Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4536
Revised: December 19, 2013
Accepted: March 19, 2014
Published online: April 28, 2014
Molecularly targeted therapeutic agents are constantly being developed and have been shown to be effective in various clinical trials. One group of representative targeted oncogenic kinases, the receptor tyrosine kinases (RTKs), has been associated with gastric cancer development. Trastuzumab, an inhibitor of ERBB2, has been approved for the treatment of gastric cancer, although other receptor tyrosine kinases, such as epidermal growth factor receptor, vascular endothelial growth factor, platelet-derived growth factor receptor, c-Met, IGF-1R and fibroblast growth factor receptor 2, are also activated in gastric cancer. The promising results of the trastuzumab clinical trial for gastric cancer resulted in the approval of trastuzumab-based therapy as a first-line treatment for human epidermal growth factor receptor 2-positive patients. On the other hand, the trial examining bevacizumab in combination with conventional chemotherapy did not meet its primary goal of increasing the overall survival time of gastric cancer patients; however, a significantly higher response rate and a longer progression-free survival were observed in the bevacizumab arm of the trial. Other clinical trials, especially phase III trials that have tested drugs targeting RTKs, such as cetuximab, panitumumab, gefitinib, erlotinib, figitumumab, sorafenib, sunitinib and lapatinib, have shown that these drugs have modest effects against gastric cancer. This review summarizes the recent results from the clinical trials of molecularly targeted drugs and suggests that further improvements in the treatment of advanced gastric cancer can be achieved through the combination of conventional drugs with the new molecularly targeted therapies.
Core tip: Since the finding of receptor tyrosine kinases (RTKs) about thirty years ago, its functions have been examined over the years as key regulators of proliferation, differentiation, and metastasis. Several RTKs are activated in advanced gastric cancer (AGC) and various RTK inhibitors have been developed as tailored therapy. The results of recent clinical trials evaluate the effectiveness of targeting RTKs. Unfortunately, recent progress in the development of RTK-targeted therapy for AGC patients has been modest. To provide maximal therapeutic benefits, well-designed clinical trials and combinations with appropriate drugs are required. In addition, new predictive biomarkers are immediately obliged to guide the selection of a drug-sensitive patients’ population.
- Citation: Morishita A, Gong J, Masaki T. Targeting receptor tyrosine kinases in gastric cancer. World J Gastroenterol 2014; 20(16): 4536-4545
- URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4536.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4536
Gastric cancer is the second leading cause of cancer-related death worldwide[1,2]. The high mortality rate is due to the lack of effective therapy for advanced stages of the disease. Conventional therapy options for gastric cancer include surgery, chemotherapy, radiation therapy and combination treatments. In the early stages, the disease can often be cured through complete surgical removal of the tumor[3]. However, because gastric cancer results in few symptoms during the early stages, most patients are usually diagnosed after the cancer has progressed to an advanced stage. Moreover, even after surgical resection, tumors will recur in many patients, resulting in short survival times. The 5-year survival rate of gastric cancer has remained at 20%-25% in the western world[4]. Therefore, the high mortality rate underscores the need for effective medical treatments for patients with advanced stages of gastric cancer[3].
Receptor tyrosine kinases (RTKs) consist of ligand-binding extracellular domains which identify the subfamilies of RTKs, a transmembrane domain and a tyrosine kinase motif, and the activation of these kinases has been shown to play an important role in the control of many fundamental process, such as growth, differentiation, adhesion, migration and apoptosis[5-8]. The activation and overexpression of RTKs were initially reported in various cancers[9,10]. Currently, RTK inhibitors have been validated through clinical trials, and some agents have received regulatory approval, such as trastuzumab for the treatment of advanced breast cancer[10], gefitinib for non-small cell lung carcinoma[11] and cetuximab for metastatic colon cancer[12]. Unlike other solid tumors, which are predominantly associated with specific signaling pathways, such as the HER-2 pathway in breast cancer, the genetic and molecular pathogenesis of gastric cancer may be more complex[13,14]. In gastric cancer, although the amplification of RTKs, such as ErbB2, c-Met and fibroblast growth factor receptor (FGFR) 2, is associated with cancer progression, the only approved inhibitor is trastuzumab, an ErbB2-targeting antibody[15]. Trastuzumab came into use after the release of promising efficacy results from the Trastuzumab for Gastric Cancer (ToGA) trial[16]. Additionally, other RTKs have also emerged as potential targets for the future treatment of gastric cancer.
In this review, we delineate the underlying molecular basis of the RTK pathways and summarize the current results of the clinical phase III trials (Table 1) and ongoing clinical trials that are targeting RTKs in patients with gastric cancer. Additionally, we also present future possibilities for the improvement of RTK inhibitor efficacy and for the identification of new strategic targets for gastric cancer treatment.
| Clinical trial | Line of treatment | RTK inhibitor | Chemotherapy | Status |
| ToGA | First | Trastuzumab | FP or XP | Completed |
| AVAGAST | First | Bevacizumab | XP | Completed |
| EXPAND | First | Cetuximab | XP | Completed |
| REAL-3 | First | Panitumumab | EOX | Completed |
| LoGIG | First | Lapatinib | OX | Ongoing |
| TYTAN | Second | Lapatinib | T | Ongoing |
RTKs are transmembrane glycoproteins that are activated by binding to their cognate ligands, resulting in the phosphorylation of tyrosine residues on the receptor and downstream signaling proteins. Fifty-eight of the 90 known protein tyrosine kinases are also receptors[5]. Various RTKs have been normally associated with intracellular signal transduction including growth, differentiation, adhesion, migration, and apoptosis (Hubbard and Till). In various types of cancer, many signaling pathways including cell proliferation, differentiation, and metabolism pathways, are activated by RTK dimerization[6,17]. In general, RTK activation occurs through ligand-induced dimerization, in which a bivalent ligand and two receptor molecules form a dimeric complex[18]. Two main processes are required for RTK activation: the enhancement of the intrinsic catalytic activity and the creation of binding sites to recruit downstream signaling proteins. Importantly, tyrosine autophosphorylation is critical for both of these processes. Autophosphorylation of tyrosine residues located in the activation loop of the kinase domain stimulates kinase activity, whereas autophosphorylation in the juxtamembrane, kinase insert and carboxy-terminal regions generates docking sites for modular domains that recognize the phosphotyrosine residues in specific sequences[19].
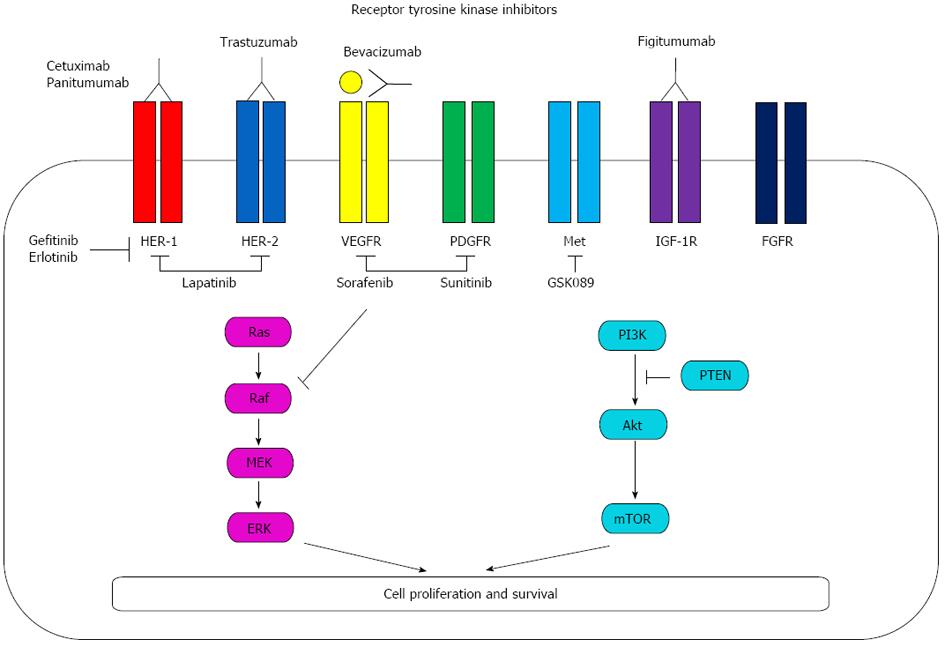
The RTK family consists of 58 kinases, and each is characterized by ligand-binding extracellular domains which identify the subfamilies of RTKs, a tansmembrane domain and a tyrosine kinase motif[5]. Of those kinases, the known RTKs are separated into 21 families, such as the epidermal growth factor receptor (EGFR), vascular endothelial growth factor receptor (VEGFR) and FGFR families, which are characterized by similar structures and the potential of dimerization in gastric cancer[20]. Additionally, it has been reported that the expression of platelet-derived growth factor (PDGF) and platelet-derived growth factor receptor (PDGFR) are involved in gastric cancer growth[21]. Each RTK inhibitor is diagramed in Figure 1 and inactivates various RTKs. Among these RTK inhibitors, monoclonal antibodies, such as cetuximab and panitumumab, trastuzumab and fugitumumab directly bind to each RTK and inhibit its signaling. Bevacizumab also binds vascular endothelial growth factor (VEGF) and inhibit VEGFR signaling. Gefitinib and erlotinib competes with the binding of ATP to the tyrosine kinase domain of EGFR and lapatinib blocks phosphorylation of HER-1 and HER-2. Sorafenib inhibits the enzyme RAF kinase and VEGFR-2/PDGFR-beta signaling cascade. Sunitinib also blocks VEGFR-2/PDGFR-beta and c-kit. In addition, GSK089 inhibits c-Met and blocks its signaling (Figure 1).
Common alterations and mutations of RTKs have been identified in gastric cancer. Interestingly, Deng et al[22] showed that druggable alterations in RTKs occurred in 37% of gastric cancer patients; the most frequently amplified RTK was FGFR2 (9.3%), followed by KRAS (8.8%), EGFR (7.7%) and ERBB2 (7.2%)[22]. Furthermore, the RTK amplification status was shown to be an independent marker of poor prognosis in gastric cancer patients according to Cox multivariate analysis, and this result was independent of chromosomal instability.
Recently, together with Tyro3, Axl and Mer receptor tyrosine kinases are aberrantly expressed in numerous human cancers. It has been reported that Axl and Mer inhibition constitutes a novel therapeutic strategy that may enhance the efficacy of standard chemotherapy in glioblastoma multiforme[23], non-small cell lung cancer[24,25] and breast cancer[26]. In gastric cancer, combination of Axl and Mer expressions correlated inversely with patient prognosis[27]. Inhibition of these RTKs may provide potential targets for AGC.
In our recent study, the levels of EGFR, ErbB2, FGFR1, FGFR2, insulin R and EphA4 were increased in human gastric cancer tissues compared with normal mucosa according to a protein array[28]. Additionally, ErbB2 was most activated in the human gastric cancer cell lines MKN45, MKN74, MKN1 and MKN7[28]. Therefore, these findings suggest that these molecules may be potential targets for selective therapy in gastric cancer. We will summarize the clinical trials for the newly established modalities related to RTK expression in gastric cancer.
The insufficient effect of chemotherapy on advanced gastric cancer has resulted in the development of new biological therapies that modulate various targets of signal transduction pathways that are overexpressed in gastric cancer. A large number of molecularly targeted drugs have been clinically developed to inhibit angiogenesis as well as to specifically inhibit the human epidermal growth factor receptor, platelet-derived growth factor receptor and c-MET receptor.
HER-2 (ErbB2) is a member of the ErbB2/HER family, which is comprised of four receptors including HER-1 (EGFR), HER-2, HER-3 and HER-4. Of these receptors, the targeting of HER-2 has been the most successful for the treatment of advanced gastric cancer. HER-2 overexpression is observed in 10%-38% of gastric cancer patients[29-31]; however, the effect of HER-2 expression on the prognosis of AGC remains controversial[32-35]. Recently, Bang et al[16] reported that HER-2-positive patients using Immunohistochemistry (IHC) scoring system had a superior outcome when treated with conventional chemotherapy with trastuzumab, which selectively binds to HER-2 and inhibits its downstream signaling pathway, in the ToGA trial. Although this result suggests HER-2 is not a negative prognostic factor, it might be confounded by various factors, such as the second line therapy or intestinal subtype. Additionally, IHC scoring system in gastric cancer is different from that of breast cancer[36].
First-line trastuzumab-based trials in AGC patients were reported in 2006[37]. Prior to the ToGA trial[16], three phase II trials evaluating the effectiveness of trastuzumab in AGC patients were presented. The results from the first phase II trial of trastuzumab combined with cisplatin and docetaxel showed that a radiological response was observed in 4/5 HER-2-positive patients (defined as IHC3+ or FISH+) with metastatic gastric cancer or gastroesophageal junction carcinoma patients[37]. In the second phase II trial, HER-2-positive (defined as IHC2+ and FISH+ or IHC3+) AGC or gastroesophageal junction adenocarcinoma patients were treated with 75 mg/m2 cisplatin and trastuzumab (8 mg/kg loading dose followed by 6 mg/kg for future cycles) every 21 d until disease regression, and there was a 35% response rate in the 17 evaluable patients who received a median of two cycles of treatment[38]. Taken together, the overall response rate (ORR) was 35%-44% in the trial arm consisting of trastuzumab combined with conventional chemotherapy.
The ToGA trial was an open-label, international, phase III, randomized controlled trial that was undertaken in 24 countries[16]. In total, 594 patients with gastric or gastroesophageal junction cancer that overexpressed HER-2 protein (as determined by immunohistochemistry or gene amplification by fluorescence in situ hybridization) were randomly assigned to the study treatments (trastuzumab plus chemotherapy, n = 298; chemotherapy alone, n = 296); of these patients, 584 were included in the primary analysis (n = 294 and n = 290, respectively). The median overall survival in the trastuzumab plus chemotherapy arm was 13.8 mo (95%CI: 12-16) compared with 11.1 mo (95%CI: 10-13) in the chemotherapy alone arm (HR = 0.74, 95%CI: 0.60-0.91, P = 0.0046). The study met not only the primary endpoint of improved overall survival but also the secondary endpoint of improved response rates and progression-free survival. Additionally, a 2.7 mo gain in median survival was observed in the intent-to-treat population. In the ToGA study, no significant overlapping toxicity was evaluated, except for cardiac dysfunction[16]. Trastuzumab is correlated with an increased risk of cardiotoxicity[39], similar to anthracyclines, which are frequently used in the treatment of breast and gastric cancers. Trastuzumab-related cardiac dysfunction is largely reversible by removal of the antibody[40] and has been classified as type II chemotherapy-related cardiac dysfunction[41]. In the ToGA study, the left ventricular ejection fraction was monitored every 12 wk during treatment. The regimen was well tolerated, and the hematological toxicity for the chemotherapy doublet was within the expected levels. Interestingly, no additional toxicity was observed, except for an asymptomatic reduction in the left ventricular ejection fraction to below the normal range, which was reported in 5.9% of the patients. Notably, although this patient group has a relatively short life expectancy, the addition of trastuzumab did not compromise the patients’ quality of life[42].
The EGFR is intrinsically expressed in various organs, including the skin, gut and renal tissues. EGFR overexpression is observed in 27%-64% of gastric cancers, especially in the more proximal tumors[43,44], and is correlated with older age, more aggressive histology and higher disease stage; additionally, EGFR expression is a poor prognostic factor[43].
Cetuximab (Erbitux, Imclone Systems) is a recombinant humanized murine monoclonal antibody against EGFR and is the most investigated anti-EGFR therapy in gastric cancer. In the first line phase II trials, six non-randomized trials investigated the addition of cetuximab to doublet chemotherapy[45-49]. The response rate of the above studies ranged from 41% to 63%, and the median overall survival ranged from 9 to 16.6 mo. A randomized phase II study comparing the addition of cetuximab to three discrete chemotherapies was reported at ASCO 2010. None of the treatment arms that included cetuximab exhibited a better survival outcome compared with the conventional control arms. In 2011, preliminary data from another phase II study demonstrated that there was no clinically significant benefit associated with the addition of cetuximab to docetaxel and oxaliplatin[50]. Additionally, the results of the large, randomized, phase III EXPAND study (NCT00678535), which investigated the addition of cetuximab to cisplatin and capecitabine chemotherapy, were presented in 2013[51]. The median progression-free survival (PFS) for the 455 patients administered the capecitabine-cisplatin plus cetuximab treatment was 4.4 mo (95%CI: 4.2-5.5) compared to 5.6 mo (95%CI: 5.1-5.7) for the 449 patients treated with capecitabine-cisplatin alone (HR = 1.09, 95%CI: 0.92-1.29; P = 0.32). Additionally, 83% of the patients in the chemotherapy plus cetuximab group and 77% of the patients in the chemotherapy group experienced grade 3-4 diarrhea, hypokalemia, hypomagnesemia, rash and hand-foot syndrome.
Panitumumab is a humanized monoclonal antibody that targets EGFR. Van Cutsem et al[52] reported a phase III trial of panitumumab plus best supportive care compared to best supportive care alone in patients with advanced colorectal cancer that failed to respond to 5-FU, irinotecan and oxaliplatin. However, there are very few reports of this agent being used to treat AGC patients. Recently, the results of a randomized, open-label, phase III trial for patients with previously untreated advanced esophagogastric cancer (REAL3) were revealed; this study examined two groups of esophagogastric cancer patients treated with epirubicin, oxaliplatin and capecitabine with or without panitumumab[53]. The median overall survival of the 275 patients with advanced esophagogastric adenocarcinoma in the epirubicin, oxaliplatin and capecitabine (EOC) treatment group was 11.3 mo (95%CI: 9.6-13.0) compared to 8.8 mo (95%CI: 7.7-9.8) in the 278 patients treated with modified-dose EOC plus panitumumab (mEOC+P) (HR = 1.37, 95%CI: 1.07-1.76; P = 0.013). The main adverse events that were observed during this trial were grade 3-4 diarrhea (48/276 mEOC+P patients 17% vs 29/266 EOC patients 11%), rash (29/276 mEOC+P patients 11% vs 2/226 EOC patients 1%), mucositis (14/276 mEOC+P patients 5% vs 0/226 EOC patients) and neutropenia (35/276 mEOC+P patients 13% vs 74/226 EOC patients 28%)[53]. On the other hand, other EGFR monoclonal antibodies, such as matuzumab and nimotuzumab, resulted in even shorter PFS times when combined with chemotherapy (compared with chemotherapy alone) in randomized phase II trials[54,55].
Gefitinib and erlotinib, which are EGFR tyrosine kinase inhibitors (TKIs), were assessed in phase II trials; however, these drugs produced unsatisfactory results when used as monotherapies for gastric cancer patients. These inhibitors were effective first-line treatments against gastroesophageal cancer (GEJ), but were not effective for gastric cancer patients, when examined in a phase II study[56]. On the other hand, combination therapy with 5-FU, oxaliplatin and erlotinib demonstrated that the ORR was greater than 50% in patients with esophageal or GEJ cancer[57].
Lapatinib is a receptor tyrosine kinase inhibitor that inhibits both HER-2 and EGFR. A phase II trial demonstrated that lapatinib could achieve an ORR of 7% and a 20% rate of disease stabilization[58]. Regarding adverse events, one patient each experienced grade 4 cardiac toxicity and vomiting in 47 patients with metastatic gastric cancer. Additionally, two patients experienced grade 4 fatigue. In another study, lapatinib had restricted single-agent activity. Only two of 21 previously treated patients had durable, stable disease[59]. Remarkably, these disappointing results were expected, as lapatinib was administered to both HER-2+ and HER-2- patients.
Two phase III trials are ongoing to determine the utility of lapatinib as a first- and second-line treatment for AGC patients. The first trial, the Lapatinib Optimization Study in ErbB2 (HER-2)-Positive Gastric Cancer (LoGIC) trial, is investigating lapatinib as a first-line treatment in combination with capecitabine and oxaliplatin[60]. The second trial, the Lapatinib (Tykerb) with paclitaxel (taxol) in Asian ErbB2+ (HER-2+) Gastric Cancer (TYTAN) trial, is investigating second-line paclitaxel treatment with or without lapatinib in Asian patients[61]. Importantly, HER-2-patients were excluded from the target AGC patients in these trials. The results of these interesting trials will determine whether lapatinib will be used to treat patients with AGC.
Bevacizumab is a monoclonal antibody that inhibits vascular endothelial growth factor-A (VEGF-A), and the broad clinical activity of bevacizumab in antiangiogenic therapies has been reported[62-66]. Various phase II trials of bevacizumab plus chemotherapy have been reported for AGC patients[67-70]. An ORR of 42%-67% was achieved, and the median TTP was 6.6-12 mo, whereas the OS time was 8.9-16.2 mo. Grade 3-4 thromboembolic diseases were reported in approximately 25% of the patients, and gastric perforation was observed in up to 8% of the patients in the phase II trials.
The phase III Avastin in Gastric Cancer (AVAGAST) trial was designed to evaluate the efficacy of adding bevacizumab to first-line capecitabine-cisplatin treatment for advanced gastric cancer. In total, 774 patients were randomly separated and administered capecitabine and cisplatin with or without bevacizumab[71]. In this trial, cisplatin was administered for the six cycles; capecitabine and bevacizumab were administered until the disease progressed or unacceptable toxicity developed. The primary end point was overall survival (OS). The ORR significantly improved with the addition of bevacizumab (46% vs 37%; P = 0.0315), and the median PFS was also significantly longer (6.7 vs 5.3 mo; HR = 0.80; 95%CI: 0.68-0.93; P = 0.0037)[71]. Additionally, the clinical outcomes were different depending on the geographical region. Survival was extended in Pan-American patients who were treated with bevacizumab; however, this was not the case for Asians or Europeans, despite the better prognosis of the latter. Differences in population genetics, patient selection and second-line chemotherapy may explain these results. Furthermore, biomarker studies will elucidate the reasons underlying the differences in efficacy. Interestingly, Ohtsu et al reported that angiogenic markers, such as plasma VEGF-A and tumor neuropilin-1, can have predictive value for the clinical outcomes of patients with gastric cancer treated with bevacizumab in the AVAGAST randomized phase III trials[72]. These results may lead to a better understanding of the study outcome. The most common grade 3-5 adverse events in both arms of the trial were neutropenia, anemia and appetite loss, and these events occurred at similar rates with or without bevacizumab[71].
Sorafenib and sunitinib are multitargeted TKIs that inhibit angiogenesis by targeting VEGFR, PDGFR and other signaling pathways. Sorafenib is a multitarget inhibitor of BRAF, VEGF, PDGFR and the Ras/Raf/MERK/ERK pathway. A phase II study was performed to assess the combination of oxaliplatin and sorafenib as a second-line therapy for AGC after treatment with cisplatin and fluoropyrimidine first-line therapy. Among 40 AGC patients, the CR was 2.5%, and the SD was 47.2%. The median PFS was 3 mo (95%CI: 2.3-4.1), and the median OS was 6.5 mo (95%CI: 5.2-9.6). The median OS was 9.7 mo when the time-to-progression during the first-line chemotherapy was > 6 mo and was decreased to 5.6 mo when the time-to-progression was < 6 mo (P = 0.04)[73]. Grade 3-4 neutropenia (9.8%), thrombocytopenia (7.3%) and neurotoxicity (4.9%) were reported. The combination of oxaliplatin and sorafenib in AGC patients previously treated with cisplatin and fluoropyrimidine appeared safe; however, these results did not support the implementation of a phase III trial[73].
Sunitinib suppresses PDGFR, Kit, rearranged during transfection (RET), Flt-3 and VEGFR. A phase II study of single-agent sunitinib as a second-line treatment for AGC patients treated with one prior chemotherapy regimen was performed, and 2.6% of the enrolled patients had a partial response, whereas 25 patients (32.1%) had stable disease. The median PFS was 2.3 mo, and the median OS was 6.8 mo. Grade 3-4 thrombocytopenia and neutropenia were reported in 34.6% and 29.4% of the patients, respectively[74]. In another phase II trial, disease stabilization was reported in five out of 14 patients[75]. These results suggested that single-agent sunitinib did not have sufficient clinical value as a second-line treatment for AGC. Sunitinib is unlikely to be further developed into a first-line treatment or to be used in combination with chemotherapy due to the complete failure of sunitinib to change the survival outcome of other solid tumors when combined with chemotherapy[76].
The overexpression and activation of c-Met, an RTK for hepatocyte growth factor, induces proliferation and anti-apoptotic signals[77]. c-Met was found to be overexpressed in human gastric cancer cells both in vitro[78] and in vivo[79]. Amplification of the MET gene can be used to determine the response to Met inhibition in vitro[55]. A phase II study of GSK1363089 (GSK089, formerly XL880), a c-Met TKI, demonstrated that this compound had minimal activity in metastatic gastric cancer patients, and liver dysfunction, fatigue and venous thromboembolism were reported as adverse events[80].
Insulin-like growth factor 1 receptor (IGF-1R) expression is correlated with poor outcome in AGC patients[81], and treatment with the IGF-1R antibody figitumumab in conjunction with docetaxel was well tolerated and in a phase I trial of advanced solid tumor patients[82]. FGFR may be a targetable RTK, as the secretion of the FGF family by fibroblasts stimulates the proliferation of scirrhous gastric cancer cells[83]. Additionally, mutations in FGFR are associated with the development of gastric cancer[84], and selective inhibitors of FGFR may be used in clinical trials for AGC patients[85].
In addition, AXL receptor tyrosine-kinase family, such as axl/ufo and nyk/mer protein kinases, co-operatively correlates with the cancer progression, metastasis and patients’ prognosis in gastric cancer[27]. The specific ligand growth arrest-specific gene 6 (Gas6) binds to Axl and Gas6-Axl signaling pathway enhanced cellular survival and invasion and suppressed apoptosis via Akt family during gastric carcinogenesis[86]. Gas6-Axl signaling could be a potential therapeutic target in gastric cancer.
Recently, Singh et al[87] reported combined blockade of HER-2 and VEGF brings about greater growth inhibition in HER-2 overexpressing gastric cancer xenografts. This result suggests that new combination therapy using inhibitors of HER-2 and VEGF may represent a new approach for the treatment of HER-2 positive AGC patients.
Effective RTK inhibitors have been developed over the years, and their potential usefulness will increase further as preclinical data using gastric cancer models continue to demonstrate their effectiveness. Several RTKs are activated in advanced gastric cancer; therefore, targeting these RTKs may lead to tailored therapy. Unfortunately, recent progress in the development of RTK-targeted therapy for AGC patients has been modest. Well-designed clinical trials and combinations with appropriate drugs are required to provide maximal therapeutic benefits. Furthermore, new predictive biomarkers are immediately needed to guide the selection of a potentially drug-sensitive cohort of patients.
P- Reviewers: Keating AK, Pablo F S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2571] [Cited by in F6Publishing: 2597] [Article Influence: 144.3] [Reference Citation Analysis (0)] |
| 2. | Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1579] [Cited by in F6Publishing: 1596] [Article Influence: 114.0] [Reference Citation Analysis (0)] |
| 3. | Yamashita K, Sakuramoto S, Nemoto M, Shibata T, Mieno H, Katada N, Kikuchi S, Watanabe M. Trend in gastric cancer: 35 years of surgical experience in Japan. World J Gastroenterol. 2011;17:3390-3397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 51] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Meyer HJ, Wilke H. Treatment strategies in gastric cancer. Dtsch Arztebl Int. 2011;108:698-705; quiz 706. [PubMed] [Cited in This Article: ] |
| 5. | Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548-5557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 748] [Cited by in F6Publishing: 719] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 6. | Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Annu Rev Biochem. 2000;69:373-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 802] [Cited by in F6Publishing: 772] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 7. | Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3110] [Cited by in F6Publishing: 3008] [Article Influence: 125.3] [Reference Citation Analysis (0)] |
| 8. | Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159-3167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1802] [Cited by in F6Publishing: 1791] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 9. | Prenzel N, Zwick E, Leserer M, Ullrich A. Tyrosine kinase signalling in breast cancer. Epidermal growth factor receptor: convergence point for signal integration and diversification. Breast Cancer Res. 2000;2:184-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Shawver LK, Slamon D, Ullrich A. Smart drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell. 2002;1:117-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 334] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 11. | Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8:303-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 400] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 12. | New treatments for colorectal cancer. FDA Consum. 2004;38:17. [PubMed] [Cited in This Article: ] |
| 13. | Wu K, Nie Y, Guo C, Chen Y, Ding J, Fan D. Molecular basis of therapeutic approaches to gastric cancer. J Gastroenterol Hepatol. 2009;24:37-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Yin M, Hu Z, Tan D, Ajani JA, Wei Q. Molecular epidemiology of genetic susceptibility to gastric cancer: focus on single nucleotide polymorphisms in gastric carcinogenesis. Am J Transl Res. 2009;1:44-54. [PubMed] [Cited in This Article: ] |
| 15. | Asaoka Y, Ikenoue T, Koike K. New targeted therapies for gastric cancer. Expert Opin Investig Drugs. 2011;20:595-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4615] [Cited by in F6Publishing: 4839] [Article Influence: 345.6] [Reference Citation Analysis (1)] |
| 17. | Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4016] [Cited by in F6Publishing: 4107] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 18. | Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3011] [Cited by in F6Publishing: 3213] [Article Influence: 229.5] [Reference Citation Analysis (0)] |
| 19. | Kuriyan J, Cowburn D. Modular peptide recognition domains in eukaryotic signaling. Annu Rev Biophys Biomol Struct. 1997;26:259-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 444] [Cited by in F6Publishing: 426] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 20. | Becker JC, Muller-Tidow C, Serve H, Domschke W, Pohle T. Role of receptor tyrosine kinases in gastric cancer: new targets for a selective therapy. World J Gastroenterol. 2006;12:3297-3305. [PubMed] [Cited in This Article: ] |
| 21. | Chung CK, Antoniades HN. Expression of c-sis/platelet-derived growth factor B, insulin-like growth factor I, and transforming growth factor alpha messenger RNAs and their respective receptor messenger RNAs in primary human gastric carcinomas: in vivo studies with in situ hybridization and immunocytochemistry. Cancer Res. 1992;52:3453-3459. [PubMed] [Cited in This Article: ] |
| 22. | Deng N, Goh LK, Wang H, Das K, Tao J, Tan IB, Zhang S, Lee M, Wu J, Lim KH. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. 2012;61:673-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 478] [Cited by in F6Publishing: 498] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 23. | Keating AK, Kim GK, Jones AE, Donson AM, Ware K, Mulcahy JM, Salzberg DB, Foreman NK, Liang X, Thorburn A. Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma cells leads to increased apoptosis and improved chemosensitivity. Mol Cancer Ther. 2010;9:1298-1307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Shieh YS, Lai CY, Kao YR, Shiah SG, Chu YW, Lee HS, Wu CW. Expression of axl in lung adenocarcinoma and correlation with tumor progression. Neoplasia. 2005;7:1058-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 207] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 25. | Wimmel A, Glitz D, Kraus A, Roeder J, Schuermann M. Axl receptor tyrosine kinase expression in human lung cancer cell lines correlates with cellular adhesion. Eur J Cancer. 2001;37:2264-2274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Holland SJ, Powell MJ, Franci C, Chan EW, Friera AM, Atchison RE, McLaughlin J, Swift SE, Pali ES, Yam G. Multiple roles for the receptor tyrosine kinase axl in tumor formation. Cancer Res. 2005;65:9294-9303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Wu CW, Li AF, Chi CW, Lai CH, Huang CL, Lo SS, Lui WY, Lin WC. Clinical significance of AXL kinase family in gastric cancer. Anticancer Res. 2002;22:1071-1078. [PubMed] [Cited in This Article: ] |
| 28. | Gong J, Morishita A, Kurokohchi K, Tani J, Kato K, Miyoshi H, Inoue H, Kobayashi M, Liu S, Murota M. Use of protein array to investigate receptor tyrosine kinases activated in gastric cancer. Int J Oncol. 2010;36:101-106. [PubMed] [Cited in This Article: ] |
| 29. | Yano T, Doi T, Ohtsu A, Boku N, Hashizume K, Nakanishi M, Ochiai A. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep. 2006;15:65-71. [PubMed] [Cited in This Article: ] |
| 30. | Koeppen HK, Wright BD, Burt AD, Quirke P, McNicol AM, Dybdal NO, Sliwkowski MX, Hillan KJ. Overexpression of HER2/neu in solid tumours: an immunohistochemical survey. Histopathology. 2001;38:96-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Jaehne J, Urmacher C, Thaler HT, Friedlander-Klar H, Cordon-Cardo C, Meyer HJ. Expression of Her2/neu oncogene product p185 in correlation to clinicopathological and prognostic factors of gastric carcinoma. J Cancer Res Clin Oncol. 1992;118:474-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Im SA, Lee KE, Nam E, Kim DY, Lee JH, Han HS, Seoh JY, Park HY, Cho MS, Han WS. Potential prognostic significance of p185(HER2) overexpression with loss of PTEN expression in gastric carcinomas. Tumori. 2005;91:513-521. [PubMed] [Cited in This Article: ] |
| 33. | Begnami MD, Fukuda E, Fregnani JH, Nonogaki S, Montagnini AL, da Costa WL, Soares FA. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol. 2011;29:3030-3036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 198] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 34. | Grabsch H, Sivakumar S, Gray S, Gabbert HE, Müller W. HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value - conclusions from 924 cases of two independent series. Cell Oncol. 2010;32:57-65. [PubMed] [Cited in This Article: ] |
| 35. | Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes--a systematic review. Int J Cancer. 2012;130:2845-2856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 36. | Moelans CB, van Diest PJ, Milne AN, Offerhaus GJ. Her-2/neu testing and therapy in gastroesophageal adenocarcinoma. Patholog Res Int. 2011;2011:674182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Nicholas G, Cripps C, Au H-J, Jonker D, Salim M, Bjarnason G, Chiritescu G, Galant V. Early results of a trial of trastuzumab, cisplatin, and docetaxel (TCD) for the treatment of metastatic gastric cancer overexpressing HER-2. Ann Oncol. 2006;17:Absract 1105. [Cited in This Article: ] |
| 38. | Cortes-Funes H, Rivera F, Ales I, Marquez A, Velasco A, Colomer R, Garcia-Carbonero R, Sastre J, Guerra J, Gravalos C. Phase II of trastuzumab and cisplatin in patients (pts) with advanced gastric cancer (AGC) with HER2/neu overexpression/amplification. In: 2007 ASCO annual meeting proceedings part I. J Clin Oncol. 2007;15; 18S (Abstr 4613). [Cited in This Article: ] |
| 39. | Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, Stewart SJ, Keefe D. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215-1221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 473] [Cited by in F6Publishing: 462] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 40. | Ewer MS, Vooletich MT, Durand JB, Woods ML, Davis JR, Valero V, Lenihan DJ. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820-7826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 517] [Cited by in F6Publishing: 476] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 41. | Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol. 2005;23:2900-2902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 445] [Cited by in F6Publishing: 415] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 42. | Tanaka Y, Yonetani Y, Shiozaki Y, Kitaguchi T, Sato N, Takeshita S, Horibe S. Retear of anterior cruciate ligament grafts in female basketball players: a case series. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH. EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology. 2008;52:738-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 44. | Ilson DH, Kelsen D, Shah M, Schwartz G, Levine DA, Boyd J, Capanu M, Miron B, Klimstra D. A phase 2 trial of erlotinib in patients with previously treated squamous cell and adenocarcinoma of the esophagus. Cancer. 2011;117:1409-1414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 45. | Pinto C, Di Fabio F, Barone C, Siena S, Falcone A, Cascinu S, Rojas Llimpe FL, Stella G, Schinzari G, Artale S. Phase II study of cetuximab in combination with cisplatin and docetaxel in patients with untreated advanced gastric or gastro-oesophageal junction adenocarcinoma (DOCETUX study). Br J Cancer. 2009;101:1261-1268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 46. | Pinto C, Di Fabio F, Siena S, Cascinu S, Rojas Llimpe FL, Ceccarelli C, Mutri V, Giannetta L, Giaquinta S, Funaioli C. Phase II study of cetuximab in combination with FOLFIRI in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma (FOLCETUX study). Ann Oncol. 2007;18:510-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 47. | Moehler M, Mueller A, Trarbach T, Lordick F, Seufferlein T, Kubicka S, Geissler M, Schwarz S, Galle PR, Kanzler S. Cetuximab with irinotecan, folinic acid and 5-fluorouracil as first-line treatment in advanced gastroesophageal cancer: a prospective multi-center biomarker-oriented phase II study. Ann Oncol. 2011;22:1358-1366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 48. | Kim C, Lee JL, Ryu MH, Chang HM, Kim TW, Lim HY, Kang HJ, Park YS, Ryoo BY, Kang YK. A prospective phase II study of cetuximab in combination with XELOX (capecitabine and oxaliplatin) in patients with metastatic and/or recurrent advanced gastric cancer. Invest New Drugs. 2011;29:366-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Han SW, Oh DY, Im SA, Park SR, Lee KW, Song HS, Lee NS, Lee KH, Choi IS, Lee MH. Phase II study and biomarker analysis of cetuximab combined with modified FOLFOX6 in advanced gastric cancer. Br J Cancer. 2009;100:298-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 50. | Richards D, Kocs DM, Spira AI, David McCollum A, Diab S, Hecker LI, Cohn A, Zhan F, Asmar L. Results of docetaxel plus oxaliplatin (DOCOX) ± cetuximab in patients with metastatic gastric and/or gastroesophageal junction adenocarcinoma: results of a randomised Phase 2 study. Eur J Cancer. 2013;49:2823-2831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 589] [Cited by in F6Publishing: 640] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 52. | Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658-1664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1444] [Cited by in F6Publishing: 1424] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 53. | Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G, Wadsley J. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 504] [Cited by in F6Publishing: 554] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 54. | Rao S, Starling N, Cunningham D, Sumpter K, Gilligan D, Ruhstaller T, Valladares-Ayerbes M, Wilke H, Archer C, Kurek R. Matuzumab plus epirubicin, cisplatin and capecitabine (ECX) compared with epirubicin, cisplatin and capecitabine alone as first-line treatment in patients with advanced oesophago-gastric cancer: a randomised, multicentre open-label phase II study. Ann Oncol. 2010;21:2213-2219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 55. | Smolen GA, Sordella R, Muir B, Mohapatra G, Barmettler A, Archibald H, Kim WJ, Okimoto RA, Bell DW, Sgroi DC. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci USA. 2006;103:2316-2321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 425] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 56. | Dragovich T, McCoy S, Fenoglio-Preiser CM, Wang J, Benedetti JK, Baker AF, Hackett CB, Urba SG, Zaner KS, Blanke CD. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol. 2006;24:4922-4927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 238] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 57. | Wainberg ZA, Lin LS, DiCarlo B, Dao KM, Patel R, Park DJ, Wang HJ, Elashoff R, Ryba N, Hecht JR. Phase II trial of modified FOLFOX6 and erlotinib in patients with metastatic or advanced adenocarcinoma of the oesophagus and gastro-oesophageal junction. Br J Cancer. 2011;105:760-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 58. | Iqbal S, Goldman B, Fenoglio-Preiser CM, Lenz HJ, Zhang W, Danenberg KD, Shibata SI, Blanke CD. Southwest Oncology Group study S0413: a phase II trial of lapatinib (GW572016) as first-line therapy in patients with advanced or metastatic gastric cancer. Ann Oncol. 2011;22:2610-2615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 59. | Arkenau HT. Gastric cancer in the era of molecularly targeted agents: current drug development strategies. J Cancer Res Clin Oncol. 2009;135:855-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 60. | Arkenau HT; ClinicalTrials. gov. LOGiC-Lapatinib Optimization Study in ErbB2 (HER2) Positive Gastric Cancer: A Phase III Global, Blinded Study Designed to Evaluate Clinical Endpoints and Safety of Chemotherapy Plus Lapatinib. [accessed January 21, 2012]. Available from: http://www.clinicaltrials.gov/ct/show/NCT00680901. [Cited in This Article: ] |
| 61. | Satoh T, Bang Y, Wang J, Xu J, Chung HC, Yeh K, Chen J, Mukaiyama A, Yoshida P, Ohtsu A. Interim safety analysis from TYTAN: A phase III Asian study of lapatinib in combination with paclitaxel as second-line therapy in gastric cancer. J Clin Oncol. 2010;28 (15 suppl): 4057. [Cited in This Article: ] |
| 62. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7832] [Cited by in F6Publishing: 7523] [Article Influence: 376.2] [Reference Citation Analysis (1)] |
| 63. | Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar B, Bajetta E. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103-2111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1757] [Cited by in F6Publishing: 1691] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 64. | Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733-4740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1901] [Cited by in F6Publishing: 1802] [Article Influence: 120.1] [Reference Citation Analysis (0)] |
| 65. | Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666-2676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2393] [Cited by in F6Publishing: 2249] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 66. | Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227-1234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1098] [Cited by in F6Publishing: 1111] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 67. | Shah MA, Ramanathan RK, Ilson DH, Levnor A, D’Adamo D, O’Reilly E, Tse A, Trocola R, Schwartz L, Capanu M. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201-5206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 346] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 68. | El-Rayes BF, Zalupski M, Bekai-Saab T, Heilbrun LK, Hammad N, Patel B, Urba S, Shields AF, Vaishampayan U, Dawson S. A phase II study of bevacizumab, oxaliplatin, and docetaxel in locally advanced and metastatic gastric and gastroesophageal junction cancers. Ann Oncol. 2010;21:1999-2004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 69. | Shah MA, Jhawer M, Ilson DH, Lefkowitz RA, Robinson E, Capanu M, Kelsen DP. Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J Clin Oncol. 2011;29:868-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 70. | Cohenuram MK, Lacy J. FOLFOX6 and bevacizumab (FOLFOX6/B) for metastatic esophageal (E), gastroesophageal (GE), and gastric (G) adenocarcinoma: A single institutionâs initial clinical experience [abstract 74]. Presented at the 2008 American Society of Clinical Oncology Gastrointestinal Cancers Symposium; Orlando, FL; January 25-27, 2008. . [Cited in This Article: ] |
| 71. | Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968-3976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 795] [Cited by in F6Publishing: 887] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 72. | Van Cutsem E, de Haas S, Kang YK, Ohtsu A, Tebbutt NC, Ming Xu J, Peng Yong W, Langer B, Delmar P, Scherer SJ. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol. 2012;30:2119-2127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 385] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 73. | Martin-Richard M, Gallego R, Pericay C, Garcia Foncillas J, Queralt B, Casado E, Barriuso J, Iranzo V, Juez I, Visa L. Multicenter phase II study of oxaliplatin and sorafenib in advanced gastric adenocarcinoma after failure of cisplatin and fluoropyrimidine treatment. A GEMCAD study. Invest New Drugs. 2013;31:1573-1579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 74. | Bang YJ, Kang YK, Kang WK, Boku N, Chung HC, Chen JS, Doi T, Sun Y, Shen L, Qin S. Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Invest New Drugs. 2011;29:1449-1458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 75. | Moehler M, Hartmann JT, Lordick F, Al-Batran S, Reimer P, Trarbach T, Ebert M, Daum S, Weihrauch M, Galle P; Upper GI Group of German AIO. An open-label, multicenter phase II trial of sunitinib for patients with chemorefractory metastatic gastric cancer. J Clin Oncol. 2010;28:e14503. [Cited in This Article: ] |
| 76. | Wong H, Yau T. Targeted therapy in the management of advanced gastric cancer: are we making progress in the era of personalized medicine? Oncologist. 2012;17:346-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 77. | Migliore C, Giordano S. Molecular cancer therapy: can our expectation be MET? Eur J Cancer. 2008;44:641-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 78. | Ponzetto C, Giordano S, Peverali F, Della Valle G, Abate ML, Vaula G, Comoglio PM. c-met is amplified but not mutated in a cell line with an activated met tyrosine kinase. Oncogene. 1991;6:553-559. [PubMed] [Cited in This Article: ] |
| 79. | Inoue T, Kataoka H, Goto K, Nagaike K, Igami K, Naka D, Kitamura N, Miyazawa K. Activation of c-Met (hepatocyte growth factor receptor) in human gastric cancer tissue. Cancer Sci. 2004;95:803-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Jhawer MP, Kindler HL, Wainberg ZA, Ford J, Kunz P, Tang K, McCallum S, Kallender H, Shah MA; on behalf of the MET111643 Investigators and Glaxo SmithKline. Assessment of two dosing schedules of GSK1363089 (GSK089), a dual MET/VEGFR2 inhibitor, in metastatic gastric cancer (GC): Interim results of a multicenter phase II study. J Clin Oncol. 2009;27:4502. [Cited in This Article: ] |
| 81. | Matsubara J, Yamada Y, Nakajima TE, Kato K, Hamaguchi T, Shirao K, Shimada Y, Shimoda T. Clinical significance of insulin-like growth factor type 1 receptor and epidermal growth factor receptor in patients with advanced gastric cancer. Oncology. 2008;74:76-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Molife LR, Fong PC, Paccagnella L, Reid AH, Shaw HM, Vidal L, Arkenau HT, Karavasilis V, Yap TA, Olmos D. The insulin-like growth factor-I receptor inhibitor figitumumab (CP-751,871) in combination with docetaxel in patients with advanced solid tumours: results of a phase Ib dose-escalation, open-label study. Br J Cancer. 2010;103:332-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 83. | Nakazawa K, Yashiro M, Hirakawa K. Keratinocyte growth factor produced by gastric fibroblasts specifically stimulates proliferation of cancer cells from scirrhous gastric carcinoma. Cancer Res. 2003;63:8848-8852. [PubMed] [Cited in This Article: ] |
| 84. | Jang JH, Shin KH, Park JG. Mutations in fibroblast growth factor receptor 2 and fibroblast growth factor receptor 3 genes associated with human gastric and colorectal cancers. Cancer Res. 2001;61:3541-3543. [PubMed] [Cited in This Article: ] |
| 85. | Squires M, Ward G, Saxty G, Berdini V, Cleasby A, King P, Angibaud P, Perera T, Fazal L, Ross D. Potent, selective inhibitors of fibroblast growth factor receptor define fibroblast growth factor dependence in preclinical cancer models. Mol Cancer Ther. 2011;10:1542-1552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 86. | Sawabu T, Seno H, Kawashima T, Fukuda A, Uenoyama Y, Kawada M, Kanda N, Sekikawa A, Fukui H, Yanagita M. Growth arrest-specific gene 6 and Axl signaling enhances gastric cancer cell survival via Akt pathway. Mol Carcinog. 2007;46:155-164. [PubMed] [Cited in This Article: ] |
| 87. | Singh R, Kim WJ, Kim PH, Hong HJ. Combined blockade of HER2 and VEGF exerts greater growth inhibition of HER2-overexpressing gastric cancer xenografts than individual blockade. Exp Mol Med. 2013;45:e52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |