hereditary non-polyposis colorectal cancer is more likely to develop tumors on the right side of the colon, whereas familial adenomatous polyposis is associated with left-sided colon cancer[16,17]. The common clinical and molecular features of right- and left-sided colon cancers are presented in Figure 1.
Figure 1 Common clinical and molecular characteristics of right- and left-sided colon tumors.
APC: Adenomatous polyposis coli; CIMP: CpG island methylator phenotype; DCC: Deleted in colorectal cancer; FAP: Familial adenomatous polyposis; HNPCC: Hereditary non-polyposis colorectal cancer; K-ras: Kirsten-ras.
Hormonal factors may explain a large percentage of right-sided colorectal cancer in females. A population-based case-control study examining sex, reproductive factors, and hormone exposure related with MSI in colon cancer (n = 4246) suggested that estrogen exposure is a protective factor against MSI, while the lack of estrogen in older women increased the risk of MSI-high colon cancer[18]. In the same study, hormone replacement therapy (HRT) was associated with the reduced risk of unstable tumors[18]. The Women’s Health Initiative Clinical Trial reported that postmenopausal women undergoing HRT showed a 40% reduction in colorectal cancer risk, whereas women undergoing HRT whilst diagnosed with colorectal cancer exhibited a higher grade/stage of colorectal cancer[19]. These results indicate that HRT could have a detrimental effect on colorectal cancer risk after tumor has developed. Taken together, previous and current HRT is likely associated with the decreased risk of colorectal cancer, while chronic endogenous estrogen exposure may be linked to the increased risk of colorectal cancer in postmenopausal women[20,21].
It has been reported that certain genetic and epigenetic differences between sexes may determine colorectal cancer risk. CIMP-high was increased from the rectum to the cecum, with a higher percentage of females developing tumors in the cecum[22]. An earlier study reported that a methylated CpG island in the 5’ region of the p161NK4a tumor suppressor was positively associated with female gender[23]. Another study found that the vascular endothelial growth factor 936 polymorphism increased the risk of colon cancer in women only[24]. In addition, the PIK3CA mutation occurs more frequently in females and in proximal colon cancer, which is associated with poorer survival[25].
However, limited number of preclinical studies used animals of both sexes to investigate the molecular mechanisms of colon cancer development, the responses to environmental stresses, and the responses to treatment. In many cases, male animals were preferably used to eliminate possible interactions between estrogen and tumor formation. Given that right-sided colon cancer, which is associated with poor prognosis, is more common in women than men, it is important to understand sex-related biological factors which affect segment-specific colon tumor formation. To study sex differences, preclinical researches need to use animals from both sexes. Epidemiological studies need to consider biological variables in determining the incidence, mortality, and survival rate of colorectal cancer to produce better screening and treatment protocols.
GENDER-SPECIFIC SCREENING TOOLS AND GUIDELINES FOR COLORECTAL CANCER
Colorectal cancer screening provides effective opportunity to prevent the disease. However, there are no gender-specific screening tools or guidelines. A previous study showed that both black race and females tend to exhibit polyps greater than 9 mm while other races and males exhibit smaller polyps upon colonoscopy[26], suggesting possible sex- and race-specific delays in diagnosis. It has been also reported that screening via flexible sigmoidoscopy can detect polyps or tumors twice as frequently in men than in women[27]. Women felt less pain and discomfort when undergoing thinner flexible sigmoidoscopy[28], suggesting there are needs to consider sex differences in the sensitivity and feasibility towards colorectal cancer screening tools.
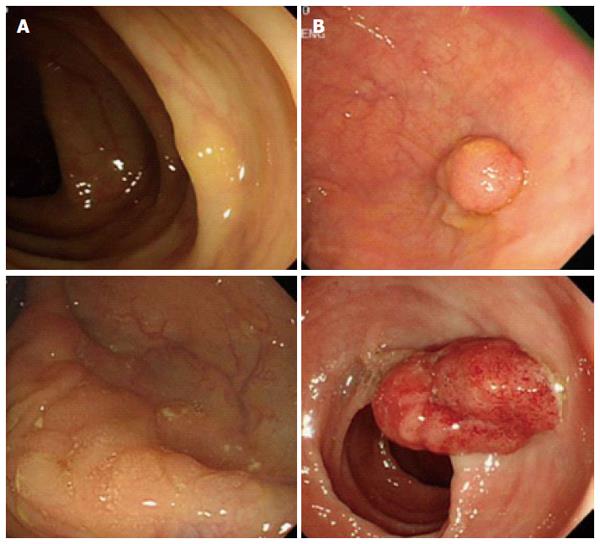
It is interesting to note that cumulative 10 year incidence and mortality of colorectal cancer in women at ages 55, 60, and 65 years follow almost identical rates of incidence and mortality of colorectal cancer in men at ages 50, 55, and 60[29], suggesting women exhibit delayed colorectal cancer development. Despite the seriousness of colon cancer in older women, sex-specific anatomical and physiological characteristics in women have made it difficult to detect tumors during screening processes. Women have the longer transverse colon and increased redundancy compare to men causing incomplete colonoscopy in women[7]. Preclinical studies suggested that dietary fiber consumption increases the length of the colon[30,31]. Therefore, higher dietary fiber consumption in women might be related to the longer colon length in women[32,33]. A large cross-sectional study (n = 4910) reported that a larger proportion of women exhibited flat- and depressed-type colorectal neoplasia while a higher percentage of men showed polypoid-type neoplasia which is more easily detectable[6]. Endoscopic observations clearly revealed morphological difference between right-sided colon cancer vs left-sided colon cancer (Figure 2).
Figure 2 Different endoscopic appearances between right- (A) and left-sided (B) colon cancers.
In addition, among other screening tools, the sensitivity of iFOBT and guaiac FOBT was substantially higher among men than among women[8]. A previous study showed that women who experienced cancer in their reproductive tract tend to develop colorectal cancer more frequently[34]. Lastly, health care disparities between gender and ethnic groups have been presented[35]. Among 1071 surgical colon cancer patients, women (n = 521, 48.6%) had less screening diagnosis (overall: 17.8% vs 22.6%, P = 0.049) with subsequently higher rates of metastatic disease on pathology. These evidences suggest that colorectal cancer screening in women needs more attention in terms of their sensitivity, and gender-specific screening guidelines for colorectal cancer need to be deliberated.
Despite distinctive sexual differences in anatomy and physiology of the colon, gender-specific screening guidelines have not been emphasized. The longer average total and transverse colon length, more frequent occurrence of flat-type right-sided colon cancer and narrower colon diameter of women compared to those of men may cause technical limitation in endoscopic examinations. Therefore, it is necessary to customize endoscopic devices for women. There is no guideline regarding the age to stop colorectal cancer screening in many parts of the world. Asia-Pacific guideline stated that colorectal cancer screening should be continued until 75 years old for both men and women[36]. Due to longer life expectancy in women, colorectal cancer screening in older women needs to be emphasized.
GENDER-SPECIFIC ASSOCIATIONS BETWEEN DIETARY FACTORS AND COLORECTAL CANCER
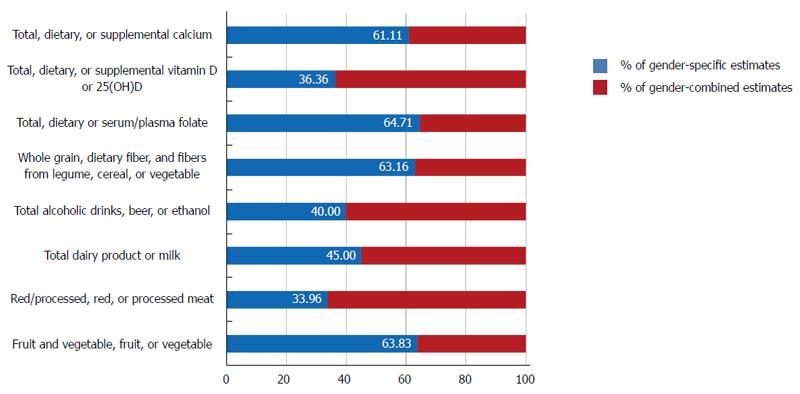
The Expert Report, “Food, Nutrition, Physical Activity, and the Prevention of Cancer: a global perspective”[37] is the most comprehensive summary of the relationships between diet and cancer based on the analysis of over 7000 scientific studies. As a continuation, the World Cancer Research Fund (WCRF) also launched the Continuous Update Project to rigorously compile the most up-to-date evidence available[38]. We reviewed all of the prospective cohort studies that analyzed cancers of the colorectum, colon or rectum as the major endpoints included in the Continuous Update Project of the WCRF[38] and examined whether the associations between dietary factors and colorectal cancer risk were reported according to gender. When we calculated the proportion of sex-specific estimates among studies that included both women and men, an average of 51.02% of the studies reviewed in this report reported sex-specific estimates, ranging from 33.96% (red/processed, red, or processed meat) to 64.71% (total, dietary and serum/plasma folate) (Figure 3). For alcoholic beverages, 40.00% of the studies reported sex-specific estimates among studies which included both women and men. Given that sociological and cultural aspects of alcohol drinking vary by sex and that the toxic threshold of ethanol may differ by sex, it may be important to provide specific summaries for women and men.
Figure 3 Proportion of sex-specific estimates reported in prospective studies that included both women and men.
Since the 1980s, many female-specific prospective cohort studies (e.g., the Nurses’ Health Study, the Iowa Women’s Health Study, the Black Women’s Health Study, the Breast Cancer Detection Demonstration Project Follow-up Cohort, the California Teachers Study, the New York University Women’s Health Study, the Shanghai Women’s Health Study, and the Sweden Mammography Cohort) have published their findings on diet and cancer prevention. These results have contributed to the accumulation of evidence regarding cancer prevention for women, particularly for cancers that occur primarily in women. However, large studies that included both women and men often did not report the sex-specific estimates. This lack of reported sex-specific estimates precludes meta-analyses of sex-specific estimates, which would have a greater statistic power and provide evidences for dietary guidelines for cancer prevention.
Studies have reported that dietary factors are associated differently with colorectal cancer depending on the location of tumors. High carbohydrate intake increased right-sided colon cancer in women, but increased rectal cancer in men[39]. High fat and protein intakes increased risks of right- and left-sided colon cancers, respectively[40,41]. Recent evidence from a large Canadian population-based case-control study suggested that high intake of polyunsaturated fat, trans-fat, cholesterol, sucrose, and lactose was associated with the increased risk of right-sided colon cancer[42]. In addition, meat consumption increased the risk of left-sided colon cancer compared to right-sided colon cancer[43-45], whereas total iron and iron from supplements were inversely associated with distal colon cancer[45]. Also, high calcium intake[46-48] and serum/plasma 25-hydroxyvitamin D level[49] were inversely associated with distal colon cancer.
Consumption of soy products containing phytoestrogens has been shown to be inversely associated with the risk of colorectal cancer[50-53]. A recent meta-analysis found that soy consumption was associated with an approximately 21% reduction in colorectal cancer risk only in women, presumably due to the structural and metabolic similarities of soy isoflavones to estrogen[50]. This result supports a previous report suggesting soy consumption differentially affects estrogen metabolism depending on the endogenous estrogen level[54]. Indeed, an experimental study reported that high phytoestrogens intake increases ER-α expression, decreases apoptosis, and induces inflammation markers in colonic mucosa of female mice possibly due to the high estrogenic background[55].
The International Agency for Research on Cancer (IARC), based on the IARC online database GLOBOCAN 2012, estimated a substantial increase to 19.3 million new cancer cases per year by 2025[1]. Given the biological and socio-cultural differences between genders, gender-specific analyses should be conducted to provide optimal cancer prevention strategies and to reduce the number of new colorectal cancer cases both in men and women. Large population-based cohort studies need to report sex-specific estimates of dietary risk factors to provide better guidelines for cancer preventive dietary intake.
SEX- AND GENDER-SPECIFIC DIFFERENCES IN COLORECTAL CANCER TREATMENT
Clinical studies have suggested that colorectal cancer treatment in premenopausal women needs attention due to its possible effects on female fertility. A retrospective study reported that 41% of women receiving adjuvant 5-fluorouracil, leucovorin, oxaliplatin (FOLFOX) chemotherapy experienced amenorrhea during chemotherapy, and 16% exhibited persistent amenorrhea 1 year after the completion of chemotherapy, which may affect early menopause and fertility[56]. In addition to chemotherapy, surgical and radiation therapies also need to be considered for female fertility preservation[57].
Furthermore, gender-specific recurrence and survival rates were detected. The genotype of the TP53 tumor suppressor gene was predictive of survival following adjuvant chemotherapy in women with stage III colon cancer[58]. In stage II and III colorectal cancer patients, polymorphisms in PLS3 and LCP1 were associated with tumor recurrence in women with proximal colorectal cancer[59]. Therefore, it is necessary to use a distinct evaluation protocol for the response of women to colorectal cancer treatment.
Taken together, research efforts towards the development of anti-cancer drugs displaying less toxicity to the reproductive system are required. Treatment protocols specifically recommended for women of child-bearing age should be suggested based on sound scientific evidence. A sex-specific decrease in the survival rate of women subjected to a specific anti-cancer drug may be associated with the genetic background. Thus, a long-term follow-up study of cancer survivors needs to be considered to ensure the safety of specific anti-cancer drug use with respect to not only reproductive function but also possible genetic effects on subsequent generations. Also, possible gender-specific barriers to cancer treatment need to be studied. This is specifically important because optimal anti-cancer drug regimen for colorectal cancer should be required based on the effect of sex on drug efficacy and toxicity. Among 1785 colon cancer patients aged more than 65 years old, women were less likely to receive 5-fluorouracil treatment and showed a shorter duration of treatment compared to men, which were possibly associated with the observation that women were more prone to dehydration than men[60]. Indeed, women experienced more severe toxicity including stomatitis, leukopenia, alopecia, and diarrhea compared to men when receiving 5-fluorouracil-based treatment[61].
It is recommended to complete all planned chemotherapy cycles to improve disease-free survival. However, recent studies reported that stage III female colon cancer patients tend to omit adjunctive chemotherapy sessions compared to male counterparts[62-64]. Also, a greater percentage of elderly female patients and female patients with a prolonged hospital stay exhibited a higher rate of discontinuation[62]. Therefore, further researches are needed to provide evidence on gender-specific barriers to colorectal cancer treatment and establish optimal anti-cancer drug regimen by gender.
CONCLUSION
Clinical and preclinical studies have indicated that there are sex- and gender-associated differences in colorectal cancer development. Both genetic and environmental factors are believed to play roles in sex and gender differences in right- vs left-sided colon cancers. Therefore, biological and pathophysiological differences in colorectal cancer development between men and women need to be clearly addressed. Despite higher incidence of right-sided colon cancer in women, a substantially higher number of preclinical studies use only male animals in colorectal cancer research. Researchers should be aware of sex-specific pathophysiological differences in colorectal cancer development and use both male and female animals for their research. In addition, there is a great deal of needs for developing gender-specific endoscopy devices with higher sensitivity due to sex-specific differences in biological and anatomic characteristics of the colon. Colorectal cancer screening guidelines may need to emphasize gender-specific points for colorectal cancer screening.
Diet is one of the most closely associated environmental factors in colorectal cancer development. Dietary factors to increase or decrease the risk of developing colorectal cancer are continuously updated based on large scale cohort studies. However, only a half of studies reported sex-specific risk estimates despite potential sex-associated differences between dietary factors and colorectal cancer risk. Given that there are sex- and gender-specific differences in the biological responses to dietary components, it is necessary to analyze and report gender-specific risk estimates to provide better guidelines for cancer prevention strategies. Furthermore, researches addressing sex-specific differences in responses to anti-cancer drugs for colorectal cancer are required to reduce side-effects on reproductive system and to investigate genetic effects on drug efficacy. By understanding sex- and gender-related biological and socio-cultural differences in colorectal cancer risk, gender-specific strategies for screening, treatment, and prevention protocols for colorectal cancer can be established to reduce the mortality and increase the quality of life.
P- Reviewer: Chiacchiera F, Divella R, Syed V S- Editor: Qi Y L- Editor: A E- Editor: Ma S