Published online Feb 7, 2014. doi: 10.3748/wjg.v20.i5.1211
Revised: November 23, 2013
Accepted: January 6, 2014
Published online: February 7, 2014
Inflammatory bowel disease (IBD) affects a part of the young population and has a strong impact upon quality of life. The underlying etiology is not known, and the existing treatments are not curative. Furthermore, a significant percentage of patients are refractory to therapy. In recent years there have been great advances in our knowledge of stem cells and their therapeutic applications. In this context, autologous hematopoietic stem cell transplantation (HSCT) has been used in application to severe refractory Crohn’s disease (CD), with encouraging results. Allogenic HSCT would correct the genetic defects of the immune system, but is currently not accepted for the treatment of IBD because of its considerable risks. Mesenchymal stem cells (MSCs) have immune regulatory and regenerative properties, and low immunogenicity (both autologous and allogenic MSCs). Based on these properties, MSCs have been used via the systemic route in IBD with promising results, though it is still too soon to draw firm conclusions. Their local administration in perianal CD is the field where most progress has been made in recent years, with encouraging results. The next few years will be decisive for defining the role of such therapy in the management of IBD.
Core tip: Treatments with mesenchymal and hematopoietic stem cells offer a potential that requires in-depth investigation. Existing studies are encouraging yet inconclusive. We are at a point of inflexion where these new therapies are seen to afford major curative potential. The coming years will be decisive. The information obtained from ongoing and future clinical trials may lead a revolution in inflammatory bowel disease management and its impact upon patients. Undoubtedly, as twenty-first century gastroenterologists, we must expand the scope of our specialty and seek multidisciplinary interaction for the benefit of our patients.
- Citation: Martínez-Montiel MDP, Gómez-Gómez GJ, Flores AI. Therapy with stem cells in inflammatory bowel disease. World J Gastroenterol 2014; 20(5): 1211-1227
- URL: https://www.wjgnet.com/1007-9327/full/v20/i5/1211.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i5.1211
Ulcerative colitis (UC) and Crohn’s disease (CD) are the two main conditions found in inflammatory bowel disease (IBD). The incidence and prevalence of IBD have gradually increased in recent years. It is estimated that in Europe 1.4 million and one million people may have UC and CD, respectively[1]. In the United States over 1.3 million people have IBD[1]. Furthermore, in the last few decades there has been an increase in the disease in low-incidence zones as South Korea, China, India, Iran Lebanon, Thailand, the French West Indies, North Africa and Japan[2,3]. IBD poses an important health problem, since its worldwide incidence is increasing[1], the condition affects young people, and persists for life - exerting a strong impact upon quality of life, in the professional setting, and in patients’ personal relations[4,5]. Furthermore, IBD is associated with considerable healthcare costs[6,7].
Although the etiology of IBD remains unclear, there have been significant improvements in our knowledge of its physiopathology, allowing advances in treatment and a change in the current therapeutic objectives. Before the introduction of anti-tumor necrosis factor-alpha (anti-TNFα) drugs, the management of IBD was centered on symptoms control. Drug substances (mesalazine, corticosteroids, thiopurines, methotrexate and cyclosporine) were used to induce and maintain disease remission. Although endoscopic disappearance of the lesions was known to occur in CD patients subjected to thiopurine therapy[8], the significance of mucosal healing was not known, and consequently did not constitute a therapeutic objective. The introduction in 1998 of anti-TNFα drugs for the treatment of CD improved the clinical outcomes of therapy, and mucosal healing was found to be associated with a good prognosis, fewer hospital admissions and surgical operations, and improved quality of life[9]. Later studies of both CD and UC showed mucosal healing to be a therapeutic objective[10-17]. In this context, current treatment focuses on achieving “deep remission” of IBD (symptoms control and endoscopic healing of the mucosal lesions)[14,15,18,19]. The purpose of treatment is to control the symptoms and the underlying intestinal inflammatory process (which is responsible for the progressive intestinal damage), and to restore normal intestinal function. Accordingly, in CD we aim to avoid structural intestinal damage, reduce disability over the long term, and improve patient quality of life. In the case of UC, we aim to reduce the percentage of colectomies and the incidence of colorectal cancer, and improve patient quality of life. All these strategies are aimed at changing the natural course of the disease.
However, despite the efforts being made to optimize use of the existing drugs, the current situation is far from ideal. Approximately one-third of all CD patients fail to respond to anti-TNFα therapy (primary non-responders)[9,11,20,21], and 10% of all CD patients do not tolerate or are primary non-responders to all the drugs used for treating the disease. Among the individuals who do respond to anti-TNFα treatment, one-third show transient loss of response and require either optimization or a switch to another biological agent (secondary non-responders)[22]. Furthermore, despite the new therapeutic strategies, the number of surgeries in CD remains stable[23]. Esophageal cancer (EC) is the second leading cause of intestinal transplantation in adults, as a consequence of the development of intestinal failure secondary to multiple surgical operations. In this context, disease recurrence in the graft is the norm[24,25]. Perianal disease can be a serious problem that proves difficult to control with the existing treatments, and refractory cases are subjected to aggressive surgery associated with a considerable psychological impact for the patient[26,27]. In addition to the above, it must be mentioned that IBD is becoming more common in the pediatric population, and patients now report to the clinic as adults with a history of multiple intestinal resections and immune modulating treatments with or without biological agents. In this population group, gastroenterologists predict that there may be a loss of response over the long term, and the future of these patients thus appears uncertain. Lastly, in some patients who do not respond to medical treatment, surgery is unable to solve the problem due to the location and extent of the lesions.
The situation is different in the case of UC, since in severe disease flare-ups and in patients resistant to medical treatment, costly surgery in the form of colectomy is a therapeutic option.
All this leads us to seek new treatment options. In recent years, efforts have increasingly focused on the study and use of cell therapies with T lymphocytes[28], tolerogenic dendritic cells[29] and both hematopoietic and mesenchymal stem cells (MSCs). The present review examines the role of stem cells in the control of IBD. An analysis is made of the physiopathological principles of stem cell therapy, the results of the studies carried out to date, and the future perspectives in this field.
Advances in our knowledge of the pathogenesis of IBD are the basis for the development of new treatments.
At present, IBD is regarded as the result of an abnormal host immune response to intraluminal antigens occurring in a genetically predisposed individual, with the production of chronic inflammation of the gastrointestinal tract, accompanied by tissue destruction. IBD is the consequence of complex interaction among genetic[30,31], environmental[1] and microbial factors[32], producing sustained inflammation at intestinal level, favored by alteration of the mucosal barrier and immune system defects[33].
Under normal conditions, the intestine presents minimal physiological inflammation, despite exposure to a large number of intraluminal microbial and food allergens, and the presence of a significant number of lymphoid cells (80% of all lymphocytes are located in the intestine)[33,34]. This situation is the result of complex mechanisms involving innate immunity[35-39], represented by the epithelial mucosal barrier, innate cell immunity (leukocytes, monocytes, macrophages and dendritic cells), and innate humoral immunity (e.g., lysozyme, complement and interferons). While this innate response is not specific, it is able to differentiate so-called pathogen-associated molecular patterns, and to activate different intracellular mechanisms that condition or orientate the adaptive response. On the other hand, dendritic cells serve as the link between innate and adaptive immunity, presenting antigens to naïve Peyer’s patch lymphocytes and inducing their differentiation[40]. The Lymphocytes are the main effector cells of the adaptive immune response, and are activated at intestinal level to eliminate pathogenic antigens. Once the antigens have been eliminated, the lymphocytes are down-regulated, thereby maintaining intestinal homeostasis[38]. Lymphocytes are memory cells, and when they are exposed again to an already identified antigen, the immune response is both faster and more potent than at first exposure. The physiological mechanisms whereby the intestine differentiates pathogenic and non-pathogenic antigens, and the pathways involved in the correct maintenance of intestinal homeostasis, are not clear. We do know that a balance between regulatory T cells (Treg) and effector T cells (Th1, Th2 and Th 17) is essential for intestinal homeostasis[41,42].
It remains unclear whether the inflammatory process damages the mucosal barrier and thus allows penetration of the intraluminal antigens, thereby activating the inflammatory process, or whether mucosal barrier alteration is the primary event that in turn triggers the inflammatory response.
In recent years there have been major advances in the knowledge of the aspects that are believed to be involved in the development of IBD.
The genetic bases of IBD were recognized early in clinical practice in view of the increased incidence of the disease in homozygous twins, in first-degree relatives, and in certain ethnic groups[43-46]. At present, genome-wide association scan studies (GWAS) have identified over 100 loci related to the development of IBD[47]. A GWAS meta-analysis has revealed 77 genes associated to CD, the existence of shared genes between CD and UC[30], and 47 loci related to UC[31]. This suggests there are overlapping genes in both disease conditions which predispose to the development of IBD [genes with expression products that encode for the interleukin (IL)-23 pathway, transcription factors such as NK2, locus 3 (NKX2-3), SMAD3, STAT3, ZMIZ1 and c-REL], by encoding for proteins involved in the innate and adaptive immune responses[48-50]. Likewise, there are genes implicated in the development of CD such as NOD2, and genes that regulate autophagia[51,52], while other genes are specifically associated to UC, such as the genes located on chromosome 6p21 (related to the major histocompatibility complex), or to mucosal barrier integrity and defense[47]. On the other hand, genetic factors have been shown to be associated to the phenotype of the disease, its pattern, evolutive course and response to drug treatment[53-57]. Genetic disorders in IBD are multiple and complex, and unfortunately we are able to predict less than 25% of hereditary correlations in IBD[58]. The exception is represented by IL-10 receptor alteration, which is associated to severe CD, and seen in children refractory to treatment and with a good response to allogenic hematopoietic stem cell transplantation (HSCT)[59].
Epidemiological studies have shown that environmental factors are essential for the development of IBD, and could account for the increase in the presence of the disease in developing countries[1]. These environmental factors could exert a direct influence upon changes in the intestinal microbiota, or on the appearance of new intraluminal antigens from foods or environmental toxic agents. Diet and antibiotics are the most important determining factors of bacterial diversity in the gastrointestinal tract, and their modification runs parallel to the socioeconomic development of countries[60]. The microbiota plays a key role in the development of IBD among genetically susceptible hosts[61]. In this regard, a recent publication addresses the possible infectious origin (produced by an unknown bacterium) of a case of colitis with histological data indicating chronicity and the presence of granulomas. The condition is considered idiopathic, and occurs in patients subjected to umbilical cord allogenic hematopoietic cell transplantation[62]. This opens a door to the future search for microbial agents in similar disease conditions characterized by an unknown etiology.
Lastly, intestinal homeostasis is lost in IBD as a result of defects in the intestinal epithelial barrier and/or imbalances between Treg and effector T cells (Th1, Th2, Th17) - giving rise to an inappropriate immune response to harmless intraluminal antigens (components of the diet) or intraluminal bacteria[42,63].
It is currently accepted that the predominant inflammatory profile in CD involves a type Th1 and Th17 activated CD4+ lymphocyte response, with an increase in interferon-γ (IFN-γ), TNFα, IL-17 and IL-22[64]. In UC, the cytokine profile is similar to that of the natural killer (NK) cell mediated Th2 response, producing IL-5 and IL-13. This latter interleukin is crucial for the development of UC, exerting cytotoxic action against the epithelial cells and positive feedback upon the NK cells, resulting in tissue damage[64,65].
Improved knowledge of the pathways leading to inflammation, and of the implicated cytokines, cells and adhesion molecules, have allowed the identification of many therapeutic targets upon which to act. Until the causal agent can be identified, these are the advances which can help us in the search for new drug substances.
Stem cell therapy aims to modify the immune response of patients with IBD and repair the tissue damage caused.
Stem cells are characterized by asymmetrical division, giving rise to a cell with the same properties as the original cell (self-renovation), and another cell of multilineage potency that can differentiate in one way or another depending on environmental conditions.
There are different types of stem cells, depending on their origin and functions[66].
These are pluripotent cells obtained from embryos, and which can produce all the tissues derived from the three embryonic layers both in vitro and in vivo.
These cells are found in all body tissues, where they may have reparatory functions. Hematopoietic stems cells (HSCs) and MSCs belong to this category. Both types are found in bone marrow, though MSCs can be obtained from different tissues (bone marrow, adipose tissue, umbilical cord, placenta, etc.), do not produce hematopoietic cells, and can differentiate towards cells belonging to the mesenchymal lineage.
These are defined as artificial pluripotent stem cells[67] that can be generated from somatic cells following the introduction of reprogramming factors (OCT314, SOX2, KLF4, c-MYC, NANOG and LIN28). These cells acquire the same properties as pluripotent stem cells, and their phenotypic differentiation can be redirected according to the culture media used. These cells pose no ethical problems, since they are not of embryonic origin. As a result, they are currently under study for the development of organs and the repair of tissues carrying the genome of the sick patient[68].
The use of embryonic stem cells and induced pluripotent stem cells has been associated to the development of teratomas following transplantation. This fact, and the present impossibility of eliminating undifferentiated cells produced as a result of division, poses a serious problem for clinical use[69].
Two types of stem cell therapy are currently used for the treatment of IBD: hematopoietic stem cell therapy and MSCs therapy (Table 1). Most treatment evaluations are made in CD patients, since in UC patients surgery is an option in the case of resistance to therapy.
| Type of therapy | Mechanisms of action |
| Hematopoietic stem cells | Autologous transplant: Elimination of reactive T lymphocytes (lymphoablation, new reconstitution of the immune system of the patient with more tolerogenic naïve lymphocytes). The genetic predisposition of the patient is not modified |
| Allogenic transplant: Replacement of the immune system with the donor immune system, correcting patient genetic predisposition. Not accepted due to high morbidity-mortality | |
| Mesenchymal stem cells (autologous or allogenic) | Systemic and local administration: Immune-modulating and trophic action |
Hematopoietic stem cells are immature cells found in bone marrow, the bloodstream and the umbilical cord. They present glycoproteins as CD34+, CD 90+, CD38-, CD133 at surface level and can differentiate towards cells pertaining to hematological cell lines[70]. In clinical practice, HSCT was initially used to treat hematological malignancies. Subsequently, as a result of experimental studies in animals[71,72] and the clinical improvement experienced by patients with immune-mediated diseases (IMDs) subjected to HSCT due to malignant conditions[73,74], HSCT has become increasingly used in selected patients with IMDs refractory to conventional therapy[75,76]. The treatments used at present in application to IBD aim to control the inflammatory process but do not act upon the origin of the disease. The therapeutic objective of autologous HSCT would be to restore the primary immune system of the patient (resetting of the immune system), after using chemotherapy to eliminate self-reactive T lymphocytes (lymphoablation) and memory cells, which would constitute the effectors of the immune dysregulation observed in CD[77], thereby inducing antigen tolerance over the long term. Based on studies carried out in other IMDs[78,79], the changes of the new immune system could be the result of reactivation of thymus gland activity (with the restoration of new polyclonal T cells) and the de novo induction of Treg derived from the thymus, which would be essential for restituting tolerance of harmless antigens[80-82]. Genetic factors are crucial in the development of IBD, and are not modified by autologous HSCT. IBD might reappear after exposure to triggering antigens, and the body would probably respond in the same way. However, and in the worst of cases, the available therapies may be prescribed earlier in an attempt to change the natural course of the disease, or while waiting for the development of new treatments.
In any case, allogenic HSCT can correct the genetic defects of the patient by conforming a new immune system (that of the donor), associated to ablation of the immune cells of the recipient[77]. This transplant strategy is currently not accepted as primary treatment for IMDs, due to its high associated mortality and complications rate.
In 1995, an international committee produced guidance with the criteria and protocols for performing HSCT in patients with severe IMDs, including IBD[83]. The committee recommended autologous HSCT instead of allogenic transplantation, due to its lower risk and toxicity.
HSCT in IMDs is carried out following the protocol used in the treatment of hematological malignancies. In autologous HSCT, the hematopoietic stem cells are obtained from peripheral blood in 95% of all cases. To this end, a first mobilization step is carried out, stimulating the production and release of stem cells from the BM towards the peripheral blood compartment. The treatment scheme most widely used in CD is cyclophosphamide 1.5-2 g/m2 and granulocyte colony stimulating factor (G-CSF) 10 μg/kg per day[84-86]. Peripheral blood stem cells are then collected through apheresis followed by cryopreservation until HSCT is performed. Before reinfusing the hematopoietic stem cells, some work groups use local protocols to purify cell culture, selecting CD34+ cells or selectively eliminating lymphocytes by means of monoclonal antibodies (anti-CD52, anti-CD3, anti-CD19 or anti-CD20)[84]. Other groups do not eliminate lymphocytes[85]. Finally, the conditioning stage (elimination of self-reactive T cells) is carried out, using cyclophosphamide and anti-thymocyte immunoglobulin[86], and hematopoietic cell infusion is carried out at the end of this stage.
The conditioning regimens used vary according to the autoimmune disease and the treating center[87]. Non-myeloablative conditioning regimens have been specially designed for IMDs. In the case of IBD, lymphoablation is performed without irreversible destruction of the hematopoietic cells of the BM, and marrow function could be recovered without the infusion of hematopoietic stem cells. Reinfusion is performed to shorten the duration of BM aplasia. The reasons for not using myeloablative regimens would be safety in first place, since lymphoablative protocols present fewer complications and a lower mortality rate. On the other hand, IBD recurrence after autologous HSCT is highly probable, since the immune system of the patient is regenerated. In turn, following a very aggressive initial phase, some IMDs undergo spontaneous remission for reasons that are unknown, and in these patients such aggressive therapy would be unnecessary[81].
The first case reporting the efficacy of autologous HSCT in the control of CD was published in 1993, and corresponded to a patient with non-Hodgkin lymphoma[88]. Subsequently, isolated cases of IBD improvement in patients receiving autologous HSCT for malignancies were published[89-92]. In 2003, the Chicago group published the first series of CD patients subjected autologous HSCT as primary treatment for IBD[93]. A later publication by this same group[84] reported the results of autologous HSCT (phase I trial) in 12 patients with CD and EC activity index (CDAI) scores of 250-450, refractory to conventional treatment, including infliximab. Eleven patients presented disease remission, defined by CDAI < 150, with a mean follow-up of 18.5 mo (range 7-37 mo). In 2008, Cassinotti et al[85] published the clinical results of autologous HSCT in four CD patients. Clinical and endoscopic remission was observed in three subjects after 16.5 mo of follow-up (phase I-II trial). Unlike the Chicago group, these authors do not perform CD34+ cell selection before hematopoietic stem cell infusion. Since then, some isolated cases as well as larger patient series have been published in which the primary objective of HSCT has been the control of refractory CD[82,94-96]. In the series published by Hasselblatt et al[96], 56% of patients achieved endoscopic healing of mucosal lesions after cell transplantation - these data being similar to those obtained in the phase III trials with anti-TNFα[9-11]. The study carried out by Oyama et al[84] reported improvement of CD following the cell mobilization phase with low dose cyclophosphamide and G-CSF, and suggested that the improvement may have been due to the action of chemotherapy rather HSCT. However, Cassinotti reported worsening of CD in three of the four patients in the period between stem cell harvesting and transplantation. The phase III Autologous Stem Cell Transplantation International Crohn’s Disease (ASTIC) study, sponsored by the European Crohn’s and Colitis Organisation (ECCO) and the European Group for Blood and Marrow Transplantation (EMBT), was designed to clarify this issue. The patients included in this study were randomized to two treatment arms: mobilization chemotherapy with G-CSF and autologous HSCT in 30 d vs mobilization chemotherapy with G-CSF and conditioning with autologous HSCT after 13 mo. The preliminary results have been presented in the ECCO of 2013, concluding that HSCT appears to be effective in CD patients, affording endoscopic improvement of the lesions. However, it involves a risk of adverse effects, and the end results referred to the trial objective are still awaiting analysis[97].
It is difficult to know the precise results of autologous HSCT in CD, due to the few patients treated to date. In the series published by Burt et al[94], which is the most numerous to date, all patients (n = 24) entered remission (CDAI < 150) after transplantation. Over subsequent follow-up, the percentage of patients in remission without the need for CD treatment was 91%, 63%, 57%, 39% and 19% after one, two, three, four and five years, respectively. Regardless of the medication taken, the percentage of patients annually in remission in the 5 years after transplantation was 70%-80%. Eighty percent of patients were in corticosteroid-free remission each year, in the 5 years after transplantation. Regarding the safety of the procedure, infectious complications during the first year were the most significant problem, though mortality was zero (one patient died in an accident).
The overall safety of HSCT in the treatment of autoimmune diseases can be assessed from the two large recently published registries: that of the EMBT, and the registry of the British Society of Blood and Marrow Transplantation (BSBMT). The results of the observational study of the EMBT, documenting the course of 900 patients subjected to autologous HSCT between 1996-2007, showed the 5-year survival rate to be 85%, with a patient disease-free rate of 43% - though there were important variations depending on disease type. In the multivariate analysis, mortality was found to be related to the experience of the center (P < 0.003) and type of autoimmune disease (P < 0.03). An age of less than 35 years (P < 0.004), transplantation after the year 2000 (P < 0.0015), and type of autoimmune disease (P < 0.0007) were associated to a disease-free patient course[87].
In 2012, Snowden et al[86] published the results on HSCT in patients with IMDs documented in the BSBMT registry between 1997-2009 (70 transplants in 69 patients: 55 autologous cell transplants and 15 allogenic transplants). The survival rate in the case of autologous transplants was 85% in the first year and 78% after 5 years. The corresponding figures for allogenic transplantation were 87% and 65%, respectively. The disease-free rate for autologous transplants was 51% in the first year and 33% after 5 years. The corresponding percentages for allogenic transplantation were 80% and 65%, respectively. There were no differences in mortality in the two groups. The leading cause of death was infection, while age was strongly correlated to survival (95% survival after 5 years among patients between 18-39 years of age).
Autologous HSCT may be a valid option in patients with treatment-refractory CD. This therapy must be provided at specialized centers where correct patient screening is performed, and in which complications referred to both transplantation and autoimmune disease are known and can be treated. Further studies are needed to determine the precise role of this procedure in CD treatment.
No descriptions were found in the literature of allogenic HSCT as primary treatment for CD until 2009, when this technique was first used to treat a patient with mutation of the IL10RA and IL10B genes (encoding for proteins ILR10R1 and ILR102, which form part of the IL-10 receptor). This mutation was identified in homozygosis in three children from the same family that developed proctitis early (at 3 mo of age), with severe perianal disease refractory to treatment. Following allogenic HSCT, sustained CD remission was observed[59].
This treatment is not currently accepted as primary therapy for IBD and, with the exception of the aforementioned case, the results found in the literature correspond to patients with neoplastic disease associated to IBD, in which the course of the latter changed after HSCT. The most numerous allogenic HSCT series to date is that published by Ditschkowski et al[98]. In this series, 11 patients (7 with CD and 4 with UC) underwent allogenic HSCT due to hematological malignancies. All patients were subjected to myeloablative treatment and total body irradiation (except in two cases). One patient died of sepsis, while the rest were found to be in complete neoplastic disease remission after 34 mo of follow-up, with no clinical relapse of IBD.
Lopez-Cubero et al[99] previously described 6 CD patients diagnosed with leukemia and subjected to allogenic HSCT. Five of them had active CD before transplantation, and two had sclerosing cholangitis. All of them received cyclophosphamide in the total body irradiation scheme. One patient died of sepsis three months after transplantation. Four of the remaining 5 patients remained symptom-free during a period of 54-183 mo after transplantation. The patients with a clinical diagnosis of primary sclerosing cholangitis showed improvement in alkaline phosphatase levels.
Similar findings have been described in a patient with CD and acute myeloid leukemia subjected to allogenic HSCT[100].
This procedure is not currently recommended in IBD, except in very specific cases such as mutation of the IL-10 gene commented above. Its efficacy and risks have been described in the above section.
MSCs, also known as stromal cells, are adult pluripotent cells initially described by Friedenstein et al[101], who isolated them from bone marrow. They are able to adhere to plastic surfaces, and can differentiate in vitro into chondrocytes, osteoblasts and adipocytes. The International Society for Cellular Therapy established the minimum criteria that must be met in order to classify a stem cell as an MSC[102]: (1) adherent to plastic under standard culture conditions; (2) express CD105, CD73 and CD90; (3) lack expression of CD45, CD34, CD14 or CD11b, CD79a or CD19 and human leukocyte antigen (HLA)-DR; and (4) differentiate to osteoblasts, adipocytes and chondroblasts in vitro.
These cells have been identified in bone marrow, adipose tissue, connective tissue, umbilical cord and placenta, and it is known that they are found in “niches” in different tissues - including the intestine[103,104]. In most clinical studies and in human therapy, MSCs are obtained from bone marrow, adipose tissue and umbilical cord. Adult bone marrow has been widely used to obtain MSCs for clinical purposes, though their presence in adult marrow is low (0.001%-0.01%), extraction is difficult, and the procedure also poses risks for the donor. Furthermore, the differentiation capacity of these cells decreases with donor age, and expansion must be performed in culture media prior to administration of the cells. As a result, alternative MSC sources have been explored[105]. A larger number of MSCs can be obtained through liposuction; in vivo expansion is not required; cells can be administered directly; and their properties are similar to those of MSCs obtained from bone marrow[106-108]. A recent study published by Melief et al[109] shows that both types of cell have similar immune modulating functions, though MSCs of adipose tissue origin present a different cytokine secretion profile, and their immune modulating effects are more potent that those of MSCs obtained from bone marrow.
The use of MSCs is based on their potential capacity to repair damaged tissues and inhibit inflammation and fibrosis[110,111]. In addition, the administration of MSCs of both autologous and allogenic origin requires no conditioning phase, since these cells are not immunogenic: they express low levels of class I HLA antigens at surface level and do not express type II HLA antigens or T cell coactivators[112]. However, in immunocompetent mice it has been seen that the half-life of these cells is notoriously shorter following second exposure. This suggests they do not evade the immune system entirely, and can be rejected[113-115]. These properties have defined MSCs as potentially useful for the treatment of autoimmune diseases and in those processes in which tissue repair is needed[112].
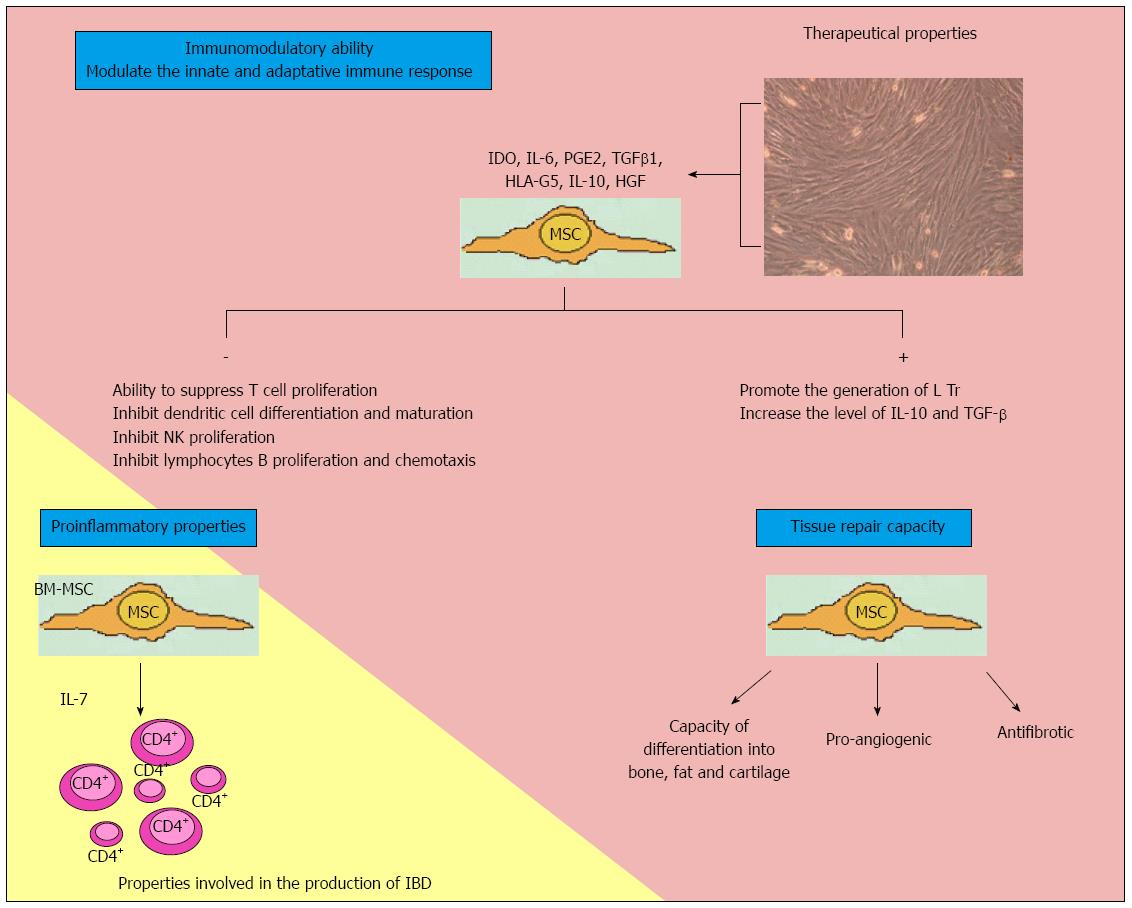
The action mechanism is based on cell contact and paracrine action involving the release of soluble factors (Figure 1).
As regards the immune regulating properties of the cells, they are capable of regulating both innate and adaptive immunity[116]. Cell culture studies have shown that MSCs are able to suppress T cell proliferation[113]. However, MSCs do not possess intrinsic immunosuppressive capacity; instead, such capacity is acquired when cells are stimulated by proinflammatory cytokines such as INFγ, TNF-α and interleukin-1β[117]. Duijvestein et al[118] have shown MSC activation by INFγ to increase the immunosuppressive capacity of cells and their in vivo therapeutic efficacy in mice with colitis induced by trinitrobenzene sulfonate.
MSCs produce a range of factors that have been implicated in immune modulating effects, such as indoleamine 2,3-dioxygenase, IL-6, IL-10, prostaglandin E2, transforming growth factor-β1, nitric oxide, heme oxygenase-1 and HLA-G5[109]. Through the production of PGE, MSCs inhibit IL-2 production and T cell proliferation, with stimulation of the production of T helper lymphocytes[119]. IL-10 and IL-6 act upon macrophages and monocytes, blocking their differentiation towards dendritic cells[120,121]. They also suppress NK cells, induce polymorphonuclear cell and cytotoxic T cell apoptosis, and promote the generation of regulatory T cells[122]. These interleukins also inhibit B lymphocyte activation[123]. Upon coming into contact with activated T cells, MSCs induce the production of IL-10 and HLA-G5[124]. In short, they inhibit innate immunity by blocking the differentiation and maturation of monocytes towards dendritic cells, inhibiting NK cells, and inducing neutrophil apoptosis. In induced colitis in mice, MSCs block T cells and increase regulatory T lymphocyte population, thereby modulating acquired immunity.
However, MSCs can acquire proinflammatory properties, depending on biological environment[125]. In this context, Nemoto et al[126], in a murine model, have shown that bone marrow MSCs constitute the main source of IL-7 production, and may play an etiopathogenic role in IBD by forming a niche for colitogenic CD4+ memory T cells in bone marrow. If these findings are confirmed in humans, anti-IL-7 treatment might become a therapeutic target.
MSCs are able to repair tissues, stimulate angiogenesis and prevent fibrosis. Healing of the intestinal lesions in IBD is the result of control of the underlying inflammatory process, and of repair mechanisms that restore the integrity of the epithelial barrier and repair intestinal damage. The initial applications of MSCs in regenerative medicine were focused on their multilineage differentiation capacity. However, recent reports have demonstrated that most of the biological effects of MSCs are mediated by paracrine mechanisms involving the secretion of cytokines, chemokines and growth factors[127-129]. Indeed, there are several observations of positive MSC biological effects in numerous disease models despite a lack of MSC differentiation and long-term engraftment into damaged or diseased tissue. These effects include a reduction of inflammation, apoptosis and fibrosis, improved wound healing and regeneration[130-133]. In addition, MSC biological effects also may be exerted by the induction and stimulation of endogenous host progenitor cells to improve the regenerative process[134-136].
When administered via the systemic route (intraarterial or intravenous) or locally, the objective is to ensure that MSCs reach damaged tissue. However, the correct dose and administration route remain to be defined, and may even differ depending on the type of lesion involved[69]. Forty-eight hours after systemic administration in animals, the cells are found to be located in the lung and liver[137], while after 9-21 mo they are detected in the lungs, liver, pancreas, spleen, kidneys and skin[69,138].
The engraftment rate in these tissues is 0.1%-2.7%, and is similar for both autologous and allogenic MSC transplantation[138].
Another aspect to be taken into consideration is that the drugs used to treat IBD do not appear to alter MSC function[139].
MSCs were first used in clinical practice to treat graft-vs-host disease (GVHD) in a patient with acute lymphoid leukemia refractory to therapy. Improvement was notable, and the patient remained free of gastrointestinal lesions after one year[140].
The company Osiris has created an MSC product based on bone marrow from healthy donors (Prochymal), and has designed a phase II open-label study on the treatment of acute GVHD. Two treatment arms were defined: one group received high-dose MSCs (8 million MSCs/kg), while the other group received low-dose MSCs (2 million MSCs/kg). Two infusions were administered in both groups, spaced three days apart. The following results were obtained: 24 out of 31 patients showed complete clinical response (absence of GVHD symptoms in skin, liver and gastrointestinal tract), while 5 showed partial response[141]. However, a phase III study involving a similar group of patients failed to obtain significant results in relation to the primary endpoint[142].
At present, MSCs are being used for repair and immune modulating purposes in clinical trials on the treatment of cardiovascular and neurological disease, in the treatment of autoimmune disorders, and in the management of post-radiotherapy tissue damage[128,130,132,135,136,143]. Laboratory-based studies reporting antineoplastic activity have recently been published[144].
Two types of treatment with MSCs have been developed in IBD: systemic administration of MSCs (intraarterial or intravenous) for the control of intestinal inflammatory disease, and the local administration of MSCs in perianal fistulizing CD.
The use of MSCs for IBD treatment is based on their reparatory and immune modulating properties.
Several phase I-II studies involving autologous and allogenic MSCs have been published in recent years on the treatment of IBD.
In 2006, Onken et al[145] published an abstract on MSCs used to treat 10 patients with active CD (CDAI > 220, C-reactive protein ≥ 5 mg/L) refractory to treatment with corticosteroids, immune modulators and infliximab. Use was made of MSCs obtained by bone marrow puncture from healthy donors (Prochymal, Osiris). The patients were randomized to two groups, both of which received two intravenous doses of MSCs, spaced one week apart. One group received high-dose MSCs (8 million MSCs/kg), while the other group received low-dose MSCs (2 million MSCs/kg). The primary endpoint was percentage clinical response, defined as a reduction in the CDAI score of ≥ 100 points. Four weeks after the end of treatment, and as a secondary endpoint, improvement was observed in the inflammatory bowel disease questionnaire (IBDQ) scores during the same period. Nine patients completed the study. All subjects presented a mean reduction in CDAI score of 105 points on day 28. The CDAI and IBDQ scores decreased to a greater extent in the high-dose group, though statistical significance was not reached[145].
A phase III, randomized and placebo-controlled trial was started by Osiris in 2007 (protocol 603, NCT00482092 clinicaltrials.gov)[146]. The aim was to include a large number of patients with active CD (CDAI score 250-450; PCR ≥ 5) and a history of intolerance or resistance to corticosteroids, immune modulators and biological drugs. The patients were randomized to four intravenous MSC infusions in two weeks. One group of patients received a total of 600 million cells (low dose), while the rest received 1200 million cells (high dose) or placebo. The primary endpoint was remission on day 28, while the secondary endpoints were clinical response, improvement in quality of life, and reduction of the number of draining fistulas. In March 2009, with 207 patients enrolled, the trial was suspended because of a high placebo response. Subsequently, the United States Food and Drug Administration authorized the reopening of the study, though no results are currently available[147].
More recently, phase I studies have been published that allow us to know the safety and possibilities of this type of therapy. In 2010, Duijvestein et al[148] published the results of a phase I trial with intravenous MSCs administered to 10 patients with CD refractory to corticosteroids, immune modulators and anti-TNFα drugs. The cells were obtained by bone marrow puncture of CD patients, and 9 subjects received two doses of 1-2 million cells/kg body weight, spaced one week apart. This study showed the autologous MSCs of CD patients to be functionally analogous to those of healthy donors, maintaining the same immune modulating properties, and without experiencing alterations due to the drugs administered to the patient. In relation to CD, the benefits obtained were few: only three patients showed improvement, with a reduction of CDAI ≥ 70. The procedure proved safe, with no significant side effects.
In 2012, Liang et al[149] reported the results obtained in 7 patients with IBD (4 with CD and 3 with UC). In three cases MSCs were obtained from the bone marrow of healthy donors, and in four cases from umbilical cord. The dose administered consisted of one million cells/kg via the intravenous route. All the patients maintained their medication (corticosteroids and immune modulators) after the infusion of MSCs. Five subjects showed remission, and the latter was maintained for over 24 mo in two of them. Two CD patients and one UC patient showed improved endoscopic indices, and in all three of these subjects biopsies revealed a decrease in the extent of IBD and in intensity of lymphoid infiltrate. Side effects were mild: one patient experienced facial flushing for 6 h after the infusion, while another experienced insomnia during the first night following infusion. One patient developed febricula and worsening of diarrhea. These symptoms disappeared without medication of any kind, and no other side effects were observed.
Regarding the safety of MSC treatment, clinical trials conducted in human subjects have found the therapy to be safe, with no toxic effects or generation of ectopic tissue. The most commonly reported side effect was transient fever[140,145,146,148-150]. MSCs may be infected with viruses (e.g., cytomegalovirus, herpes virus), and a case of infection with Epstein-Barr virus has been reported in a patient with a lymphoproliferative process previously subjected to bone marrow conditioning treatment, followed by the administration of MSCs due to an episode of GVHD[151,152]. As mentioned, a possible case of bacterial infection transmitted by umbilical cord hematopoietic stem cells has recently been reported[62].
The above data are promising, yet the number of treated patients is small, the source of MSCs diverse, and the regimens and doses different. It is therefore not possible to draw firm conclusions at this time. Strictly protocolized trials need to be conducted to draw valid conclusions.
MSCs treatment for perianal CD has been carried out using MSCs obtained from bone marrow or adipose tissue. We do not know exactly the mechanism of how MSCs work in perianal fistulas. As we explained before, MSCs and expanded allogeneic adipose-derived stem cells (ASCs) have the potential to control systemic and local inflammatory pathways by suppressing the proliferation of activated lymphocytes. By controlling the local inflammatory process they could induce cicatrization of the fistula due to reparative properties.
Regarding the first cell source, the experience gained corresponds only to an Italian study published in 2008[153]. The authors treated 9 CD patients - 8 with complex perianal fistulas and one with multiple enterocutaneous fistulas - using MSCs from bone marrow, serially injected into the fistular canal every four weeks, with an average of four doses in total. Remission was achieved in 7 of the 10 patients, while improvement was noted in the remaining three, with marked and significant reductions in the perianal disease activity index and CDAI scores. The findings were confirmed by endoscopy and magnetic resonance imaging (MRI), and evident improvement of the rectal inflammation was also evidenced. The authors postulated that part of the effect may have resulted from stem cells reaching the lymph nodes, influencing T lymphocyte differentiation and[154] apoptosis.
Regarding ASCs, a large number of studies have been published over the last decade. The first such study dates back to 2003[155]. Based on the previously reported myogenic, adipogenic or chondrogenic differentiation potential of these cells[156], ASC injection into a rectovaginal fistula of a CD patient resulted in sealing of the fistula. From this point onwards, a series of studies were conducted to explore the usefulness of these cells in this field. The first publication was a phase I study[157] carried outperformed in 2005 to evaluate the safety and viability of the treatment. ASCs were inoculated in 8 complex fistulas in 5 CD patients, with evaluation of the response after 8 wk. The results showed re-epithelization of the external fistular orifice, with complete sealing of 6 fistulas (75%) and a partial decrease in suppuration in the remaining two (25%). No serious adverse effects were reported. Biopsies obtained from the zone in two patients after 7 and 12 mo showed no evidence of dysplastic transformation.
Following the results obtained, a new phase II, randomized, open-label, controlled multicenter study was published in 2009[158]. This study included 35 patients with complex cryptogenic fistulas, including 14 associated to CD, randomized to either fibrin glue or fibrin glue plus 20 million ASCs. Evaluations were made after 8 wk and one year, with the possibility of administering a second dose of 40 million ASCs in cases showing failure in week 8. Sealing of the fistula, defined as closure of the external orifice and the absence of suppuration in response to digital pressure, was recorded in 11 patients (46%) in the ASC treatment arm, vs in two patients (8%) in the non-cell therapy arm, after 8 wk. In turn, following both doses, sealing was recorded in 17 patients (71%) in the ASC treatment arm and in four patients (16%) in the fibrin glue treatment arm (RR = 4.43, 95%CI: 1.74-11.27, P < 0.001). Effectiveness was found to be similar on considering a cryptoglandular origin of the fistulas vs fistulas associated to CD, though statistical significance was not reached in this latter subgroup due to the small number of patients involved. No adverse effects related to ASC use were reported.
The results of the FATTI phase III, randomized, single-blind, multicenter clinical trial were published in 2012[159]. In this study IBD constituted an exclusion criterion, and a total of 200 subjects diagnosed with simple cryptoglandular fistulas (confirmed by MRI) were included. The patients were randomized to three groups: 20 million ASCs (group A), 20 million ASCs with fibrin glue (group B), and fibrin glue with placebo (group C). The possibility existed of a second dose of 40 million ASCs in week 12. The primary endpoint was fistular sealing (re-epithelization of the external orifice, with absence of drainage and no collections as evidenced by MRI) in week 12 and in weeks 24-26. Of the 200 randomized individuals, 183 received treatment, and 165 completed the study [per protocol (PP) analysis]. After 12 wk fistular sealing was observed in 26.5%, 38.33% and 15.25% of patients in groups A, B and C, respectively (P = 0.01). A second dose was administered in 61.5% of patients, with fistular sealing in 39.1%, 43.3% and 37.3%, respectively (P = 0.79). Although the findings were not as promising as in previous studies, posterior analysis stratified by center revealed far better results for patients administered ASCs on comparing the center with the greatest experience vs the rest of the participating centers: 45.55%, 83.3% and 18.8% (P = 0.025 for treatment) vs 35.8%, 33.3% and 42.6% for groups A, B and C, respectively. The multivariate analysis moreover showed the fact of having received treatment at the center with most experience to be a significant factor. The authors postulated that the different surgical protocol used in the treatment, as well as the experience of the surgeon in using ASCs in perianal fistulas, may have been decisive. As regards the safety of treatment, there were no significant differences in adverse effects among the three groups. A total of 37 serious adverse effects were recorded - three of which were related to the procedures used, but none to use of ASCs.
In that same year, the first study on donor adipose tissue expanded mesenchymal stem cells (eASCs) was published[160]. Up until that time, all treatments had been made with autologous MSCs, except in one case where donor cells were used[161]. This study used an eASC formulation prepared by the company Cellerix (now Tigenix). Twenty-four patients with CD and complex perianal fistulas were enrolled. The primary endpoint was fistular closure in weeks 12 and 24, defined as the absence of suppuration, re-epithelization of the external orifice, and the absence of collections as evidenced by MRI. A starting dose of 20 million cells was injected into the fistula, followed by a second dose of 40 million cells in the event of incomplete closure in week 12. Of the 24 patients included in the study, 16 completed the treatment period and, of these, 69.2% experienced decreased suppuration of at least one of the fistulas, 56.3% showed closure of the treated fistulas, and 30% presented closure of all fistular trajectories. The results were confirmed by MRI. There were two serious adverse effects (fever and perianal abscess), possibly related to the intervention, although both showed full recovery.
At present, a phase III study is underway involving eASCs for the treatment of complex fistulas in CD patients. This trial will contribute relevant information and may lead to future marketing of the treatment. A new treatment option is thus under development that may revolutionize the management of perianal fistulizing CD. The safety of this cell product appears to have been confirmed by the different studies published to date. Ten years have passed since publication of the first report, and follow-up studies have been made of some of the published series, without the detection of anomalies[162].
At present, treatments with mesenchymal and hematopoietic stem cells offer a potential that requires in-depth investigation. Existing studies are encouraging yet inconclusive. We are at a point of inflexion where these new therapies are seen to afford major curative potential. The coming years will be decisive. The information obtained from ongoing and future clinical trials may lead a revolution in IBD management and its impact upon patients. Undoubtedly, as twenty-first century gastroenterologists, we must expand the scope of our specialty and seek multidisciplinary interaction for the benefit of our patients.
P- Reviewers: Actis GC, Bonaz BL, Schmelzer E S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1390] [Cited by in F6Publishing: 1425] [Article Influence: 109.6] [Reference Citation Analysis (0)] |
| 2. | Burisch J, Pedersen N, Cukovic-Cavka S, Brinar M, Kaimakliotis I, Duricova D, Shonová O, Vind I, Avnstrøm S, Thorsgaard N, Andersen V, Krabbe S, Dahlerup JF, Salupere R, Nielsen KR, Olsen J, Manninen P, Collin P, Tsianos EV, Katsanos KH, Ladefoged K, Lakatos L, Björnsson E, Ragnarsson G, Bailey Y, Odes S, Schwartz D, Martinato M, Lupinacci G, Milla M, De Padova A, D’Incà R, Beltrami M, Kupcinskas L, Kiudelis G, Turcan S, Tighineanu O, Mihu I, Magro F, Barros LF, Goldis A, Lazar D, Belousova E, Nikulina I, Hernandez V, Martinez-Ares D, Almer S, Zhulina Y, Halfvarson J, Arebi N, Sebastian S, Lakatos PL, Langholz E, Munkholm P; for the EpiCom-group. East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut. 2013;Apr 20; Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 3. | Thia KT, Loftus EV, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008;103:3167-3182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 371] [Cited by in F6Publishing: 393] [Article Influence: 24.6] [Reference Citation Analysis (1)] |
| 4. | Wilson B, Greco M, Hommes DW, Vermiere S, Bell C. A European Crohn’s and ulcerative colitis patient life IMPACT survey. Abstract PO875, 19 the United European Gastroenterology Week;. 2011;October 22-26; Stockholm, Sweden. [Cited in This Article: ] |
| 5. | Hommes D, Colombel JF, Emery P, Greco M, Sandborn WJ. Changing Crohn’s disease management: need for new goals and indices to prevent disability and improve quality of life. J Crohns Colitis. 2012;6 Suppl 2:S224-S234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Kappelman MD, Rifas-Shiman SL, Porter CQ, Ollendorf DA, Sandler RS, Galanko JA, Finkelstein JA. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology. 2008;135:1907-1913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 469] [Cited by in F6Publishing: 510] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 7. | Gibson TB, Ng E, Ozminkowski RJ, Wang S, Burton WN, Goetzel RZ, Maclean R. The direct and indirect cost burden of Crohn’s disease and ulcerative colitis. J Occup Environ Med. 2008;50:1261-1272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | D’Haens G, Geboes K, Rutgeerts P. Endoscopic and histologic healing of Crohn’s (ileo-) colitis with azathioprine. Gastrointest Endosc. 1999;50:667-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 7] [Reference Citation Analysis (0)] |
| 9. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2987] [Cited by in F6Publishing: 2935] [Article Influence: 133.4] [Reference Citation Analysis (0)] |
| 10. | Rutgeerts P, Van Assche G, Sandborn WJ, Wolf DC, Geboes K, Colombel JF, Reinisch W, Kumar A, Lazar A, Camez A. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology. 2012;142:1102-1111.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 399] [Cited by in F6Publishing: 416] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 11. | Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1598] [Cited by in F6Publishing: 1523] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 12. | Baert F, Moortgat L, Van Assche G, Caenepeel P, Vergauwe P, De Vos M, Stokkers P, Hommes D, Rutgeerts P, Vermeire S. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138:463-468; quiz e10-1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 603] [Cited by in F6Publishing: 607] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 13. | Frøslie KF, Jahnsen J, Moum BA, Vatn MH. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 834] [Cited by in F6Publishing: 822] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 14. | Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, Lang Y, Marano CW, Strauss R, Oddens BJ, Feagan BG. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194-1201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 643] [Cited by in F6Publishing: 666] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 15. | Meucci G, Fasoli R, Saibeni S, Valpiani D, Gullotta R, Colombo E, D’Incà R, Terpin M, Lombardi G. Prognostic significance of endoscopic remission in patients with active ulcerative colitis treated with oral and topical mesalazine: a prospective, multicenter study. Inflamm Bowel Dis. 2012;18:1006-1010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D’Haens G, Wolf DC, Kron M, Tighe MB, Lazar A, Thakkar RB. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257-65.e1-3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 817] [Cited by in F6Publishing: 863] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 17. | Reinisch W, Sandborn WJ, Hommes DW, D’Haens G, Hanauer S, Schreiber S, Panaccione R, Fedorak RN, Tighe MB, Huang B. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60:780-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 588] [Cited by in F6Publishing: 626] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 18. | Colombel JRP, Sandbord WJ, Camez A. Deep remission for adalimumab-treated patients with moderate to severe ileocolonic Crohn’s disease: result s from EXTEND. Abstract OP31, 5th Congress of the European Crohn’s and Colitis Organization. . [Cited in This Article: ] |
| 19. | Zallot C, Peyrin-Biroulet L. Deep remission in inflammatory bowel disease: looking beyond symptoms. Curr Gastroenterol Rep. 2013;15:315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | Sandborn WJ, Feagan BG, Stoinov S, Honiball PJ, Rutgeerts P, Mason D, Bloomfield R, Schreiber S. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357:228-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 800] [Cited by in F6Publishing: 760] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 21. | Schreiber S, Khaliq-Kareemi M, Lawrance IC, Thomsen OØ, Hanauer SB, McColm J, Bloomfield R, Sandborn WJ. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357:239-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 758] [Cited by in F6Publishing: 693] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 22. | Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106:685-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 250] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 23. | Bernstein CN, Loftus EV, Ng SC, Lakatos PL, Moum B. Hospitalisations and surgery in Crohn’s disease. Gut. 2012;61:622-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 24. | Grant D, Abu-Elmagd K, Reyes J, Tzakis A, Langnas A, Fishbein T, Goulet O, Farmer D. 2003 report of the intestine transplant registry: a new era has dawned. Ann Surg. 2005;241:607-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 379] [Cited by in F6Publishing: 394] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 25. | Harpaz N, Schiano T, Ruf AE, Shukla D, Tao Y, Fishbein TM, Sauter BV, Gondolesi GE. Early and frequent histological recurrence of Crohn’s disease in small intestinal allografts. Transplantation. 2005;80:1667-1670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Hurst RD, Molinari M, Chung TP, Rubin M, Michelassi F. Prospective study of the features, indications, and surgical treatment in 513 consecutive patients affected by Crohn’s disease. Surgery. 1997;122:661-667; discussion 667-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 89] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Galandiuk S, Kimberling J, Al-Mishlab TG, Stromberg AJ. Perianal Crohn disease: predictors of need for permanent diversion. Ann Surg. 2005;241:796-801; discussion 801-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Desreumaux P, Foussat A, Allez M, Beaugerie L, Hébuterne X, Bouhnik Y, Nachury M, Brun V, Bastian H, Belmonte N. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology. 2012;143:1207-17.e1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 29. | Thomson AW, Robbins PD. Tolerogenic dendritic cells for autoimmune disease and transplantation. Ann Rheum Dis. 2008;67 Suppl 3:iii90-iii96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118-1125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1894] [Cited by in F6Publishing: 1924] [Article Influence: 137.4] [Reference Citation Analysis (0)] |
| 31. | Fiocchi C. Genes and ‘in-vironment’: how will our concepts on the pathophysiology of inflammatory bowel disease develop in the future? Dig Dis. 2012;30 Suppl 3:2-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Chassaing B, Darfeuille-Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1720-1728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 338] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 33. | MacDonald TT, Monteleone I, Fantini MC, Monteleone G. Regulation of homeostasis and inflammation in the intestine. Gastroenterology. 2011;140:1768-1775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 34. | Blumberg R, Cho J, Lewis J, Wu G. Inflammatory bowel disease: an update on the fundamental biology and clinical management. Gastroenterology. 2011;140:1701-1703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901-1909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 410] [Cited by in F6Publishing: 429] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 36. | Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 601] [Cited by in F6Publishing: 622] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 37. | Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009;2:340-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 38. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1967] [Cited by in F6Publishing: 2038] [Article Influence: 135.9] [Reference Citation Analysis (5)] |
| 39. | Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3061] [Cited by in F6Publishing: 3050] [Article Influence: 152.5] [Reference Citation Analysis (0)] |
| 40. | Hart AL, Al-Hassi HO, Rigby RJ, Bell SJ, Emmanuel AV, Knight SC, Kamm MA, Stagg AJ. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129:50-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 346] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 41. | Makita S, Kanai T, Oshima S, Uraushihara K, Totsuka T, Sawada T, Nakamura T, Koganei K, Fukushima T, Watanabe M. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004;173:3119-3130. [PubMed] [Cited in This Article: ] |
| 42. | Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 352] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 43. | Cho JH, Weaver CT. The genetics of inflammatory bowel disease. Gastroenterology. 2007;133:1327-1339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 44. | Orholm M, Binder V, Sørensen TI, Rasmussen LP, Kyvik KO. Concordance of inflammatory bowel disease among Danish twins. Results of a nationwide study. Scand J Gastroenterol. 2000;35:1075-1081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 225] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 45. | Halme L, Paavola-Sakki P, Turunen U, Lappalainen M, Farkkila M, Kontula K. Family and twin studies in inflammatory bowel disease. World J Gastroenterol. 2006;12:3668-3672. [PubMed] [Cited in This Article: ] |
| 46. | Yang H, McElree C, Roth MP, Shanahan F, Targan SR, Rotter JI. Familial empirical risks for inflammatory bowel disease: differences between Jews and non-Jews. Gut. 1993;34:517-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 265] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 47. | Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology. 2011;140:1704-1712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 293] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 48. | Franke A, Balschun T, Karlsen TH, Hedderich J, May S, Lu T, Schuldt D, Nikolaus S, Rosenstiel P, Krawczak M. Replication of signals from recent studies of Crohn’s disease identifies previously unknown disease loci for ulcerative colitis. Nat Genet. 2008;40:713-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 296] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 49. | Fisher SA, Tremelling M, Anderson CA, Gwilliam R, Bumpstead S, Prescott NJ, Nimmo ER, Massey D, Berzuini C, Johnson C. Genetic determinants of ulcerative colitis include the ECM1 locus and five loci implicated in Crohn’s disease. Nat Genet. 2008;40:710-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 333] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 50. | Anderson CA, Massey DC, Barrett JC, Prescott NJ, Tremelling M, Fisher SA, Gwilliam R, Jacob J, Nimmo ER, Drummond H. Investigation of Crohn’s disease risk loci in ulcerative colitis further defines their molecular relationship. Gastroenterology. 2009;136:523-9.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 51. | Brant SR, Wang MH, Rawsthorne P, Sargent M, Datta LW, Nouvet F, Shugart YY, Bernstein CN. A population-based case-control study of CARD15 and other risk factors in Crohn’s disease and ulcerative colitis. Am J Gastroenterol. 2007;102:313-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Parkes M, Barrett JC, Prescott NJ, Tremelling M, Anderson CA, Fisher SA, Roberts RG, Nimmo ER, Cummings FR, Soars D. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat Genet. 2007;39:830-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 870] [Cited by in F6Publishing: 867] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 53. | Adler J, Rangwalla SC, Dwamena BA, Higgins PD. The prognostic power of the NOD2 genotype for complicated Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2011;106:699-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 54. | Alvarez-Lobos M, Arostegui JI, Sans M, Tassies D, Plaza S, Delgado S, Lacy AM, Pique JM, Yagüe J, Panés J. Crohn’s disease patients carrying Nod2/CARD15 gene variants have an increased and early need for first surgery due to stricturing disease and higher rate of surgical recurrence. Ann Surg. 2005;242:693-700. [PubMed] [Cited in This Article: ] |
| 55. | Fiedler T, Büning C, Reuter W, Pitre G, Gentz E, Schmidt HH, Büttner J, Ockenga J, Gerloff T, Meisel C. Possible role of MDR1 two-locus genotypes for young-age onset ulcerative colitis but not Crohn’s disease. Eur J Clin Pharmacol. 2007;63:917-925. [PubMed] [Cited in This Article: ] |
| 56. | Arijs I, Li K, Toedter G, Quintens R, Van Lommel L, Van Steen K, Leemans P, De Hertogh G, Lemaire K, Ferrante M. Mucosal gene signatures to predict response to infliximab in patients with ulcerative colitis. Gut. 2009;58:1612-1619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 271] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 57. | Annese V, Valvano MR, Palmieri O, Latiano A, Bossa F, Andriulli A. Multidrug resistance 1 gene in inflammatory bowel disease: a meta-analysis. World J Gastroenterol. 2006;12:3636-3644. [PubMed] [Cited in This Article: ] |
| 58. | Park JH, Wacholder S, Gail MH, Peters U, Jacobs KB, Chanock SJ, Chatterjee N. Estimation of effect size distribution from genome-wide association studies and implications for future discoveries. Nat Genet. 2010;42:570-575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 489] [Cited by in F6Publishing: 491] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 59. | Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schäffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033-2045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1121] [Cited by in F6Publishing: 1015] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 60. | Dominguez-Bello MG, Blaser MJ, Ley RE, Knight R. Development of the human gastrointestinal microbiota and insights from high-throughput sequencing. Gastroenterology. 2011;140:1713-1719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 256] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 61. | Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119-124. [PubMed] [Cited in This Article: ] |
| 62. | Bhatt AS, Freeman SS, Herrera AF, Pedamallu CS, Gevers D, Duke F, Jung J, Michaud M, Walker BJ, Young S. Sequence-based discovery of Bradyrhizobium enterica in cord colitis syndrome. N Engl J Med. 2013;369:517-528. [PubMed] [Cited in This Article: ] |
| 63. | Frolkis A, Dieleman LA, Barkema H, Panaccione R, Ghosh S, Fedorak RN, Madsen K, Kaplan GG. Environment and the inflammatory bowel diseases. Can J Gastroenterol. 2013;27:e18-e24. [PubMed] [Cited in This Article: ] |
| 64. | Christophi GP, Rong R, Holtzapple PG, Massa PT, Landas SK. Immune markers and differential signaling networks in ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2012;18:2342-2356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 65. | Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756-1767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 738] [Cited by in F6Publishing: 781] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 66. | Smith A. Glossary a glossary for stem-cell biology. Nature. 2006;441:1060. [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 67. | Egashira T, Yuasa S, Fukuda K. Novel insights into disease modeling using induced pluripotent stem cells. Biol Pharm Bull. 2013;36:182-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 68. | Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A, Feldman O, Gepstein A, Arbel G, Hammerman H. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471:225-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 794] [Cited by in F6Publishing: 834] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 69. | Swenson E, Theise N. Stem cell therapeutics: potential in the treatment of inflammatory bowel disease. Clin Exp Gastroenterol. 2010;3:1-10. [PubMed] [Cited in This Article: ] |
| 70. | Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1:635-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 394] [Cited by in F6Publishing: 380] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 71. | Ikera S. Treatment of autoimmune diseases by haematopoietic stem cell transplantation. Exp Hematol. 2001;29:661-669. [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 72. | van Bekkum DW. Stem cell transplantation for autoimmune disorders. Preclinical experiments. Best Pract Res Clin Haematol. 2004;17:201-222. [PubMed] [Cited in This Article: ] |
| 73. | Gratwohl A, Passweg J, Gerber I, Tyndall A. Stem cell transplantation for autoimmune diseases. Best Pract Res Clin Haematol. 2001;14:755-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Marmont AM. Stem cell transplantation for autoimmune disorders. Coincidental autoimmune disease in patients transplanted for conventional indications. Best Pract Res Clin Haematol. 2004;17:223-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Tyndall A, Gratwohl A. Adult stem cell transplantation in autoimmune disease. Curr Opin Hematol. 2009;16:285-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Tyndall A. Successes and failures of stem cell transplantation in autoimmune diseases. Hematology Am Soc Hematol Educ Program. 2011;2011:280-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 77. | Leung Y, Geddes M, Storek J, Panaccione R, Beck PL. Hematopoietic cell transplantation for Crohn’s disease; is it time? World J Gastroenterol. 2006;12:6665-6673. [PubMed] [Cited in This Article: ] |
| 78. | Muraro PA, Douek DC, Packer A, Chung K, Guenaga FJ, Cassiani-Ingoni R, Campbell C, Memon S, Nagle JW, Hakim FT. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J Exp Med. 2005;201:805-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 342] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 79. | Alexander T, Thiel A, Rosen O, Massenkeil G, Sattler A, Kohler S, Mei H, Radtke H, Gromnica-Ihle E, Burmester GR. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood. 2009;113:214-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 80. | Clerici M, Cassinotti A, Onida F, Trabattoni D, Annaloro C, Della Volpe A, Rainone V, Lissoni F, Duca P, Sampietro G. Immunomodulatory effects of unselected haematopoietic stem cells autotransplantation in refractory Crohn’s disease. Dig Liver Dis. 2011;43:946-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 81. | García-Bosch O, Ricart E, Panés J. Review article: stem cell therapies for inflammatory bowel disease - efficacy and safety. Aliment Pharmacol Ther. 2010;32:939-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 82. | van Deen WK, Oikonomopoulos A, Hommes DW. Stem cell therapy in inflammatory bowel disease: which, when and how? Curr Opin Gastroenterol. 2013;29:384-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | Tyndall A, Gratwohl A. Blood and marrow stem cell transplants in auto-immune disease: a consensus report written on behalf of the European League against Rheumatism (EULAR) and the European Group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 1997;19:643-645. [PubMed] [Cited in This Article: ] |
| 84. | Oyama Y, Craig RM, Traynor AE, Quigley K, Statkute L, Halverson A, Brush M, Verda L, Kowalska B, Krosnjar N. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn’s disease. Gastroenterology. 2005;128:552-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 190] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 85. | Cassinotti A, Annaloro C, Ardizzone S, Onida F, Della Volpe A, Clerici M, Usardi P, Greco S, Maconi G, Porro GB. Autologous haematopoietic stem cell transplantation without CD34+ cell selection in refractory Crohn’s disease. Gut. 2008;57:211-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 86. | Snowden JA, Saccardi R, Allez M, Ardizzone S, Arnold R, Cervera R, Denton C, Hawkey C, Labopin M, Mancardi G. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2012;47:770-790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 87. | Farge D, Labopin M, Tyndall A, Fassas A, Mancardi GL, Van Laar J, Ouyang J, Kozak T, Moore J, Kötter I. Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years’ experience from the European Group for Blood and Marrow Transplantation Working Party on Autoimmune Diseases. Haematologica. 2010;95:284-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 246] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 88. | Drakos PE, Nagler A, Or R. Case of Crohn’s disease in bone marrow transplantation. Am J Hematol. 1993;43:157-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 89. | Castro J, Bentch HL, Smith L, Kalter S, Bachier C. Prolonged clinical remission in patients with inflammatory bowel disease after high dose chemotherapy and autologous blood stem cell transplantation. Blood. 1996;88:133a. [Cited in This Article: ] |
| 90. | Kashyap A, Forman SJ. Autologous bone marrow transplantation for non-Hodgkin’s lymphoma resulting in long-term remission of coincidental Crohn’s disease. Br J Haematol. 1998;103:651-652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 91. | Musso M, Porretto F, Crescimanno A, Bondì F, Polizzi V, Scalone R. Crohn’s disease complicated by relapsed extranodal Hodgkin’s lymphoma: prolonged complete remission after unmanipulated PBPC autotransplant. Bone Marrow Transplant. 2000;26:921-923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 92. | Söderholm JD, Malm C, Juliusson G, Sjödahl R. Long-term endoscopic remission of crohn disease after autologous stem cell transplantation for acute myeloid leukaemia. Scand J Gastroenterol. 2002;37:613-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 93. | Craig RM, Traynor A, Oyama Y, Burt RK. Hematopoietic stem cell transplantation for severe Crohn’s disease. Bone Marrow Transplant. 2003;32 Suppl 1:S57-S59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 94. | Burt RK, Craig RM, Milanetti F, Quigley K, Gozdziak P, Bucha J, Testori A, Halverson A, Verda L, de Villiers WJ. Autologous nonmyeloablative hematopoietic stem cell transplantation in patients with severe anti-TNF refractory Crohn disease: long-term follow-up. Blood. 2010;116:6123-6132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 95. | Cassionotti A, Onida F, Annaloro C, Samprieto G, Fociani P, Fichera M, Maconi G, Lombardini M, Bezzio C, Foschi D. Autologous hematopoietic stem cell transplantation without CD34 selection for refractory Crohn’s disease: the Milan experience after 5 year. J Cronhs Colitis. 2012;6:S153. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Hasselblatt P, Drognitz K, Potthoff K, Bertz H, Kruis W, Schmidt C, Stallmach A, Schmitt-Graeff A, Finke J, Kreisel W. Remission of refractory Crohn’s disease by high-dose cyclophosphamide and autologous peripheral blood stem cell transplantation. Aliment Pharmacol Ther. 2012;36:725-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 97. | Hawkey C, Allez M, Ardizzone S, Clark M, Clark L, Colombel JF, Danese S, Farge-Bancel D, Labopin M, Lindsay J. Clinical and endoscopic improvement following hemopoietic stem cell transplantation in the ASTIC trial. J Crohns Colitis. 2013;7:S4. [DOI] [Cited in This Article: ] |
| 98. | Ditschkowski M, Einsele H, Schwerdtfeger R, Bunjes D, Trenschel R, Beelen DW, Elmaagacli AH. Improvement of inflammatory bowel disease after allogeneic stem-cell transplantation. Transplantation. 2003;75:1745-1747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 99. | Lopez-Cubero SO, Sullivan KM, McDonald GB. Course of Crohn’s disease after allogeneic marrow transplantation. Gastroenterology. 1998;114:433-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 167] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 100. | Talbot DC, Montes A, Teh WL, Nandi A, Powles RL. Remission of Crohn’s disease following allogeneic bone marrow transplant for acute leukaemia. Hosp Med. 1998;59:580-581. [PubMed] [Cited in This Article: ] |
| 101. | Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luriá EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83-92. [PubMed] [Cited in This Article: ] |
| 102. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11055] [Cited by in F6Publishing: 11776] [Article Influence: 692.7] [Reference Citation Analysis (1)] |
| 103. | da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204-2213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1715] [Cited by in F6Publishing: 1652] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 104. | Lanzoni G, Roda G, Belluzzi A, Roda E, Bagnara GP. Inflammatory bowel disease: Moving toward a stem cell-based therapy. World J Gastroenterol. 2008;14:4616-4626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 50] [Cited by in F6Publishing: 52] [Article Influence: 3.3] [Reference Citation Analysis (2)] |
| 105. | Macias MI, Grande J, Moreno A, Domínguez I, Bornstein R, Flores AI. Isolation and characterization of true mesenchymal stem cells derived from human term decidua capable of multilineage differentiation into all 3 embryonic layers. Am J Obstet Gynecol. 2010;203:495.e9-495.e23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 106. | Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 685] [Cited by in F6Publishing: 663] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 107. | Najar M, Raicevic G, Boufker HI, Fayyad Kazan H, De Bruyn C, Meuleman N, Bron D, Toungouz M, Lagneaux L. Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: Combined comparison of adipose tissue, Wharton’s Jelly and bone marrow sources. Cell Immunol. 2010;264:171-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 108. | Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, Eom Y, Lee JE, Kim YJ, Yang SK, Jung HL. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009;259:150-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 285] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 109. | Melief SM, Zwaginga JJ, Fibbe WE, Roelofs H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2013;2:455-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 290] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 110. | Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499-3506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1289] [Cited by in F6Publishing: 1288] [Article Influence: 75.8] [Reference Citation Analysis (1)] |
| 111. | Satija NK, Singh VK, Verma YK, Gupta P, Sharma S, Afrin F, Sharma M, Sharma P, Tripathi RP, Gurudutta GU. Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J Cell Mol Med. 2009;13:4385-4402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 112. | Voswinkel J, Francois S, Gorin NC, Chapel A. Gastro-intestinal autoimmunity: preclinical experiences and successful therapy of fistulizing bowel diseases and gut Graft versus host disease by mesenchymal stromal cells. Immunol Res. 2013;56:241-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 113. | Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1730] [Cited by in F6Publishing: 1613] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 114. | Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755-1761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1050] [Cited by in F6Publishing: 1032] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 115. | Dalal J, Gandy K, Domen J. Role of mesenchymal stem cell therapy in Crohn’s disease. Pediatr Res. 2012;71:445-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 116. | English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol. 2013;91:19-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 368] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 117. | Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1552] [Cited by in F6Publishing: 1473] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 118. | Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, van Zuylen VL, Bosse T, Vos AC, de Jonge-Muller ES, Roelofs H. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:1549-1558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 250] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 119. | Voswinkel J, Francois S, Simon JM, Benderitter M, Gorin NC, Mohty M, Fouillard L, Chapel A. Use of mesenchymal stem cells (MSC) in chronic inflammatory fistulizing and fibrotic diseases: a comprehensive review. Clin Rev Allergy Immunol. 2013;45:180-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 120. | English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156:149-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 517] [Cited by in F6Publishing: 505] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 121. | Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576-6583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 478] [Cited by in F6Publishing: 479] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 122. | Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, Le AD. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787-7798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 495] [Cited by in F6Publishing: 532] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 123. | Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1263] [Cited by in F6Publishing: 1235] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 124. | De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12:574-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 467] [Cited by in F6Publishing: 466] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 125. | Dazzi F, Lopes L, Weng L. Mesenchymal stromal cells: a key player in ‘innate tolerance’? Immunology. 2012;137:206-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 126. | Nemoto Y, Kanai T, Takahara M, Oshima S, Nakamura T, Okamoto R, Tsuchiya K, Watanabe M. Bone marrow-mesenchymal stem cells are a major source of interleukin-7 and sustain colitis by forming the niche for colitogenic CD4 memory T cells. Gut. 2013;62:1142-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 127. | Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16:203-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 438] [Cited by in F6Publishing: 448] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 128. | Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 533] [Cited by in F6Publishing: 549] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 129. | Burdon TJ, Paul A, Noiseux N, Prakash S, Shum-Tim D. Bone marrow stem cell derived paracrine factors for regenerative medicine: current perspectives and therapeutic potential. Bone Marrow Res. 2011;2011:207326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 130. | Kong P, Xie X, Li F, Liu Y, Lu Y. Placenta mesenchymal stem cell accelerates wound healing by enhancing angiogenesis in diabetic Goto-Kakizaki (GK) rats. Biochem Biophys Res Commun. 2013;438:410-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 131. | van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47:1634-1643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 400] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 132. | Burchfield JS, Dimmeler S. Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis Tissue Repair. 2008;1:4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 133. | Qi Y, Jiang D, Sindrilaru A, Stegemann A, Schatz S, Treiber N, Rojewski M, Schrezenmeier H, Vander Beken S, Wlaschek M. TSG-6 Released from Intradermally Injected Mesenchymal Stem Cells Accelerates Wound Healing and Reduces Tissue Fibrosis in Murine Full-Thickness Skin Wounds. J Invest Dermatol. 2014;134:526-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 134. | Chen QQ, Yan L, Wang CZ, Wang WH, Shi H, Su BB, Zeng QH, Du HT, Wan J. Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World J Gastroenterol. 2013;19:4702-4717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 84] [Cited by in F6Publishing: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 135. | Sémont A, Demarquay C, Bessout R, Durand C, Benderitter M, Mathieu N. Mesenchymal stem cell therapy stimulates endogenous host progenitor cells to improve colonic epithelial regeneration. PLoS One. 2013;8:e70170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 136. | Manuguerra-Gagné R, Boulos PR, Ammar A, Leblond FA, Krosl G, Pichette V, Lesk MR, Roy DC. Transplantation of mesenchymal stem cells promotes tissue regeneration in a glaucoma model through laser-induced paracrine factor secretion and progenitor cell recruitment. Stem Cells. 2013;31:1136-1148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 137. | Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 700] [Cited by in F6Publishing: 682] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 138. | Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999-3001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 568] [Cited by in F6Publishing: 535] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 139. | Hoogduijn MJ, Crop MJ, Korevaar SS, Peeters AM, Eijken M, Maat LP, Balk AH, Weimar W, Baan CC. Susceptibility of human mesenchymal stem cells to tacrolimus, mycophenolic acid, and rapamycin. Transplantation. 2008;86:1283-1291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 140. | Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439-1441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2044] [Cited by in F6Publishing: 1970] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 141. | Kebriaei P, Isola L, Bahceci E, Holland K, Rowley S, McGuirk J, Devetten M, Jansen J, Herzig R, Schuster M. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:804-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 307] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 142. | Martin PJ, Uberti JP, Soiffer RJ, Klingemann H, Waller EK, Daly AS, Herrmann RP, Kebriaei P. Prochymal improves response rates in patients with steroid-refractory acute graft versus host disease ( SR-GVHD) involving the liver and gut: results of a randomized, placebo-controlled, multicenter Phase III trial in GVHD. Biol Blood Marrow Tr. 2010;16:S169-70. [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 143. | Bornstein R, Macias MI, de la Torre P, Grande J, Flores AI. Human decidua-derived mesenchymal stromal cells differentiate into hepatic-like cells and form functional three-dimensional structures. Cytotherapy. 2012;14:1182-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 144. | Vegh I, Grau M, Gracia M, Grande J, de la Torre P, Flores AI. Decidua mesenchymal stem cells migrated toward mammary tumors in vitro and in vivo affecting tumor growth and tumor development. Cancer Gene Ther. 2013;20:8-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 145. | Onken J, Gallup D, Hanson J, Pandak M, Custer L. Sucessful outpatient treatment of refractory Cron’s disease using adult mesenchymal stem cells. American College of Gastroenterology Annual Meeting; 2006; Las Vegas. Available from: http://www.acgmeuniverse.orgAccessed July 2, 2011. [Cited in This Article: ] |
| 146. | Osiris Therapeutics. A phase III, multicenter, placebo-controlled, randomized, double-blind study to evaluate the safety and efficacy of Prochimal [tm] (ex vivo cultured adult human mesenchymal stem cells) intravenous infusion for induction of remission in subjects experiencing treatment-refractory moderate-to-severe Crohn’s disease. ClinicalTrials. Gov2010, NCTO0482092. Available from: http://www.Clinicaltrials.gov/ct2/show/NCT00482092. [Cited in This Article: ] |
| 147. | Mannon PJ. Remestemcel-L: human mesenchymal stem cells as an emerging therapy for Crohn’s disease. Expert Opin Biol Ther. 2011;11:1249-1256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 148. | Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, Koning F, Zwaginga JJ, Fidder HH. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59:1662-1669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 471] [Cited by in F6Publishing: 448] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 149. | Liang J, Zhang H, Wang D, Feng X, Wang H, Hua B, Liu B, Sun L. Allogeneic mesenchymal stem cell transplantation in seven patients with refractory inflammatory bowel disease. Gut. 2012;61:468-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 150. | Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 759] [Cited by in F6Publishing: 784] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 151. | Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 530] [Cited by in F6Publishing: 487] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 152. | Ringdén O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lönnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390-1397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 872] [Cited by in F6Publishing: 831] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 153. | Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, Minelli A, Alvisi C, Vanoli A, Calliada F. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut. 2011;60:788-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 390] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 154. | Ahrendt M, Hammerschmidt SI, Pabst O, Pabst R, Bode U. Stromal cells confer lymph node-specific properties by shaping a unique microenvironment influencing local immune responses. J Immunol. 2008;181:1898-1907. [PubMed] [Cited in This Article: ] |
| 155. | García-Olmo D, García-Arranz M, García LG, Cuellar ES, Blanco IF, Prianes LA, Montes JA, Pinto FL, Marcos DH, García-Sancho L. Autologous stem cell transplantation for treatment of rectovaginal fistula in perianal Crohn’s disease: a new cell-based therapy. Int J Colorectal Dis. 2003;18:451-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 156. | Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5703] [Cited by in F6Publishing: 5568] [Article Influence: 242.1] [Reference Citation Analysis (0)] |
| 157. | García-Olmo D, García-Arranz M, Herreros D, Pascual I, Peiro C, Rodríguez-Montes JA. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416-1423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 604] [Cited by in F6Publishing: 556] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 158. | Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, De-La-Quintana P, Garcia-Arranz M, Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 598] [Cited by in F6Publishing: 541] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 159. | Herreros MD, Garcia-Arranz M, Guadalajara H, De-La-Quintana P, Garcia-Olmo D. Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: a phase III randomized clinical trial (FATT 1: fistula Advanced Therapy Trial 1) and long-term evaluation. Dis Colon Rectum. 2012;55:762-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 160. | de la Portilla F, Alba F, García-Olmo D, Herrerías JM, González FX, Galindo A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28:313-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 247] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 161. | García-Olmo D, Herreros D, De-La-Quintana P, Guadalajara H, Trébol J, Georgiev-Hristov T, García-Arranz M. Adipose-derived stem cells in Crohn’s rectovaginal fistula. Case Rep Med. 2010;2010:961758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 162. | Guadalajara H, Herreros D, De-La-Quintana P, Trebol J, Garcia-Arranz M, Garcia-Olmo D. Long-term follow-up of patients undergoing adipose-derived adult stem cell administration to treat complex perianal fistulas. Int J Colorectal Dis. 2012;27:595-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |