Published online Jul 14, 2013. doi: 10.3748/wjg.v19.i26.4234
Revised: April 12, 2013
Accepted: May 16, 2013
Published online: July 14, 2013
AIM: To investigate if there is an association between hepatitis B virus (HBV) or hepatitis C virus (HCV) infection and the risk of pancreatic cancer.
METHODS: All relevant studies published before 11 October, 2012 were identified by a systematic search of MEDLINE, EMBASE, BIOSIS Previews and the Cochrane Library databases and with cross-referencing. The observational studies that reported RR or OR estimates with 95%CIs for the association between HBV or HCV and pancreatic cancer were included. A random-effects model was used to summarize meta-analytic estimates. The Newcastle-Ottawa quality assessment scale was applied to assess the quality of the methodology in the included studies.
RESULTS: A total of 8 eligible studies were selected for meta-analysis. Overall, chronic hepatitis B and inactive hepatitis B surface antigen (HBsAg) carrier state (HBsAg positive) had a significantly increased risk of pancreatic cancer with OR of 1.20 (95%CI: 1.01-1.39), especially in the Chinese population (OR = 1.30, 95%CI: 1.05-1.56). Past exposure to HBV (possible occult HBV infection) had an increased OR of pancreatic cancer risk (OR = 1.24, 95%CI: 1.05-1.42), especially among those patients without natural immunity [anti hepatitis B core (HBc) positive/hepatitis B surface antibody (anti HBs) negative], with OR of 1.67 (95%CI: 1.13-2.22). However, past exposure to HBV with natural immunity (anti-HBc positive/anti-HBs positive) had no association with pancreatic cancer development, with OR 0.98 (95%CI: 0.80-1.16), nor did the HBV active replication (hepatitis B e antigen positive status), with OR 0.98 (95%CI: 0.27-1.68). The risk of pancreatic cancer among anti-HBs positive patients was significantly lower than among anti-HBs negative patients (OR = 0.54, 95%CI: 0.46-0.62). Past exposure to HCV also resulted in an increased risk of pancreatic cancer (OR = 1.26, 95%CI: 1.03-1.50). Significant between-study heterogeneity was observed. Evidence of publication bias for HBV/HCV infection-pancreatic cancer association was not found.
CONCLUSION: Chronic HBV and HCV infection increases pancreatic cancer risk. Our findings underscore the need for more studies to confirm this potential relationship.
Core tip: Based on the meta-analysis, we identified that chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection is associated with pancreatic cancer, especially among Chinese population. Patients with past exposure to HBV/HCV should be screened for hepatocellular carcinoma and other malignancies, especially pancreatic cancer.
- Citation: Xu JH, Fu JJ, Wang XL, Zhu JY, Ye XH, Chen SD. Hepatitis B or C viral infection and risk of pancreatic cancer: A meta-analysis of observational studies. World J Gastroenterol 2013; 19(26): 4234-4241
- URL: https://www.wjgnet.com/1007-9327/full/v19/i26/4234.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i26.4234
Pancreatic cancer is one of the most lethal and devastating human malignancies and the fourth leading cause of cancer-related fatality worldwide. Because of absence of early symptoms, lack of sensitive and specific tests to screen the cancer in the initial phases, limited therapeutic options and rapid progression, nearly all patients die of the disease within one year of diagnosis with the overall 5-year survival rate being less than 5%[1]. Therefore, it is crucial to identify the intrinsic genes as well as other risk factors, that may influence the progression of the cancer, and to develop more accurate screening programs for early monitoring and intervention strategies. Although several risk factors associated with pancreatic cancer have been explored, the causative factors for pancreatic cancer are far from being understood, one of which is chronic hepatitis infection[2,3].
The prevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) infection varies worldwide, ranging from less than 0.5% in Western countries to 7% and 25% in East Asian and African countries[4,5]. Infection with HBV is a huge global public health concern, especially in China. According to the World Health Organization, there are 2 billion people infected with HBV globally, with China accounting for 65% of the HBV infective public health burden of the world[6]. HBV/HCV have been detected not only in hepatotropic tissue but also in extrahepatic sites such as the pancreas[7,8]. Several studies reported conflicting results regarding the association between HBV infection and the risk of subsequent pancreatic cancer. Studies by Berrington de Gonzalez et al[9] and Hong et al[10] found no relationship between chronic infection with HBV and the development of pancreatic cancer, while several published studies found an association between presence of HBV infection and incidence of pancreatic cancer, not only in countries with a lower prevalence of HBV infection such as the United States but also in countries with a higher number of HBV infections such as China[11,12]. Meanwhile, little is known about the association between the presence of HCV infection and the risk of pancreatic cancer[3].
The purpose of the present study is to summarize all available evidence of observational studies in order to better define the impact of HBV and HCV infection on the risk of pancreatic cancer in patients following the meta-analysis of observational studies in epidemiological guidelines.
A comprehensive literature search was carried out on observational studies and trials, and no language or time restriction was applied. All literature from January 1, 1980 to October 18, 2012 was searched using the following databases: Pubmed, ISI web of Science, Embase and Cochrane library. The following main keywords or corresponding MeSH terms were used: hepatitis, virus, viral, pancreas, cancer OR adenocarcinoma OR neoplasm OR tumor. A manual search was also performed for references cited in the selected articles.
Studies were included in the meta-analysis if (1) they were the principal published reports of original data from case-control or cohort studies; (2) they were independent from other studies to avoid giving double weight to estimate the same study; (3) the exposure of interest was a history of HBV/HCV infection; (4) the outcome of interest was pancreatic cancer incidence or mortality; and (5) they had sufficient information to allow adequate estimation of OR or RR and 95%CI to estimate cancer risk under HBV/HCV exposure. Two authors (Fu JJ and Xu JH) independently evaluated all of the studies retrieved from database, then compared their results. Any disagreements were resolved by consensus.
The following data were extracted from each study: authors, publication year, study design, country of origin, sample size, measure of outcome, duration of follow-up, marker of hepatitis serostatus, covariates adjusted for in the analysis, and the effect estimates with corresponding 95%CIs.
The Newcastle-Ottawa quality assessment scale (NOS)[13] was applied to assess the quality of the methodology in the included studies. A star system was used to judge the data according to the selection populations, comparability of groups and exposure/outcome of interest. The NOS scale consists of 8 questions with 9 possible points. The assessment score ranged from 0 to 9. Studies with a total score of 6 or lower indicated low quality while study scores of 7 or higher were considered to be of high quality. Two reviewers (Xu JH and Fu JJ) independently evaluated and cross-checked the qualities of the included studies (Table 1).
| Ref. | Quality indicators from NOS | Score | ||||||||
| Selection | Comparability | Exposure/outcome | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| Hong et al[10] | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | 6 |
| Hassan et al[11] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | 7 |
| Wang et al[18] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | 7 |
| Zhu et al[17] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | 7 |
| Ben et al[12] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | 7 |
| Berrington de Gonalez et al[9] | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | 6 |
| El-Serag et al[3] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 8 |
| Iloeje et al[2] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
Statistical analyses were completed with STATA version 10.0 (STATA, College Station, TX, United States). Summary odd ratio estimates with the corresponding 95%CIs were combined and weighted to produce pooled ORs using a random-effects model, which considers both within- and between-study variations[14]. Q and I2 statistics were both examined to investigate the source of heterogeneity across studies. I2 values of 25, 50 and 75% were assigned to low, moderate, and high heterogeneities, respectively[15]. The Begg’s adjusted rank correlation test and the Egger’s regression test (significant at P < 0.1) were performed to test for evidence of publication bias[16].
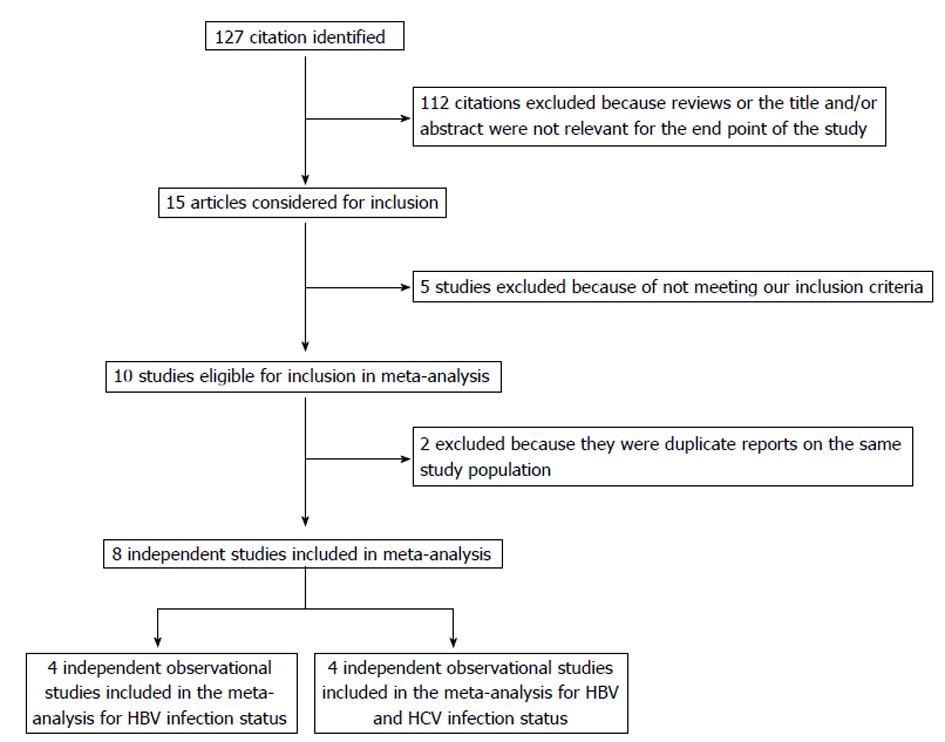
The participant flow diagram for the study inclusion in the meta-analysis is shown in Figure 1. A total of 8 articles were retrieved and checked for relevance in terms of infectious status, population studied, and reporting of pancreatic cancer risk data[2,3,9-12,17,18]. Seven of other articles were not included in the meta-analysis for the following reasons: (1) two referred to the same cohort[19,20]; (2) two were editorials responding to originated studies[21,22]; (3) one reported HCV infection and pancreatic cancer incidence in the abstract but no OR (95%CI) information was found[23]; and (4) two studies were manual search cited in the selected articles, but did not meet our inclusion criteria after reading the text[24,25].
The main characteristics of the 8 studies pooled in the present analysis are reported in Table 2. All studies except one prospective study were retrospective. Five studies were case-control, and three were cohort studies conducted between 1988 and 2010 and published between 2008 and 2012. These studies included a total of 744 120 investigated patients and 3758 cases of pancreatic cancer events. Four studies were conducted in China, two in South Korea and two in the United States.
| Ref. | Population | Study design | Country | Ethnicity | Case (n) | No. of control | Confirmation of HBV/HCV | Confirmation of PC | Matching criteria |
| Berrington de Gonalez et al[9] | Population-based | Cohort | South Korea | Asian | 2194 | 628978 | HBsAg | clinic diagnosed | Age, sex |
| Hong et al[10] | Hospital-based | CC | South Korea | Asian | 506 | 1008 | Anti-HCV, Anti-HBs, HBsAg | histologically confirmed | Age, sex |
| Hassan et al[11] | Hospital-based | CC | United States | White | 476 | 879 | Anti-HCV,Anti-HBs, Anti-HBc | pathologically confirmed | Age, sex, race |
| El-Serag et al[3] | Population-based | Cohort | United States | Asian | 140 | 477 | Anti-HCV, HBV | ICD-9 | Age, sex |
| Iloeje et al[2] | Population-based | Cohort | Taiwan | Asian | 48 | 22471 | HBsAg, HBV DNA | pathologically confirmed | Age, sex, smoking, alcohol |
| Wang et al[18] | Hospital-based | CC | China | Asian | 645 | 711 | HBsAg, Anti-HBs, Anti-HBc | pathologically confirmed | Age, sex |
| Zhu et al[17] | Hospital-based | CC | China | Asian | 80 | 77 | HBsAg, Anti-HCV, Anti-HBc | pathologically confirmed | Age, sex |
| Ben et al[12] | Hospital-based | CC | China | Asian | 943 | 1128 | HBsAg, Anti-HBs, Anti-HBc | pathologically confirmed | Age, sex, smoking, DM, BMI |
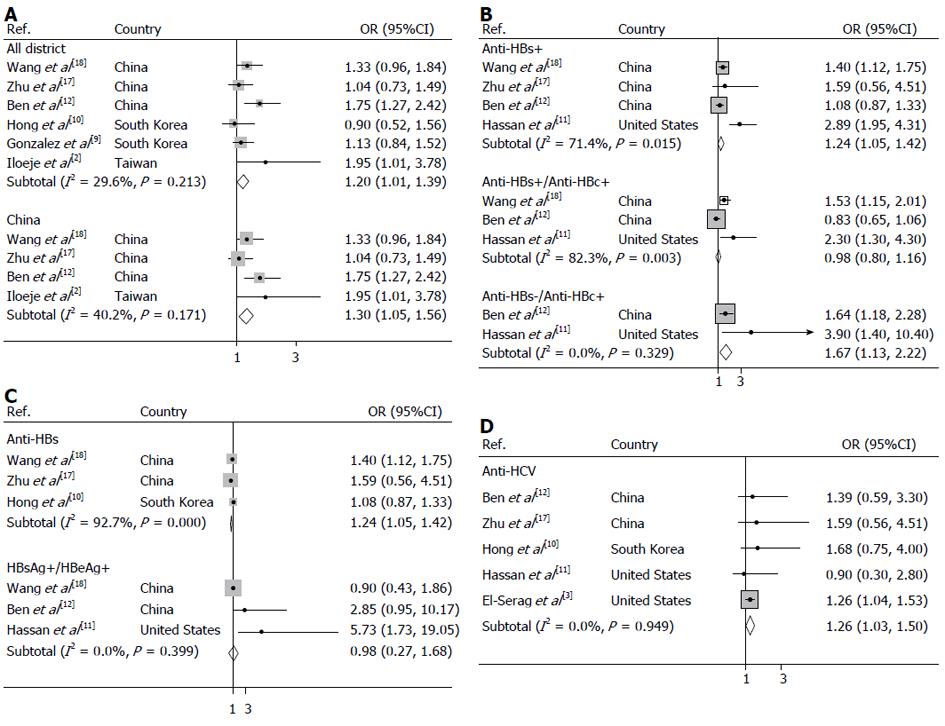
Hepatitis B surface antigen (HBsAg) was seropositive in 4492 patients across all of the groups. Meta-analysis of 6 studies in a random-effects model found that compared to individuals without a history of chronic hepatitis B, those with chronic hepatitis B and in inactive HBsAg carrier state (HBsAg positive) had a 20% greater risk of pancreatic cancer (OR = 1.20, 95%CI: 1.01-1.39), with moderate heterogeneity among studies (test for heterogeneity I2 = 29.6%, P = 0.213). To further evaluate the HBsAg carrier state associated with pancreatic cancer in the Chinese population, a subgroup type was used to analyze the data. As shown in Figure 2, 4 studies conducted in the Chinese population revealed that the odds ratio of pancreatic cancer for HBsAg positivity was 1.30 (95%CI: 1.05-1.56). Moderate heterogeneity (I2 = 40.2%, P = 0.171) was seen across studies.
Prior infection with hepatitis B, as determined by the presence of anti- hepatitis B core (HBc), resulted in a significantly increased risk of pancreatic cancer showing OR of 1.24 (95%CI: 1.05-1.42) (I2 = 71.4%, Pheterogeneity = 0.015) which is summarized in Figure 2. Furthermore, an increased risk of pancreatic cancer was observed for hepatitis B surface antibody (anti-HBs)-seronegative/anti-HBc-seropositive carriers who were previously exposed to HBV without natural immunity, with OR of 1.67 (95%CI: 1.13-2.22) (I2 = 0.0%, Pheterogeneity = 0.329), but not for past exposure to HBV carriers with natural immunity (anti-HBs-seropositive/anti-HBc-seropositive), with OR of 0.98 (95%CI: 0.80-1.16) (I2 = 82.3%, Pheterogeneity = 0.003).
We observed non-significant positive associations between markers of active viral replication and pancreatic cancer risk, as illustrated in Figure 2. The risk of developing pancreatic cancer was 0.98 (95%CI: 0.27-1.68) (I2 = 0.0%, Pheterogeneity = 0.399) for HBsAg-seropositive/hepatitis B e antigen (HBeAg)- seropositive subjects compared with that of HBsAg-seronegative subjects while a significant positive association between the protective markers of HBV, anti-HBs and pancreatic cancer risk was found for studies conducted in the pooled analysis with OR of 0.54 (95%CI: 0.46-0.62) (I2 = 92.7%, Pheterogeneity = 0.000).
As summarized in Figure 2, the incidence of pancreatic cancer risks were also significantly increased in previously HCV infected population, with OR of 1.26 (95%CI: 1.03-1.50) (I2= 0.0%, Pheterogeneity = 0.949).
We also carried out stratified analyses to assess the impact of confounding factors of the RRs on the chronic carriers of HBV subgroup. As shown in Table 3, when we restricted the meta-analysis to those studies adjusted for smoking, the association between chronic HBV infection and pancreatic cancer risk was positive, the pooled OR was 1.32 (95%CI: 1.08-1.56). No positive association between chronic HBV infection and pancreatic cancer risk was found in the studies that were not adjusted for smoking, the pooled OR was 0.99 (95%CI: 0.68-1.30). In the stratified analysis, the association between chronic HBV infection and pancreatic cancer risk was also similar between the studies that were adjusted for alcohol drinking (the pooled OR = 1.51, 95%CI: 1.17-1.85) and those that were not adjusted for alcohol drinking (the pooled OR = 1.05, 95%CI: 0.83-1.28). Meanwhile, there was no positive association between chronic HBV infection and pancreatic cancer risk in the studies adjusted for diabetes (the pooled OR = 1.25, 95%CI: 0.95-1.55), neither was in the studies that were adjusted for diabetes (the pooled OR, 1.24, 95%CI: 0.50-1.98).
| Stratifying variables | Studies (n) | OR (95%CI) | Tests for heterogeneity | ||
| χ2 | P value | I2 | |||
| Adjusted for smoking | |||||
| Yes | 4 | 1.32 (1.08-1.56) | 4.14 | 0.246 | 27.60% |
| No | 2 | 0.99 (0.68-1.30) | 0.18 | 0.670 | 0.00% |
| Adjusted for drinking | |||||
| Yes | 3 | 1.51 (1.17-1.85) | 1.70 | 0.428 | 0.00% |
| No | 3 | 1.05 (0.83-1.28) | 0.53 | 0.766 | 0.00% |
| Adjusted for diabetes | |||||
| Yes | 4 | 1.25 (0.95-1.55) | 5.22 | 0.156 | 42.50% |
| No | 2 | 1.24 (0.50-1.98) | 1.54 | 0.214 | 35.20% |
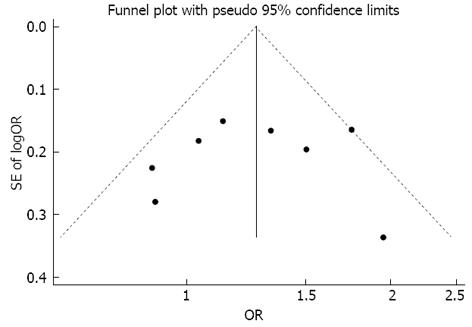
No publication bias was apparent following an assessment by funnel plot (Figure 3, Begg’s test P = 0.711, Egger’s test P = 0.868).
This is the first comprehensive meta-analysis of observational studies on the association between chronic hepatitis viral infection and pancreatic cancer risk. We found that HBV and HCV infection is associated with 20% and 23% higher risk of pancreatic cancer, respectively. Our results reveal that prior infection with hepatitis B, especially in those without natural immunity would significantly increase the risk of pancreatic cancer. However, active hepatitis B viral replication does not increase the pancreatic cancer incidence. These observations provide evidence supporting the importance role of chronic HBV and HCV infection in the development of pancreatic cancer. In light of the fact that pancreatic cancer is a highly fatal tumor with a 5-year survival rate of less than 5% and that the number of people with HBV is 2 billion[6,26], our findings have substantial clinical and public significance on a global scale. It points to the need for further investigation on the etiological causes involved in human pancreatic carcinogenesis, the recognition of pancreatic damage mechanisms caused by chronic hepatic viral infection, and for long-term, large scale clinical studies to confirm this clinical association.
If the positive association between the chronic or inactive HBV or HCV carriers and the development of pancreatic cancer is a true, what mechanism could explain such a link From the anatomical point of view, the proximity of the liver to the pancreas, as well as the sharing of the two organs blood vessels and ducts may make the pancreas a potential reservoir of hepatitis viruses. HBV or HCV may travel through the blood stream and be deposited in non-liver tissue[27,28]. In fact, by means of situ hybridization and immunohistochemical techniques, the serological markers of present or past HBV infection, HBsAg was detected in chronic inflammatory pancreatic acinar cells and in the pancreatic duct epithelia with pancreatic adenocarcinoma[29]. The same was true with HCV antigen, which was also found in pancreatic acinar cells[30]. These findings demonstrated the possibility of HBV infection and evidence of a chronic inflammatory reaction in non-hepatic tissues.
HBV and HCV replication intermediates in pancreatic cells support the assumption that the permissiveness of these extrahepatic cells for viral replication might also induce the chronic inflammatory response, thus eventually promoting tumor development. Anti-HBc-positive status, the sero biological marker of past exposure to HBV had an increased risk of developing pancreatic cancer. The observation provides some biological plausibility to the idea that long-lasting persistence of viral infection could indeed replicate in the pancreas. In fact, HBV and HCV are oncogenic viruses, and both are able to integrate the viral RNA or DNA into the genome of the infected cells[31,32]. DNA integration may play a key role in the regulation of the cell cycle, inducing carcinogenesis associated with HBV infection[33].
The third reason why our finding of a relationship between HBV infection and pancreatic cancer incidence may not be surprising is that the presence of HBV infection protection marker, seroconversion from HBsAg to anti-HBs, which is considered a sign of disease protection, leads to a significantly decreased risk of pancreatic cancer showing an OR of 0.54 in the pooled studies.
As with all meta-analyses of observational studies, our findings might have some limitations. First, because five of eight studies used a case-control design[10-12,17,18], the findings provided by this meta-analysis should be viewed with caution since more recall and selection bias might be seen in case-control studies. In addition, all the case-control studies were hospital-based and therefore may not fully represent the general population of pancreatic cancer patients, thereby introducing potential for selection bias into our meta-analysis.
Second, when investigating the association between hepatic virus infection and the risk of pancreatic cancer, the potential residual confounding and the allocation bias, with hepatic virus infection being at different stage and baseline risk of pancreatic cancer would affect the results. For example, it is difficult to understand the biological explanation for finding the cancer risk in subjects with chronic infection and not in those with current and active replication of the virus as shown by a positive HBeAg. The progression from active hepatitis virus infection to chronic inflammatory response targeted to pancreatic carcinogenesis is still unknown, for there is a lack of such data[34]. This incomplete information on HBV/HCV in the pathogenesis of progressive stages limits our knowledge on the true relationship of these oncogenic viruses with pancreatic cancer development. Although an increased risk of pancreatic cancer was observed for anti-HBs-seronegative/anti-HBc-seropositive carriers who were previously exposed to HBV without natural immunity, it is very difficult to interpret a pooled analysis of only 2 studies. Another issue is that none of the studies directly tested for the presence of markers of hepatitis virus infection in the pancreatic tissue. Therefore, a correlation between the level of the markers of HBV/HCV infection in peripheral blood and that in pancreatic tissue could not be established, which would throw some doubt into the reliability of the summary of RRs. More research is necessary to assess a dose-response association to examine the influence of viral load on the progression of pancreatic cancer to support biological plausibility.
Third, possible confounding factors and biases that may not have been fully adjusted for in this study exist. In fact, risk factors such as cigarette smoking, alcohol intake and diabetes, all of which could increase risk of pancreatic cancer associated with a history of chronic hepatitis virus infection. Only 4[2,10-12], 3[2,11], and 4[10-12,17] studies provided risk estimated adjusting for smoking, alcohol intake, and diabetes, respectively. The positive association between chronic HBV infection and pancreatic cancer risk was found after adjustment by smoking and alcohol drinking, but no positive correlation was maintained after adjustment by diabetes, suggesting that residual confounding by diabetes modified the association between chronic HBV infection and pancreatic cancer risk.
Finally, it is also important to realize that there is still a significant heterogeneity observed across studies, mostly due to the diversity of the study designs and the varying incidence of pancreatic cancer and HBV/HCV infection rates may vary from continent to continent.
Even with these limitations, our meta-analysis supports the hypothesis that chronic HBV and HCV infection may significantly increase pancreatic cancer risk. The findings of this study raise the question of whether the early detection and provision of aggressive antiviral treatment for chronic hepatitis virus infection could prevent the development of pancreatic cancer, and whether patients with past exposure to HBV/HCV should be screened for malignancies other than HCC particularly in patients at high risk of HBV/HCV infection.
In conclusion, our meta-analysis favors the association between HBV/HCV infection and pancreatic cancer risk. However, observational studies were moderately heterogeneous and biased. Additional long-term prospective evidence for HBV/HCV infection among higher risk of pancreatic disease patients should be monitored and new evaluations on the effects of early intervention including HBV/HCV treatment, especially in occult HBV infection (anti-HBc-seropositive status), on the molecular carcinogenesis of pancreatic cancer are warranted.
Data from epidemiological studies related to the association of hepatitis B virus (HBV) and hepatitis C virus (HCV) and pancreatic cancer risk are inconsistent, with some studies supporting the excess pancreatic cancer with HBV/HCV infection compared to non-infected controls, and some studies showing differently. The aim of this meta-analysis was to clarify the association of chronic hepatitis viruses with the risk of pancreatic cancer.
To date, several studies have assessed the association between the chronic HBV/HCV and pancreatic cancer risk in different ethics; however, the results are inconsistent and inconclusive. No quantitative summary of the evidence has ever been performed.
Based on the meta-analysis, the authors identified that chronic HBV and HCV infection is associated with pancreatic cancer, especially among Chinese population. Early intervention of HBV and HCV infection might decrease pancreatic cancer incidence.
The results support the hypothesis that chronic HBV/HCV infection significantly increases pancreatic cancer risk. Findings of this analysis are comparable with previous studies, and long-term prospective studies. Patients with past exposure to HBV/HCV should be screened for hepatocellular carcinoma and other malignancies, especially pancreatic cancer.
Anti-hepatitis B core antigen (HBc)+/anti-hepatitis B surface antigen (HBs)± are serum biomarkers of possible occult HBV infection. Anti-HBc+/anti-HBs+ is the status of past exposure to HBV with evidence of HBV immunity or recovery, but possibly harboring persistent HBV infection. Anti-HBc+/ anti-HBs- is the status of past exposure to HBV without natural immunity.
The meta-analysis was aimed at assessing the association between HBV/HCV chronic infection and risk of pancreatic cancer. This is an appealing issue, leading to interesting results.
P- Reviewers Bechtold ML, Chan DSY S- Editor Gou SX L- Editor Ma JY E- Editor Ma S
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25175] [Article Influence: 1936.5] [Reference Citation Analysis (3)] |
| 2. | Iloeje UH, Yang HI, Jen CL, Su J, Wang LY, You SL, Lu SN, Chen CJ. Risk of pancreatic cancer in chronic hepatitis B virus infection: data from the REVEAL-HBV cohort study. Liver Int. 2010;30:423-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 17] [Reference Citation Analysis (0)] |
| 3. | El-Serag HB, Engels EA, Landgren O, Chiao E, Henderson L, Amaratunge HC, Giordano TP. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology. 2009;49:116-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 4. | Dehesa-Violante M, Nuñez-Nateras R. Epidemiology of hepatitis virus B and C. Arch Med Res. 2007;38:606-611. [PubMed] [Cited in This Article: ] |
| 5. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [PubMed] [Cited in This Article: ] |
| 6. | World Health Organization. Hepatitis B. Fact sheet no. 2012, 204. Available from: http//www.who.int/mediacentre/factsheets/fs204/en. [Cited in This Article: ] |
| 7. | Hoefs JC, Renner IG, Askhcavai M, Redeker AG. Hepatitis B surface antigen in pancreatic and biliary secretions. Gastroenterology. 1980;79:191-194. [PubMed] [Cited in This Article: ] |
| 8. | Chen MY, Huang ZQ, Chen LZ, Gao YB, Peng RY, Wang DW. Detection of hepatitis C virus NS5 protein and genome in Chinese carcinoma of the extrahepatic bile duct and its significance. World J Gastroenterol. 2000;6:800-804. [PubMed] [Cited in This Article: ] |
| 9. | Berrington de Gonzalez A, Yun JE, Lee SY, Klein AP, Jee SH. Pancreatic cancer and factors associated with the insulin resistance syndrome in the Korean cancer prevention study. Cancer Epidemiol Biomarkers Prev. 2008;17:359-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Hong SG, Kim JH, Lee YS, Yoon E, Lee HJ, Hwang JK, Jung ES, Joo MK, Jung YK, Yeon JE. [The relationship between hepatitis B virus infection and the incidence of pancreatic cancer: a retrospective case-control study]. Korean J Hepatol. 2010;16:49-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Hassan MM, Li D, El-Deeb AS, Wolff RA, Bondy ML, Davila M, Abbruzzese JL. Association between hepatitis B virus and pancreatic cancer. J Clin Oncol. 2008;26:4557-4562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Ben Q, Li Z, Liu C, Cai Q, Yuan Y, Wang K, Xiao L, Gao J, Zhang H. Hepatitis B virus status and risk of pancreatic ductal adenocarcinoma: a case-control study from China. Pancreas. 2012;41:435-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Health Research Institute. Available from: http//www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Cited in This Article: ] |
| 14. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [PubMed] [Cited in This Article: ] |
| 15. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [PubMed] [Cited in This Article: ] |
| 16. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] [Cited in This Article: ] |
| 17. | Zhu F, Li HR, Du GN, Chen JH, Cai SR. Chronic hepatitis B virus infection and pancreatic cancer: a case-control study in southern China. Asian Pac J Cancer Prev. 2011;12:1405-1408. [PubMed] [Cited in This Article: ] |
| 18. | Wang DS, Chen DL, Ren C, Wang ZQ, Qiu MZ, Luo HY, Zhang DS, Wang FH, Li YH, Xu RH. ABO blood group, hepatitis B viral infection and risk of pancreatic cancer. Int J Cancer. 2012;131:461-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Wang DS, Wang ZQ, Zhang L, Qiu MZ, Luo HY, Ren C, Zhang DS, Wang FH, Li YH, Xu RH. Are risk factors associated with outcomes in pancreatic cancer. PLoS One. 2012;7:e41984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Iloeje UH, Yang HI, Chen CJ. Natural history of chronic hepatitis B: what exactly has REVEAL revealed. Liver Int. 2012;32:1333-1341. [PubMed] [Cited in This Article: ] |
| 21. | Sherman M. Pancreatic cancer in chronic hepatitis B. Liver Int. 2010;30:339-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | de Gonzalez AB, Jee SH, Engels EA. No association between hepatitis B and pancreatic cancer in a prospective study in Korea. J Clin Oncol. 2009;27:648; author reply 648-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Malaguarnera M, Gargante MP, Risino C, Ranno S, Berretta M, Cannizzaro MA, Costanzo M, Fricia T, Rampello E, Romano M. Hepatitis C virus in elderly cancer patients. Eur J Intern Med. 2006;17:325-329. [PubMed] [Cited in This Article: ] |
| 24. | Katakura Y, Yotsuyanagi H, Hashizume K, Okuse C, Okuse N, Nishikawa K, Suzuki M, Iino S, Itoh F. Pancreatic involvement in chronic viral hepatitis. World J Gastroenterol. 2005;11:3508-3513. [PubMed] [Cited in This Article: ] |
| 25. | Yoffe B, Bagri AS, Tran T, Dural AT, Shtenberg KM, Khaoustov VI. Hyperlipasemia associated with hepatitis C virus. Dig Dis Sci. 2003;48:1648-1653. [PubMed] [Cited in This Article: ] |
| 26. | Heinemann V, Haas M, Boeck S. Systemic treatment of advanced pancreatic cancer. Cancer Treat Rev. 2012;38:843-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 27. | Hohenberger P. Detection of HBs-Ag in the pancreas in cases of pancreatic carcinoma. Hepatogastroenterology. 1984;31:239-241. [PubMed] [Cited in This Article: ] |
| 28. | Alvares-Da-Silva MR, Francisconi CF, Waechter FL. Acute hepatitis C complicated by pancreatitis: another extrahepatic manifestation of hepatitis C virus. J Viral Hepat. 2000;7:84-86. [PubMed] [Cited in This Article: ] |
| 29. | Dejean A, Lugassy C, Zafrani S, Tiollais P, Brechot C. Detection of hepatitis B virus DNA in pancreas, kidney and skin of two human carriers of the virus. J Gen Virol. 1984;65:651-655. [PubMed] [Cited in This Article: ] |
| 30. | Yan FM, Chen AS, Hao F, Zhao XP, Gu CH, Zhao LB, Yang DL, Hao LJ. Hepatitis C virus may infect extrahepatic tissues in patients with hepatitis C. World J Gastroenterol. 2000;6:805-811. [PubMed] [Cited in This Article: ] |
| 31. | Mason A, Wick M, White H, Perrillo R. Hepatitis B virus replication in diverse cell types during chronic hepatitis B virus infection. Hepatology. 1993;18:781-789. [PubMed] [Cited in This Article: ] |
| 32. | Laskus T, Radkowski M, Wang LF, Vargas H, Rakela J. Search for hepatitis C virus extrahepatic replication sites in patients with acquired immunodeficiency syndrome: specific detection of negative-strand viral RNA in various tissues. Hepatology. 1998;28:1398-1401. [PubMed] [Cited in This Article: ] |
| 33. | Brechot C, Pourcel C, Louise A, Rain B, Tiollais P. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature. 1980;286:533-535. [PubMed] [Cited in This Article: ] |
| 34. | Fiorino S, Lorenzini S, Masetti M, Deleonardi G, Grondona AG, Silvestri T, Chili E, Del Prete P, Bacchi-Reggiani L, Cuppini A. Hepatitis B and C virus infections as possible risk factor for pancreatic adenocarcinoma. Med Hypotheses. 2012;79:678-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |