Published online Mar 28, 2013. doi: 10.3748/wjg.v19.i12.1975
Revised: February 21, 2013
Accepted: February 28, 2013
Published online: March 28, 2013
AIM: To compare the prognostic assessment of lymph node ratio and absolute number based staging system for gastric cancer after D2 resection.
METHODS: The clinical, pathologic, and long-term follow-up data of 427 patients with gastric cancer that underwent D2 curative gastrectomy were retrospectively analyzed. The relationships between the metastatic lymph node ratio (MLR), log odds of positive lymph nodes (LODDS), and positive lymph nodes (pN) staging methods and the long-term prognoses of the patients were compared. In addition, the survival curves, accuracy, and homogeneity were compared with stratification to evaluate the prognostic assessment of the 3 methods when the number of tested lymph nodes was insufficient (< 10 and 10-15).
RESULTS: MLR [hazard ratio (HR) = 1.401, P = 0.012], LODDS (HR = 1.012, P = 0.034), and pN (HR = 1.376, P = 0.005) were independent risk factors for gastric cancer patients. The receiver operating characteristic (ROC) curves showed that the prognostic accuracy of the 3 methods was comparable (P > 0.05). Spearman correlation analysis confirmed that MLR, LODDS, and pN were all positively correlated with the total number of tested lymph nodes. When the number of tested lymph node was < 10, the value of survival curves staged by MLR and LODDS was superior to those of pN staging. However, the difference in survival curves between adjacent stages was not significant. In addition, the survival rate of stage 4 patients using the MLR and LODDS staging methods was 26.7% and 27.3% with < 10 lymph node, respectively which were significantly higher than the survival rate of patients with > 15 tested lymph nodes (< 4%). The ROC curve showed that the accuracy of the prognostic assessment of MLR, LODDS, and pN staging methods was comparable (P > 0.05), and the area under the ROC curve of all 3 methods were increased progressively with the enhanced levels of examined lymph nodes. In addition, the homogeneity of the 3 methods in patients with ≤ 15 tested lymph nodes also showed no significant difference.
CONCLUSION: Neither MLR or LODDS could reduce the staging bias. A sufficient number of tested lymph nodes is key to ensure an accurate prognosis for patients underwent D2 radical gastrectomy.
- Citation: Xu J, Bian YH, Jin X, Cao H. Prognostic assessment of different metastatic lymph node staging methods for gastric cancer after D2 resection. World J Gastroenterol 2013; 19(12): 1975-1983
- URL: https://www.wjgnet.com/1007-9327/full/v19/i12/1975.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i12.1975
Gastric carcinoma is one of the most common cancers in many Asian countries including South Korea and Japan, and the second most common cause of cancer-related death worldwide[1]. There are nearly 470 000 newly diagnosed cases every year in China. Of these cases, approximately 75% of the patients will die, making gastric cancer the third leading cause of cancer deaths[2]. Because of its long-term efficacy, D2 radical gastrectomy has been accepted in most countries, including those in the Europe and the United States, as the standard surgery for gastric cancer[3-5]. The pathological staging of gastric cancer after D2 radical gastrectomy is not only closely related to the long-term survival of patients but is also the main basis to guide subsequent adjuvant therapy. In the currently accepted criteria of postoperative tumor-node-metastasis (TNM) staging of gastric cancer, the staging of regional lymph node metastasis (N) is of great significance. This staging is currently controversial and changes frequently. In both the latest 7th edition of the American Joint Cancer Committee (AJCC)[6] and the 14th edition of the Statute of Gastric Cancer Treatment in Japan[7] in 2010, the absolute number of positive lymph nodes (pN) in the perigastric region was used as the staging basis, and the staging criteria for each stage were unified. Meanwhile, many studies have supported the N staging by computing the metastatic lymph node ratio. Currently, there are 2 main methods in the staging of relative number of positive lymph nodes, the metastatic lymph node ratio (MLR)[8] and the log odds of positive lymph nodes (LODDS)[9]. The former calculates the ratio of the number of pN over the total number of the tested lymph nodes, while the latter calculates the log value, log[(pnod + 0.5)/(tnod - pnod + 0.5)], of the ratio between positive and negative lymph nodes. Previous studies have shown that, especially when the number of the tested lymph nodes was insufficient, the staging of MLR and LODDS could more accurately assess the prognosis of patients with gastric cancer than staging using the absolute value (pN)[9-13]. However, a unified standard of specific staging for relative number of positive lymph nodes is not currently available, and whether this ratio is superior to the pN staging is also unknown[14,15]. Therefore, the clinical data and long-term follow-up results of the gastric cancer patients that received D2 radical gastrectomy were retrospectively analyzed in this study, and the values of the above staging methods for regional lymph node metastasis in assessing patient prognosis were compared.
The clinical data of 427 gastric cancer patients who were admitted and underwent standard D2 radical gastrectomy at Affiliated Renji Hospital, Shanghai Jiaotong University School of Medicine from June 2005 to December 2008 and had complete follow-up data were collected. All patients underwent either distal partial gastrectomy, proximal partial gastrectomy or total gastrectomy with regional lymph nodes dissection to D2 with curative intent by the same gastrointestinal professional operation team. However, due to the defects of pathological examination, the number of examined lymph nodes of most patients (65.1%) failed to reach the 7th edition of AJCC requirement, which recommended at least 16 lymph nodes should be retrieved for adequate staging. The clinical and pathological data are shown in Table 1. All surviving cases were followed for 39-81 mo with a median follow-up time of 55 mo. The last follow-up was on March 11, 2012. The overall survival rate was 52.5% for all patients. The survival rate was 38.9% for the patients with lymph node metastasis and 80.6% for the patients without lymph node metastasis. The overall median survival time was 44 mo.
| Factors | n |
| Gender (male/female) | 281/146 |
| Age ( ≤ 60 yr/> 60 yr) | 200/227 |
| Site (antrum/body/fundus/others) | 234/163/22/8 |
| Size (< 3 cm/3-6 cm/≥ 6 cm) | 36/216/175 |
| Histological grade (well/moderately/poorly) | 11/313/103 |
| Depth of invasion (T1/T2/≥ T3) | 4/79/344 |
| Lymphatic/venous invasion (absence/presence) | 359/68 |
| Perineural invasion (absence/presence) | 396/31 |
| Examined lymph nodes (< 10/10-15/> 15) | 126/152/149 |
Of the 427 patients, those without lymph node metastasis were staged as MLR 0. For the remaining patients, the ratio of the number of pN over the number of tested lymph nodes was calculated, and 20 layers were established from 0 to 1 in 5% intervals. The log-rank test was used to compare differences in the survival curves of 2 adjacent layers. The layers with no differences were merged. Finally, based on prognosis, all patients with lymph node metastases were staged MLR 1-4. Similarly, the patients were staged LODDS 0-4 by the log-rank survival test. The pN staging criteria was defined in accordance to the 2010 AJCC/UICC 7th edition TNM staging criteria. The staging criteria and the number of cases for each group are shown in Table 2.
| Grade | MLR | LODDS | pN | |||
| 0 | Nr = 0 | 139 (32.6) | Nr < -1 | 129 (30.2) | 0 | 139 (32.6) |
| 1 | 0 < Nr ≤ 0.2 | 79 (18.5) | -1 ≤ Nr < -0.5 | 87 (20.4) | 1-2 | 78 (18.3) |
| 2 | 0.2 < Nr ≤ 0.4 | 58 (13.6) | -0.5 ≤ Nr < 0 | 85 (19.9) | 3-6 | 94 (22.0) |
| 3/3a | 0.4 < Nr ≤ 0.7 | 104 (24.4) | 0 ≤ Nr < 0.5 | 76 (17.8) | 7-15 | 86 (20.1) |
| 4/3b | 0.7 < Nr ≤ 1 | 47 (11.0) | Nr ≥ 0.5 | 50 (11.7) | > 15 | 30 (7.0) |
The cumulative survival rate was obtained using a Kaplan-Meier curve, and the differences in cumulative survival rates were compared by the log-rank test. The multivariate prognostic analysis was conducted with the Cox proportional risk regression model. The correlation between MLR, LODDS, and pN, as well as the total number of the tested lymph nodes, was analyzed with the Spearman correlation analysis. The accuracy of the prognosis assessment of each staging method was compared using the receiver operating characteristic curve (ROC) and the area under the curve (AUC). The group in each pN stage was re-grouped in accordance with MLR and LODDS, and the overall survival differences within groups and between groups were analyzed using the log-rank survival test to compare the homogeneity of the 3 staging methods. All statistical analyses were completed with SPSS 17.0 software; P < 0.05 was considered significant.
The results of univariate analysis of the correlation between various prognostic factors related to lymph node status and the prognosis of gastric cancer patients after D2 radical gastrectomy showed that the total number of the tested lymph nodes, MLR, LODDS, and pN staging all had an impact on the patient prognosis (Table 3). When the above factors were individually fitted into the Cox proportional risk model, the results showed that MLR [hazard ratio (HR) = 1.401, P = 0.012], LODDS (HR = 1.012, P = 0.034), and pN (HR = 1.376, P = 0.005) were independent risk factors for the prognosis of patients with gastric cancer.
| Variable | 5-yr survival rate | Log rankχ2value | P value | |
| Examined lymph nodes | ||||
| < 10 | 126 (29.5) | 57.1% | 4.256 | 0.039 |
| 10-15 | 152 (35.6) | 55.9% | ||
| > 15 | 149 (34.9) | 45.0% | ||
| pN | ||||
| 0 | 139 (32.6) | 80.6% | 97.014 | 0.000 |
| 1-2 | 78 (18.3) | 57.7% | ||
| 3-6 | 94 (22.0) | 44.7% | ||
| 7-15 | 86 (20.1) | 27.9% | ||
| > 15 | 30 (7.0) | 3.3% | ||
| MLR | ||||
| Nr = 0 | 139 (32.6) | 80.6% | 103.984 | 0.000 |
| 0 < Nr ≤ 0.2 | 79 (18.5) | 62.0% | ||
| 0.2 < Nr ≤ 0.4 | 58 (13.6) | 50.0% | ||
| 0.4 < Nr ≤ 0.7 | 104 (24.4) | 26.9% | ||
| 0.7 < Nr ≤ 1 | 47 (11.0) | 12.8% | ||
| LODDS | ||||
| Nr < -1 | 129 (30.2) | 80.6% | 96.214 | 0.000 |
| -1 ≤ Nr < -0.5 | 87 (20.4) | 63.2% | ||
| -0.5 ≤ Nr < 0 | 85 (19.9) | 43.5% | ||
| 0 ≤ Nr < 0.5 | 76 (17.8) | 27.6% | ||
| Nr ≥ 0.5 | 50 (11.7) | 14.0% | ||
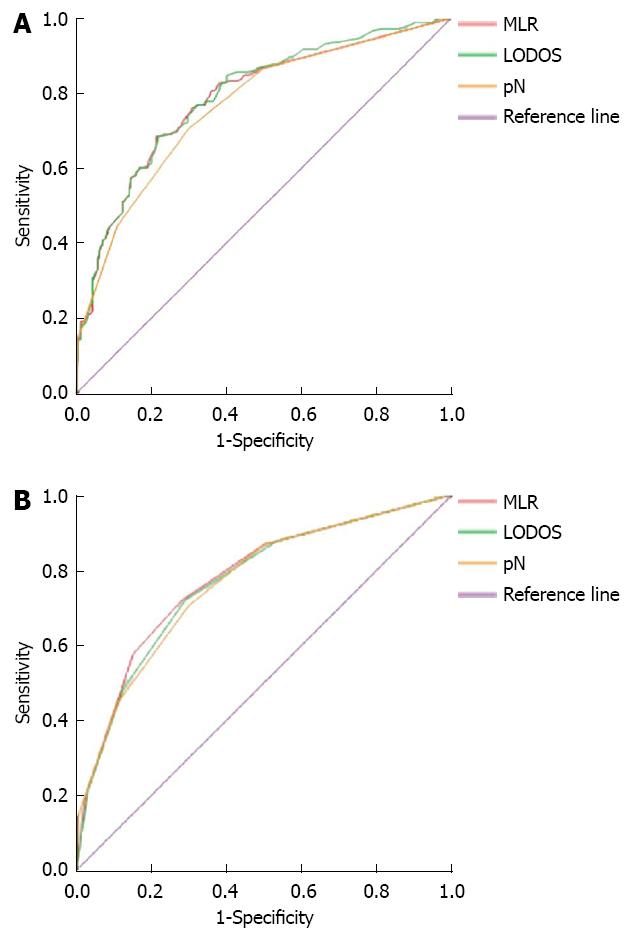
The 5-year survival of the 427 patients after surgery was used as the gold standard to draw the ROC curve to compare the accuracy of the 3 staging methods in the prognostic assessment of gastric cancer patients. In the groups with no staging, the corresponding area under the curve for MLR, LODDS, and pN was 0.784 ± 0.022, 0.790 ± 0.022, and 0.765 ± 0.023 respectively (Figure 1A), with no significant differences (P > 0.05). In the groups with staging, the corresponding areas under the curve for MLR, LODDS, and pN were 0.775 ± 0.023, 0.767 ± 0.023, and 0.765 ± 0.023, respectively (Figure 1B), with no significant differences.
The results of Spearman correlation analysis showed that MLR, LODDS, and pN staging were all positively correlated with the total number of the tested lymph nodes, with a correlation coefficient of 0.177, 0.053, and 0.410, respectively, and all P values were < 0.01, which suggested that all of the 3 staging methods were more or less affected by the total mumber of tested lymph nodes. pN was positively correlated with MLR and LODDS with a correlation coefficient of 0.919 and 0.871, respectively, and the P values were both < 0.001.
Some previous studies have suggested that, for the patients with an insufficient number of tested lymph nodes, the prognosis-assessment value of MLR staging was superior to that of the staging based on absolute number of positive lymph nodes[9-12]. Therefore, all patients were divided into 3 subgroups according to the total number of tested lymph nodes: the number of the tested lymph nodes was < 10 (n = 126), 10-15 (n = 152) or > 15 (n = 149). A comparison was performed to compare the differences in the postoperative survival curve, the prognostic accuracy, and the homogeneity of the 3 staging methods in the patients with < 15 tested lymph nodes.
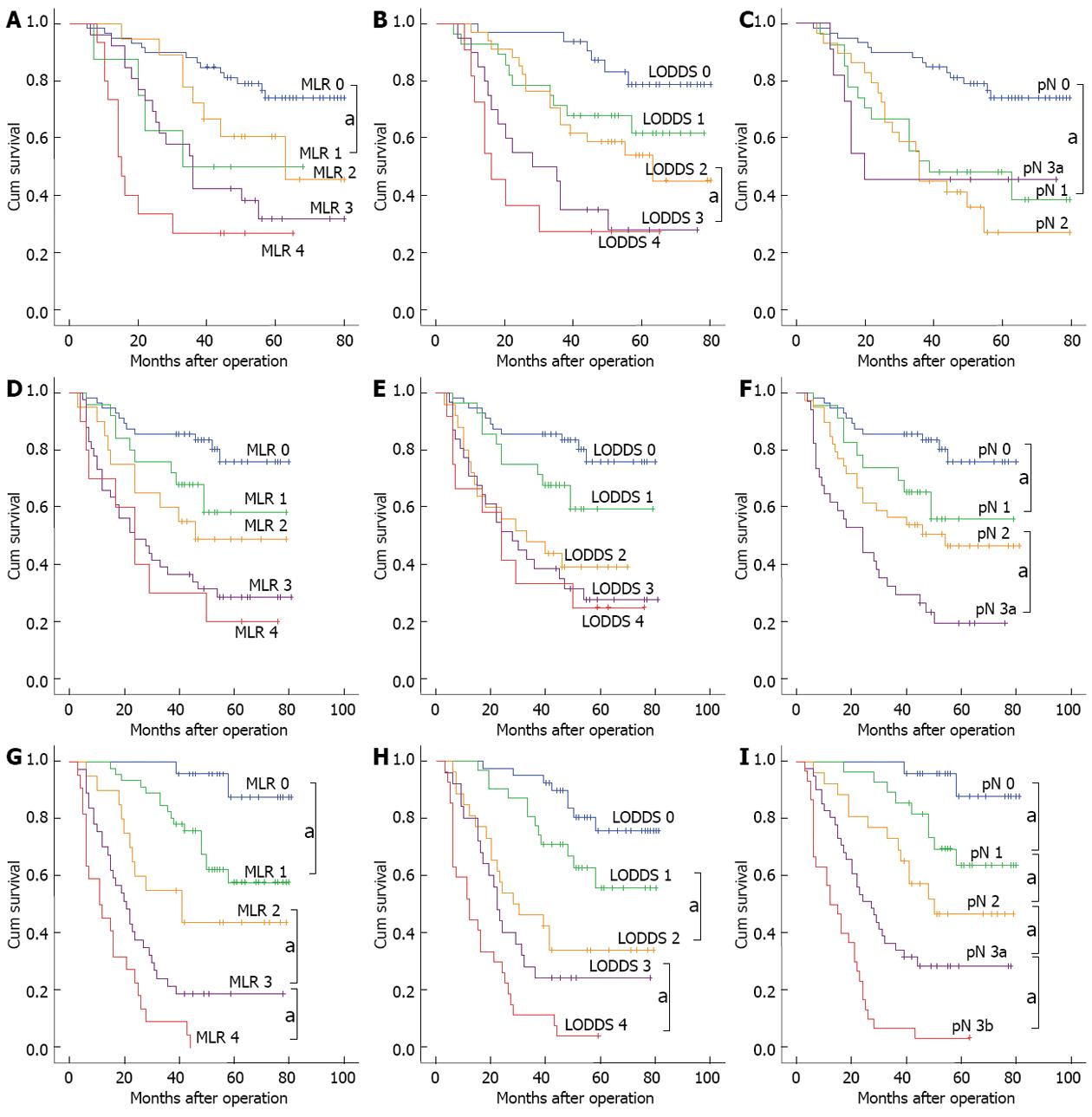
For the patient group with < 10 tested lymph nodes, the 5-year survival rate of patients exhibited a downward trend with the enhanced levels of MLR and LODDS staging. A log-rank test was conducted to compare the difference between adjacent stages, and the results showed that only the difference in survival curves between stage MLR 0 and MLR 1, and stage LODDS 2 and LODDS 3 was significant, with the P values of 0.026 and 0.028 respectively; The difference of remaining survival curves between adjacent stages was not significant. The value of prognostic assessment based on pN staging system was not satisfactory, and the 5-year survival rate for each pN stage was 76.3% for pN 0, 44.4% for pN 1, 34.5% for pN 2, and 45.5% for pN 3a.
For the patient group with 10-15 tested lymph nodes, no significant difference in survival curves between any adjacent stages was found in the subgroup of MLR. Similar with MLR staging, the difference in survival curves also was not significant between any adjacent stages of LODDS.
For the group of patients with > 15 tested lymph nodes, the 5-year survival rates for each stage between MLR, LODDS and were comparable. However, the survival curves of pN staging appeared to better assess prognosis than the ratio-based staging methods, with significant difference in survival curves between any various stages (P < 0.05; Figure 2).
The ROC curves showed that, regardless of staging, the corresponding areas under the curves of the MLR, LODDS, and pN staging methods were all increased progressively with the enhanced levels of examined lymph nodes. the AUC using the MLR, LODDS and pN staging methods increased from 0.716 ± 0.047, 0.718 ± 0.046 and 0.688 ± 0.048 with < 10 lymph node to 0.843 ± 0.031, 0.818 ± 0.034 and 0.836 ± 0.032 with > 15 tested lymph nodes, which were significantly larger than former groups. However, the AUC was not significantly different between the 3 methods within groups.
The various pN groups in which patients had < 10 or 10-15 tested lymph nodes were re-grouped according to MLR staging, and the results were shown in Table 4. When the numbers of retrieved lymph nodes were less than 10, only for patients in stage pN 1, the difference in the 5-year survival rate among different MLR stages was significant (P < 0.05). Furthermore, there was no significant difference in the 5-year survival rate among the different pN groups within the one MLR group. In 10-15 retrieved-node group, there was no significant difference of 5-year survival rates between the different MLR groups in one pN group. In addition, the difference of 5-year survival rates between different pN groups in one MLR group was also not significant.
| MLR 0 | MLR 1 | MLR 2 | MLR 3 | MLR 4 | χ2 | P value | |||||||
| n | 5-YSR | n | 5-YSR | n | 5-YSR | n | 5-YSR | n | 5-YSR | ||||
| < 10 LN | pN 0 | 59 | 76.30% | - | - | - | - | - | - | - | - | - | - |
| pN 1 | - | - | 8 | 50% | 14 | 57.10% | 2 | 0% | 3 | 0% | 22.293 | 0 | |
| pN 2 | - | - | - | - | 4 | 50% | 22 | 31.80% | 3 | 33.30% | 0.51 | 0.775 | |
| pN 3a | - | - | - | - | - | - | 3 | 66.70% | 8 | 37.50% | 0.78 | 0.377 | |
| χ2 | - | - | 0.258 | 9.278 | 1.658 | ||||||||
| P | - | - | 0.611 | 0.098 | 0.437 | ||||||||
| 10-15 LN | pN 0 | 56 | 80.40% | - | - | - | - | - | - | - | - | - | - |
| pN 1 | - | - | 24 | 62.50% | - | - | - | - | - | - | - | - | |
| pN 2 | - | - | 1 | 100% | 20 | 50% | 18 | 44.40% | - | - | 0.755 | 0.686 | |
| pN 3a | - | - | - | - | - | - | 23 | 17.40% | 10 | 20% | 0.068 | 0.794 | |
| χ2 | - | 0.836 | - | 3.613 | - | ||||||||
| P | - | 0.658 | - | 0.057 | - | ||||||||
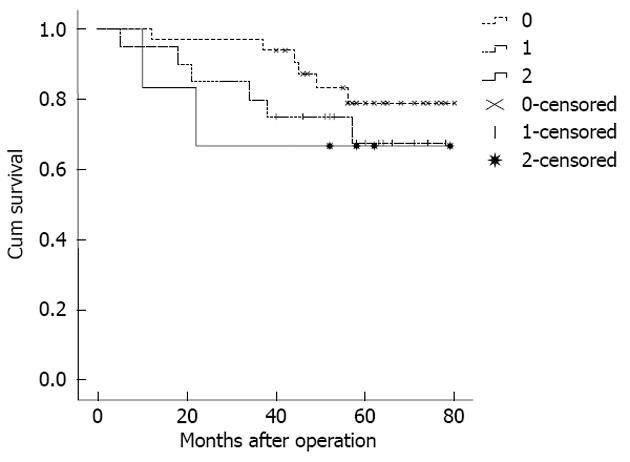
Because the staging of the patients in MLR 0 (no pN detected) was the same as in pN 0, some studies have stated that the prognostic assessment of LODDS staging was more accurate for these patients[9,16]. Therefore, when comparing the homogeneity of pN staging and LODDS staging, according to different numbers of the tested lymph nodes, the stage pN 0 was divided into two layers. The results showed that the pN0 patients with < 10 tested lymph nodes could be further staged LODDS 0-2, and the 5-year survival rate for the 3 stages was 81.8%, 70.0%, and 66.7%, respectively (Figure 3). However, the log-rank survival test showed that the differences between the 3 stages were not significant (P = 0.476). Furthermore, the pN 0 patients with 10-15 tested lymph nodes had the same LODDS stage. Generally, the difference in the 5-year survival rates between the different LODDS groups in one pN group was not significant. In addition, the difference in the 5-year survival rates between the different pN groups in one LODDS group was also not significant (Table 5).
| LODDS 0 | LODDS 1 | LODDS 2 | LODDS 3 | LODDS 4 | χ2 | P value | |||||||
| n | 5-YSR | n | 5-YSR | n | 5-YSR | n | 5-YSR | n | 5-YSR | ||||
| < 10 LN | pN 0 | 33 | 81.80% | 20 | 70% | 6 | 66.7.0% | - | - | - | - | 1.486 | 0.476 |
| pN 1 | - | - | 10 | 50% | 12 | 58.30% | 4 | 0 | 1 | 0 | 22.349 | 0 | |
| pN 2 | - | - | - | - | 14 | 42.90% | 13 | 30.80% | 2 | 0 | 4.202 | 0.122 | |
| pN 3a | - | - | - | - | - | - | 3 | 66.70% | 8 | 37.5% | 0.78 | 0.377 | |
| χ2 | - | 1.44 | 0.969 | 5.689 | 1.083 | ||||||||
| P | - | 0.23 | 0.619 | 0.128 | 0.582 | ||||||||
| 10-15 LN | pN 0 | 56 | 80.40% | - | - | - | - | - | - | - | - | - | - |
| pN 1 | - | - | 24 | 62.50% | - | - | - | - | - | - | - | - | |
| pN 2 | - | - | 4 | 75% | 23 | 43.50% | 12 | 50% | - | - | 1.241 | 0.538 | |
| pN 3a | - | - | - | - | 2 | 0% | 19 | 15.80% | 12 | 25% | 3.413 | 0.182 | |
| χ2 | - | 0.222 | 6.785 | 3.614 | - | ||||||||
| P | - | 0.638 | 0 | 0.057 | - | ||||||||
Due to the decent long-term survival rate, surgical resection, represented by standard D2 radical gastrectomy, is currently the preferred treatment for gastric cancer. However, in recent years, with the rise of the concept of individualized treatment and the application of new adjunct treatment in clinical practice, an accurate prognostic assessment of patients with gastric cancer after surgery is essential to the development of relevant follow-up treatment strategies[17,18]. Currently, the postoperative pathological TNM staging is accepted and widely applied as the prognostic evaluation indicator in clinical practice. With regard to the N portion of the TNM staging, there has been considerable controversy ranging from earlier staging based on anatomical sites of metastatic lymph nodes[19] to the specific staging criteria based on the number of regional metastatic lymph nodes[20,21]. The N staging criteria were not unified until the 7th edition of the AJCC[4] and the 14th edition of the Statute of Gastric Cancer Treatment in Japan[5] unified the criteria for the first time in 2010. However, many researchers still believe that when the staging is based on the absolute number of metastatic lymph nodes, the number of pN is easily influenced by the numbers of removed and tested lymph nodes. When the number of tested lymph nodes is insufficient, staging bias may occur, affecting the accuracy of the prognostic assessment[22,23]. The N staging based on MLR can overcome the above shortcomings[24,25]. Therefore, when comparing the prognostic assessment of different lymph node metastasis staging methods in gastric cancer patients after D2 radical gastrectomy, this study focused on the impact of the 3 staging methods on long-term survival rate when the number of pathologically tested lymph nodes after surgery was insufficient.
To date, neither MLR nor LODDS staging has accurate and widely accepted criteria; therefore, the log-rank survival test was first conducted to verify the staging criteria of MLR and LODDS (Table 2). The 5-year survival rates of various stages according to the above criteria were similar to those of the corresponding pN stages (TNM staging criteria in the 7th edition of AJCC/UICC). The correlation analysis of the 3 staging methods also showed that MLR and LODDS were significantly positively correlated with pN. The ROC curves also showed that the accuracy of prognosis assessment of the 3 staging methods in gastric cancer patients was not significantly different. The subsequent univariate and multivariate analyses both showed that the MLR, LODDS, and pN staging methods were all closely related to patient prognosis-they were all independent risk factors for the prognoses of gastric cancer patients. The above results suggest that MLR, LODDS, and pN staging methods can all be used for the prognostic assessment of gastric cancer, and the assessment efficacies of the 3 methods were similar.
Although the total number of tested lymph nodes in the Cox proportional risk regression model was not a significant independent risk factor for patient prognosis, univariate analysis showed that as the number of tested lymph nodes increased, the 5-year survival rate of patients exhibited a downward trend (P = 0.039); moreover, a correlation analysis showed that MLR, LODDS, and pN were all positively correlated with the number of tested lymph nodes. When only the correlation coefficient of the number of tested lymph nodes was considered (pN > MLR > LODDS), the impact of the number of tested lymph nodes on the MLR and LODDS was smaller than that of the absolute number of pN, which suggests that compared with pN staging system, the MLR and LODDS were less affected by the total mumber of tested lymph nodes. The subsequent results of the survival curve of patients with insufficient tested lymph nodes also showed that, when the number of tested lymph node was < 10, the MLR and LODDS staging methods appeared to better assess prognosis than pN staging. However, as shown in Figure 2, although the MLR and LODDS staging methods could more accurately assess the 5-year postoperative survival rate of gastric cancer patients at the early-middle stages (stage 0-2), the difference in the prognostic assessment of the patients at middle-late stages (stage 3 and 4) was not significant. The main reason was that, although the ratio could reduce the impact of sampling error compared with the absolute number, as the number of pN increased, the impact of the sampling error due to the insufficient number of tested lymph nodes also increased. Therefore, the difference in the prognoses of patients in the middle-late stages was not significant. In addition, for the patients in the middle-late stages of MLR and LODDS, especially those in stage 4, the 5-year survival rates were all ≥ 20%, which was significantly higher than that of the patients with > 15 (< 4%) tested lymph nodes. The reason for this result may be that when the total number of tested lymph nodes was insufficient, the sampling sites were too concentrated near the lesion; therefore, the ratio of pN was higher, resulting in an overestimation of the actual pathological staging of patients. Moreover, the comparison of the 5-year survival rate of the 3 methods in patients with ≤ 15 tested lymph nodes also confirmed that the accuracy and homogeneity of the staging methods based on the MLR, LODDS or the absolute number of pN were similar, with no significant difference. At the same time, the comparison of the survival curve and ROC curve of the 3 staging methods in the < 10, 10-15 and > 15 group showed that the difference in the 5-year survival rate between stages and the assessment accuracy of survival rate were all increased progressively with the enhanced levels of examined lymph nodes, and the 3 staging methods exhibited no significant difference. The above results all confirmed that, regardless of the staging method, a sufficient number of tested lymph nodes was the key factor. When the number of tested lymph nodes was ≤ 15, the staging based on MLR or LODDS could not compensate for the inadequacy of pN staging, and thus could not accurately assess patient prognosis.
Although the number of cases did not affect the results of the statistical analysis significantly, it could be observed from the survival curve that the LODDS staging method appeared to better assess prognosis for patients at MLR and pN stage 0 with an insufficient number of tested lymph nodes. However, the advantage of the LODDS staging method was only apparent when the number of tested lymph nodes was < 10. Additionally, because the 5-year survival rate for patients in stage pN0 was relatively high, the survival rates of patients in various LODDS stages were not significantly different after re-staging. Finally, the calculation method of LODDS was complicated. All of the above factors limited the practical application of LODDS staging method, and the practical value was low.
Some studies have found that the staging methods based on the MLR and LODDS could more accurately predict the prognosis of gastric cancer patients than the staging method based on the absolute number (pN), especially when the number of tested lymph nodes was insufficient[9-13]. The above conclusions in this study appeared to be inconsistent with those previous findings. Different surgical methods may be the main cause of the contradictory findings[26]. In a study recently published in Annals of Surgery in 2012[13], the postoperative clinical, pathologic and follow-up data of 18 043 gastric cancer patients retrieved from the Surveillance, Epidemiology, and End Results database of United States were retrospectively analyzed, and the results showed that when the number of tested lymph nodes was insufficient, the MLR staging method could more accurately assess the patient prognosis than the pN staging method. However, only 10% of the patients in this study underwent D2 radical gastrectomy, and the scope of lymph node removal in the remaining patients was D1 or below. The insufficient number of tested lymph nodes was mainly limited by the scope of lymph node removal. Some studies have confirmed that the average number of removed lymph nodes during D2 radical gastrectomy could reach 32[27]. The smaller the number of tested lymph nodes, the greater the sampling error. A sufficient number of tested lymph nodes is key to reducing sampling error. Therefore, to accurately assess the prognosis of patients after D2 radical gastrectomy, no staging method can replace a sufficient number of tested lymph nodes.
In summary, the MLR, LODDS and pN are all independent risk factors for the long-term postoperative survival of gastric cancer patients. The accuracy of the prognostic assessment of the MLR and LODDS staging methods is comparable to that of the pN staging method in gastric cancer patients. However, for the patients that undergo a D2 radical gastrectomy, when the number of tested lymph nodes is insufficient (≤ 15), neither the staging method based on metastatic lymph node ratio nor the pN staging method can avoid staging bias. Therefore, as D2 radical gastrectomy is increasingly accepted, a sufficient number of tested lymph nodes is the only key to ensure an accurate prediction of gastric cancer patient prognosis.
Gastric cancer is one of the leading fatal malignancies worldwide. D2 radical gastrectomy has been accepted in most countries as the standard surgery for gastric cancer. In the currently widely applied criteria of postoperative tumor-node-metastasis staging system, the staging of regional lymph node metastasis (N) is of great significance for accurate prognosis-assessment.
Currently, the metastatic lymph node ratio has been considered as an alternative to the absolute number of positive lymph nodes. Although the 7th edition of the American Joint Cancer Committee and the 14th edition of the Statute of Gastric Cancer Treatment in Japan unified the pN staging criteria for the first time in 2010, many researchers still believe that the prognostic assessment of ratio staging was superior to that of the staging based on absolute number of positive lymph nodes, which demands the examination of at least 15 lymph nodes.
The clinical, pathologic, and long-term follow-up data of 427 patients underwent D2 radical gastrectomy were stratified and compared to evaluate the prognostic assessment of the 3 metastatic lymph node staging methods. The findings from this study suggested that neither metastatic lymph node ratio nor log odds of positive lymph nodes could avoid the staging bias due to the insufficient number of tested lymph nodes for patients underwent D2 radical gastrectomy.
This study compared three lymph node based N staging systems for gastric cancer patients with radical resection and D2 lymphadenectomy, and then demonstrated that a sufficient number of tested lymph nodes was key to ensure an accurate prognosis-assessment. The results are clinical significance in the prognostic assessment of patients with gastric cancer after surgery.
This is a retrospective research of 427 gastric cancer patients undergoing radical resection plus D2 lymphadenectomy, with a median follow-up of 55 mo. The authors have analyzed patient outcomes in considerable depth, their data is well characterized. They provide an in depth analysis of factors contributing to survival and have utilized multivariate analysis in doing this. The information in the manuscript is highly relevant and useful.
P- Reviewer Szczepanik AM S- Editor Wen LL L- Editor A E- Editor Lu YJ
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7953] [Cited by in F6Publishing: 8022] [Article Influence: 534.8] [Reference Citation Analysis (2)] |
| 2. | Peng J, Wang Y. Epidemiology, pathology and clinical management of multiple gastric cancers: a mini-review. Surg Oncol. 2010;19:e110-e114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1140] [Cited by in F6Publishing: 1280] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 4. | Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 698] [Cited by in F6Publishing: 720] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 5. | Degiuli M, Sasako M, Calgaro M, Garino M, Rebecchi F, Mineccia M, Scaglione D, Andreone D, Ponti A, Calvo F. Morbidity and mortality after D1 and D2 gastrectomy for cancer: interim analysis of the Italian Gastric Cancer Study Group (IGCSG) randomised surgical trial. Eur J Surg Oncol. 2004;30:303-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 206] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 6. | Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumors. 7th ed. Oxford: Wiley-Blackwell 2009; . [Cited in This Article: ] |
| 7. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. 14th ed. Tokyo: Kanehara and Co. Ltd 2010; . [Cited in This Article: ] |
| 8. | Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol. 2002;9:775-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 203] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 9. | Sun Z, Xu Y, Li de M, Wang ZN, Zhu GL, Huang BJ, Li K, Xu HM. Log odds of positive lymph nodes: a novel prognostic indicator superior to the number-based and the ratio-based N category for gastric cancer patients with R0 resection. Cancer. 2010;116:2571-2580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Sun Z, Zhu GL, Lu C, Guo PT, Huang BJ, Li K, Xu Y, Li DM, Wang ZN, Xu HM. The impact of N-ratio in minimizing stage migration phenomenon in gastric cancer patients with insufficient number or level of lymph node retrieved: results from a Chinese mono-institutional study in 2159 patients. Ann Oncol. 2009;20:897-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Inoue K, Nakane Y, Iiyama H, Sato M, Kanbara T, Nakai K, Okumura S, Yamamichi K, Hioki K. The superiority of ratio-based lymph node staging in gastric carcinoma. Ann Surg Oncol. 2002;9:27-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Lee SR, Kim HO, Son BH, Shin JH, Yoo CH. Prognostic significance of the metastatic lymph node ratio in patients with gastric cancer. World J Surg. 2012;36:1096-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Wang J, Dang P, Raut CP, Pandalai PK, Maduekwe UN, Rattner DW, Lauwers GY, Yoon SS. Comparison of a lymph node ratio-based staging system with the 7th AJCC system for gastric cancer: analysis of 18,043 patients from the SEER database. Ann Surg. 2012;255:478-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 14. | Espín F, Bianchi A, Llorca S, Feliu J, Palomera E, García O, Remon J, Suñol X. Metastatic lymph node ratio versus number of metastatic lymph nodes as a prognostic factor in gastric cancer. Eur J Surg Oncol. 2012;38:497-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Xiao LB, Yu JX, Wu WH, Xu FF, Yang SB. Superiority of metastatic lymph node ratio to the 7th edition UICC N staging in gastric cancer. World J Gastroenterol. 2011;17:5123-5130. [PubMed] [Cited in This Article: ] |
| 16. | Qiu MZ, Qiu HJ, Wang ZQ, Ren C, Wang DS, Zhang DS, Luo HY, Li YH, Xu RH. The tumor-log odds of positive lymph nodes-metastasis staging system, a promising new staging system for gastric cancer after D2 resection in China. PLoS One. 2012;7:e31736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 576] [Cited by in F6Publishing: 607] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 18. | Ohtsu A. Chemotherapy for metastatic gastric cancer: past, present, and future. J Gastroenterol. 2008;43:256-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Karpeh MS, Leon L, Klimstra D, Brennan MF. Lymph node staging in gastric cancer: is location more important than Number? An analysis of 1,038 patients. Ann Surg. 2000;232:362-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 268] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 20. | Katai H, Yoshimura K, Maruyama K, Sasako M, Sano T. Evaluation of the New International Union Against Cancer TNM staging for gastric carcinoma. Cancer. 2000;88:1796-1800. [PubMed] [Cited in This Article: ] |
| 21. | Rausei S, Dionigi G, Boni L, Rovera F, Dionigi R. How does the 7th TNM edition fit in gastric cancer management? Ann Surg Oncol. 2011;18:1219-1221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Kulig J, Sierzega M, Kolodziejczyk P, Popiela T. Ratio of metastatic to resected lymph nodes for prediction of survival in patients with inadequately staged gastric cancer. Br J Surg. 2009;96:910-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Lemmens VE, Dassen AE, van der Wurff AA, Coebergh JW, Bosscha K. Lymph node examination among patients with gastric cancer: variation between departments of pathology and prognostic impact of lymph node ratio. Eur J Surg Oncol. 2011;37:488-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Kim CY, Yang DH. Adjustment of N stages of gastric cancer by the ratio between the metastatic and examined lymph nodes. Ann Surg Oncol. 2009;16:1868-1874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Celen O, Yildirim E, Berberoglu U. Prognostic impact of positive lymph node ratio in gastric carcinoma. J Surg Oncol. 2007;96:95-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Maduekwe UN, Lauwers GY, Fernandez-Del-Castillo C, Berger DL, Ferguson CM, Rattner DW, Yoon SS. New metastatic lymph node ratio system reduces stage migration in patients undergoing D1 lymphadenectomy for gastric adenocarcinoma. Ann Surg Oncol. 2010;17:1267-1277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Ahn HS, Lee HJ, Hahn S, Kim WH, Lee KU, Sano T, Edge SB, Yang HK. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. 2010;116:5592-5598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |