Published online Dec 28, 2012. doi: 10.3748/wjg.v18.i48.7271
Revised: October 30, 2012
Accepted: November 14, 2012
Published online: December 28, 2012
AIM: To investigate the impact of intestinal ischemia/reperfusion (I/R) injury and lymph drainage on distant organs in rats.
METHODS: Thirty-two Sprague-Dawley male rats, weighing 280-320 g, were randomly divided into blank, sham, I/R, and ischemia/reperfusion and drainage (I/R + D) groups (n = 8). All rats were subjected to 60 min ischemia by clamping the superior mesenteric artery, followed by 120 min reperfusion. The rats in the I/R + D group received intestinal lymph drainage for 180 min. In the sham group, the abdominal cavity was opened for 180 min, but the rats received no treatment. The blank group served as a normal and untreated control. A chromogenic limulus assay kit was used for quantitative detection of serum endotoxin. The serum concentrations of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-1β, soluble cell adhesion molecules (sICAM-1), and high mobility group protein box 1 (HMGB1) were determined with an enzyme-linked immunosorbent assay kit. Histological evaluations of the intestine, liver, kidney, and lung were performed by hematoxylin and eosin staining and immunohistochemistry. HMGB1 protein expression was assayed by western blot analysis.
RESULTS: The serum levels of endotoxin and HMGB1 in the I/R and I/R + D groups were significantly higher than those in the sham group (endotoxin, I/R and I/R + D vs sham: 0.033 ± 0.004 EU/mL, 0.024 ± 0.003 EU/mL vs 0.017 ± 0.009 EU/mL, respectively, P < 0.05; HMGB1, I/R and I/R + D vs sham: 5.473 ± 0.963 EU/mL, 4.906 ± 0.552 EU/mL vs 0.476 ± 0.406 EU/mL, respectively, P < 0.05). In addition, endotoxin and HMGB1 were significantly lower in the I/R + D group compared to the I/R group (P < 0.05). The serum inflammatory factors IL-6, IL-1β, and sICAM-1 in the I/R and I/R + D groups were significantly higher than those in the sham group (IL-6, I/R and I/R + D vs sham: 41.773 ± 9.753 pg/mL, 19.204 ± 4.136 pg/mL vs 11.566 ± 2.973 pg/mL, respectively, P < 0.05; IL-1β, I/R and I/R + D vs sham: 144.646 ± 29.378 pg/mL, 65.829 ± 10.888 pg/mL vs 38.178 ± 7.157 pg/mL, respectively, P < 0.05; sICAM-1, I/R and I/R + D vs sham: 97.360 ± 12.714 ng/mL, 48.401 ± 6.547 ng/mL vs 33.073 ± 5.957 ng/mL, respectively; P < 0.05). The serum TNF-α in the I/R group were significantly higher than in the sham group (45.863 ± 11.553 pg/mL vs 18.863 ± 6.679 pg/mL, respectively, P < 0.05). These factors were significantly lower in the I/R + D group compared to the I/R group (P < 0.05). The HMGB1 immunohistochemical staining results showed no staining or apparent injury in the blank group, and slight staining at the top of the microvillus was detected in the sham group. In the I/R group, both the top of villi and the basement membrane were stained for HMGB1 in most areas, and injury in the I/R + D group was less than that in the I/R group. HMGB1 expression in the liver, kidney, and lung of rats in the I/R + D group was significantly lower than the rats in the I/R group (P < 0.05).
CONCLUSION: Lymph drainage could block the “gut-lymph” pathway, improve intestinal barrier function, and attenuate distant organ injury incurred by intestinal I/R.
- Citation: He GZ, Zhou KG, Zhang R, Wang YK, Chen XF. Impact of intestinal ischemia/reperfusion and lymph drainage on distant organs in rats. World J Gastroenterol 2012; 18(48): 7271-7278
- URL: https://www.wjgnet.com/1007-9327/full/v18/i48/7271.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i48.7271
The gut is an important functional organ for the immune and endocrine systems, as well as its role as a protective barrier. Intestinal ischemia/reperfusion (I/R) injury is the “motor” of systemic inflammatory response syndrome (SIRS), acute respiratory distress syndrome (ARDS), and multiple organ dysfunction syndrome (MODS)[1-3]. Severe trauma, acute necrotizing pancreatitis, major surgery, extensive burns, and other stresses are all associated with intestinal barrier dysfunction. Although extensive investigative efforts have focused on clarifying the pathogenesis of SIRS, ARDS and MODS induced by intestinal I/R, the specific mechanism still remains controversial[4].
Recently, numerous studies have shown that the transport of inflammatory mediators occurs through the intestinal lymphatics in trauma-hemorrhage shock (T/HS). Deitch et al[5] demonstrated that toxic gut-derived substances enter the mesenteric lymph to cause lung injury. In addition, ligating the lymph duct in a variety of species after hemorrhagic shock can prevent distant organ injury[6]. Damle et al[7], Watkins et al[8] and Jordan et al[9] have shown that lymph is the key link between T/HS and MODS. The production and release of inflammatory factors through the “gut-lymph” pathway to the circulatory system can cause acute lung injury (ALI) and a systemic inflammation state[10-13]. Our recent work, together with findings by other investigators, suggests that thoracic/mesenteric lymphatic duct ligation prior to intestinal I/R injury protects the lung from injury and modulates the serum levels of endotoxin, D-lactate, diamine oxidase, and cytokines[14,15]. However, the composition of lymph that is responsible for distant organ injury remains unknown.
Some experiments have demonstrated that T/HS lymph is sterile and does not contain measurable levels of endotoxin[16], but does contain some biologically active non-microbial protein and lipid species[17,18]. Recent studies proposed that mesenteric lymph-induced distant organ injury in intestinal I/R was directly mediated by gut-derived endogenous ligands, one of which is the high mobility group protein box 1 (HMGB1). In intestinal I/R injury, intestinal epithelium cells and macrophages synthesize and release toll-like receptor 4 (TLR4) endogenous ligands, which can be recognized by and combine with TLR4[19]. HMGB1 is also the key factor of inflammation of aseptic injury (including T/HS and liver I/R injury)[20,21]. In liver I/R injury, HMGB1 can directly combine with TLR2 or TLR4 and cause inflammation[22]. The binding of HMGB1 and TLR4 may also be the most important trigger of the inflammatory response to I/R injury in the heart, kidney, brain, lung, and other organs[23-26]. Recent studies suggest that HMGB1 acts as a late mediator of lethal sepsis and an early mediator of inflammation and necrosis following I/R injury[19,27,28].
This study is a continuation of our previous study. We hypothesized that the HMGB1-TLR4 combination plays an important role in the distant organ injury caused by intestinal I/R injury. The purpose of this study was to determine the impact of intestinal I/R injury and lymph drainage on the intestine and distant organs in rats, and to clarify whether HMGB1 in the intestinal mesenteric lymph after I/R mediates distant organ injury.
Thirty-two male Sprague-Dawley rats that were specific pathogen-free grade and weighed 280-320 g were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. The rats were housed under barrier-sustained conditions at a temperature of 25 °C with 12 h light/dark cycles, and had free access to water and food for five days prior to the operation. The rats were randomly divided into four groups: blank, sham, ischemia/reperfusion and drainage (I/R + D), and I/R (n = 8 for each group). All rats were maintained in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals. The research protocols were approved by the Academic Committee of Peking Union Medical College and Chinese Academy of Medical Sciences.
Prior to the operation, all rats were fasted overnight, but were allowed access to water ad libitum. The rats were anesthetized with an intraperitoneal injection of 1% sodium pentobarbital (50 mg/kg). A midline incision was performed to bluntly separate the superior mesenteric artery (SMA) and intestinal lymphatic trunk. In the I/R and I/R + D groups, the SMA was occluded for 60 min with an artery clamp, followed by reperfusion for 120 min. In the I/R + D group, a small incision was made on the proximal end of the intestinal lymphatic trunk. A catheter (Jinan Medical Silicone Tube Plant, China) was inserted into the incision obliquely 3-5 mm toward the distal end. A small amount of medical adhesive (Beijing Fuaile Science and Technology Development Co. Ltd, China) was used on the serosa adjacent to the right kidney to fix the catheter. Outflow of lymph from the catheter was collected with a sterile test-tube (Nunc, Denmark) for 180 min. In the sham group, the abdominal cavity was opened for 180 min but the rats received no treatment. The blank group served as a normal and untreated control. The lymph (0.6-1.2 mL per rat) was collected for 180 min.
After the operation, the catheter was removed. Blood was then extracted from the inferior vena cava and centrifuged at 3000 g for 15 min at 4 °C; the serum was separated and stored at -80 °C for further analysis. After the rats were fully exsanguinated, a 3 cm proximal section of the jejunum and 3 cm distal section of the ileum were excised, rinsed in ice-cold normal saline, and dried on filter paper. The liver, kidney, and lung were stored at -80 °C.
Measurement of endotoxin: A chromogenic limulus assay kit (Yi Hua Medical Technology Co. Ltd, Shanghai, China) was used for the quantitative detection of serum endotoxin, and the assay was performed according to the manufacturer’s directions.
Cytokines and HMGB1 assay: The serum concentrations of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-1β, soluble cell adhesion molecules (sICAM-1), and HMGB1 were determined using enzyme-linked immunosorbent assay kits (Sun Biomedical Technology Co., Ltd., Beijing, China) according to the kit protocols.
Hematoxylin and eosin staining of rats tissue slices: Samples of the intestine, liver, kidney, and lung were fixed in 10% formalin solution and sectioned (4 mm) after dehydration, cleaning, and paraffin embedding. The sections were flattened, mounted, and heated on blank glass slides. Histological evaluations were performed by hematoxylin and eosin staining and pathological examination.
HMGB1 immunohistochemistry: The slices of intestine, liver, kidney, and lung embedded in paraffin were used for histological examination. A mouse anti-HMGB1 primary antibody (Beijing Biosynthesis Biotechnology Co., Ltd., China) and biotinylated secondary antibody (Beijing Biosynthesis Biotechnology Co., Ltd., China) were used for immunohistochemical staining. Brownish-yellow stained areas were recognized as regions with positive antigen expression.
HMGB1 protein expression-Western blotting analysis: Total protein extract was prepared, and samples were separated using sodium dodecyl sulfate polyacrylamide gels. Proteins were then transferred to nitrocellulose membranes overnight at room temperature and blocked for 8 h with 5% bovine serum albumin. The membranes were then incubated overnight in anti-HMGB1 primary antibody (1 μg/mL, ABCAM Ltd, Cambridge, United Kingdom) diluted in blocking solution (1:500, Beijing Biosynthesis Biotechnology Co., Ltd., China). Membranes were washed in Tris-buffered saline with Tween and incubated in horseradish peroxidase-conjugated mouse secondary antibodies in 5% milk (1:3000, Santa Cruz Inc., United States) for 1 h at room temperature. Protein bands were visualized by chemiluminescence.
Quantitative data were presented as mean ± SD. Statistical software SPSS l7.0 (SPSS, Inc., Chicago, IL, United States) was used to test the homogeneity of variance. Multiple comparisons were performed with one-way analysis of variance followed by a least-significant difference test. Statistical significance was set at P < 0.05.
The serum levels of HMGB1 and endotoxin in the I/R and I/R + D groups were significantly higher than those in the blank and sham groups (P < 0.05). In addition, the level of endotoxin in the I/R group was higher than that in the I/R + D group (P < 0.05). The level of HMGB1 in the I/R group was slightly higher than that in the I/R + D group, but the difference was not significant (P > 0.05) (Table 1).
The levels of inflammatory cytokines IL-6, IL-1β and sICAM-1 in the I/R and I/R + D groups and TNF-α in the I/R group were remarkably higher than those in the blank and sham groups (P < 0.05). The levels of inflammatory factors in the I/R + D group were markedly lower compared to those in the I/R group (P < 0.05). There was no significant difference in cytokine levels between the blank and sham groups (P > 0.05) (Table 2).
| Groups | TNF-α(pg/mL) | IL-1β(pg/mL) | IL-6 (pg/mL) | sICAM-1 (ng/mL) |
| Blank | 13.799 ± 6.456 | 22.476 ± 8.498 | 8.687 ± 0.761 | 30.901 ± 6.962 |
| Sham | 18.863 ± 6.679 | 38.178 ± 7.157 | 11.566 ± 2.973 | 33.073 ± 5.957 |
| I/R + D | 25.381 ± 9.281ae | 65.829 ± 10.888ace | 19.204 ± 4.136ace | 48.401 ± 6.547ace |
| I/R | 45.863 ± 11.553ac | 144.646 ± 29.378ac | 41.773 ± 9.753ac | 97.360 ± 12.714ac |
Intestinal morphology showed little change in the sham group compared to the blank group. In contrast, the jejunum and ileum mucosa in the I/R group showed swelling and atrophy, and appeared fragile and black in color in some segments of the intestine. In the I/R + D group, the intestinal mucosa showed slight swelling, no breakage, and less apparent damage than the I/R group.
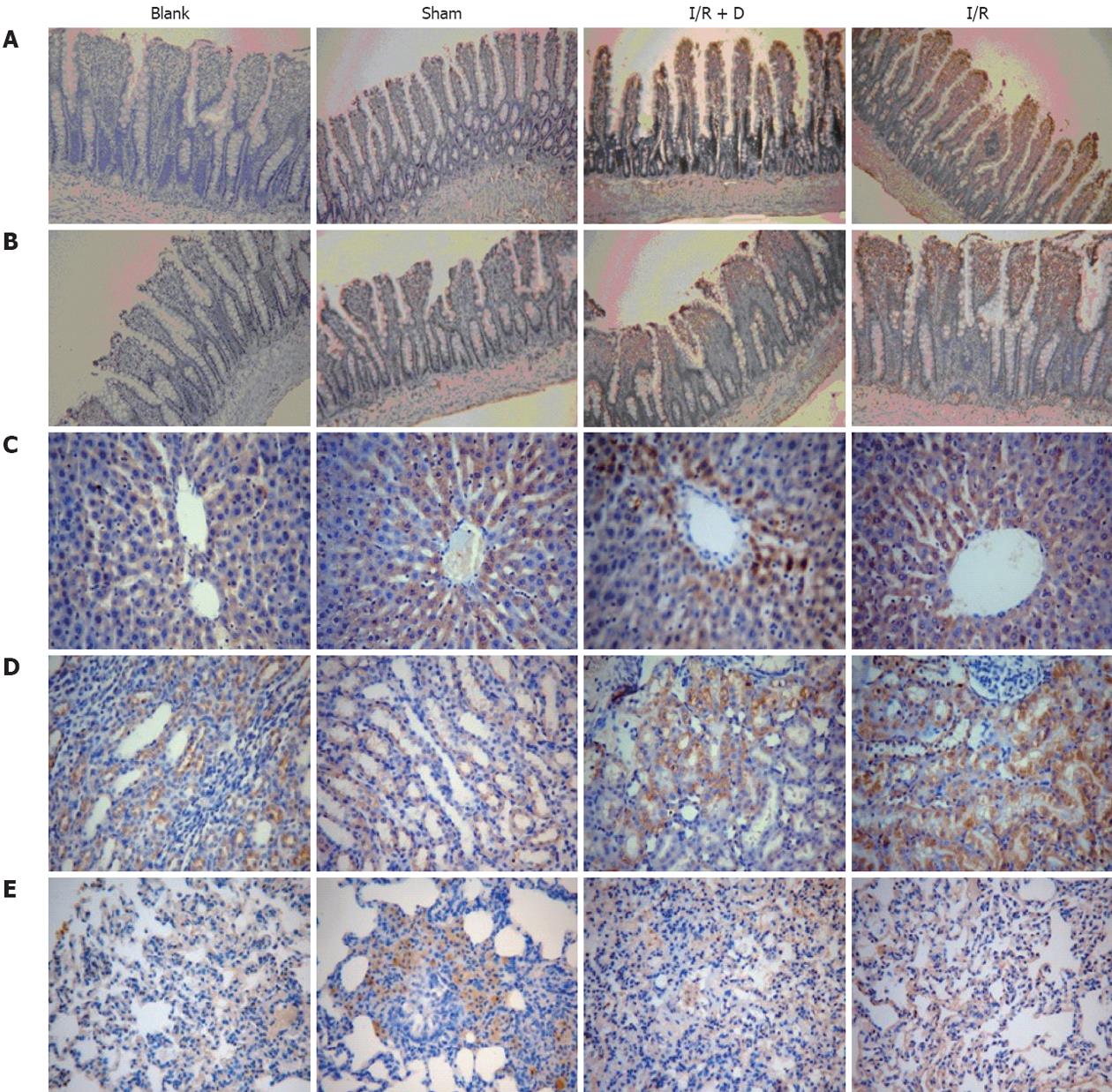
Analysis of the jejunum and ileum in the blank group confirmed that there was no HMGB1 staining or apparent injury. A small amount of staining at the top of the microvillus was detected in the sham group, indicating slight injury. In the I/R group, both the top of villi and the basement membrane were stained for HMGB1 in most areas. Injury in the I/R + D group was less than that in the I/R group, although we did find some areas showing positive staining (Figure 1A and B).
There was a significant level of HMGB1 staining in the liver of I/R group, while only a few liver cells were stained in the I/R + D group. HMGB1 staining in the medullary region and the outer medulla of the kidney were obviously increased in the I/R group compared to controls. Analysis of HMGB1 expression in the lung showed that a large number of cells, including endothelial cells and macrophages, were positively stained. Immunohistochemistry in the blank and sham groups showed almost no yellow staining in the liver, kidney, or lung (Figure 1C-E).
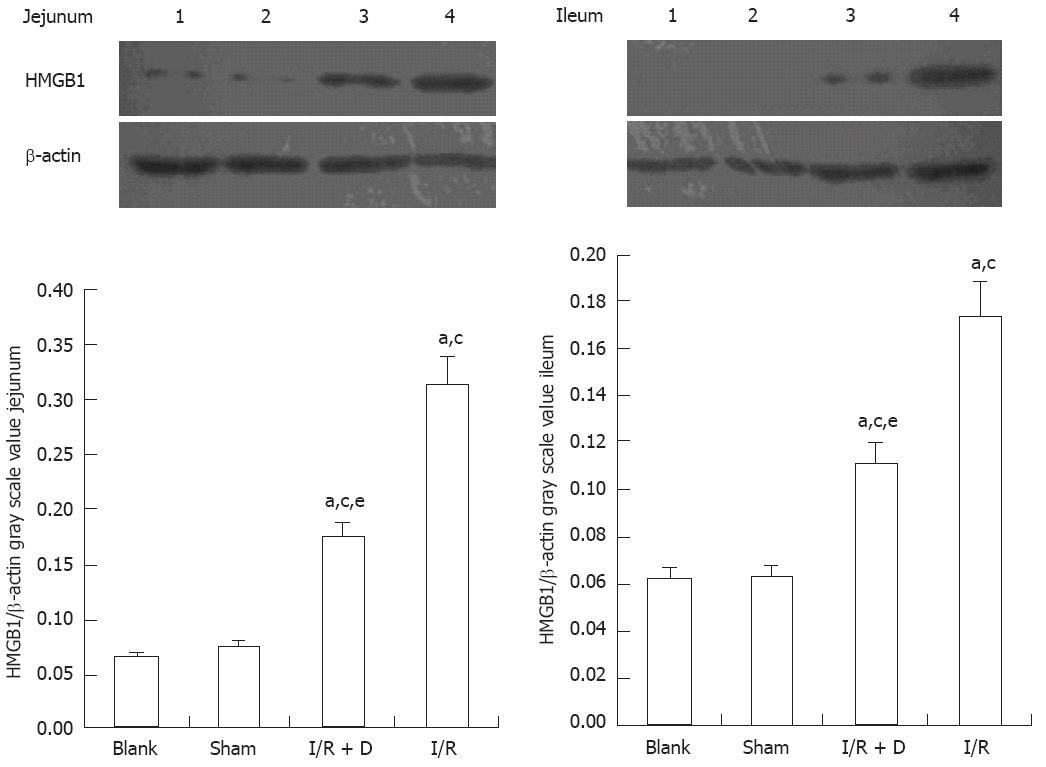
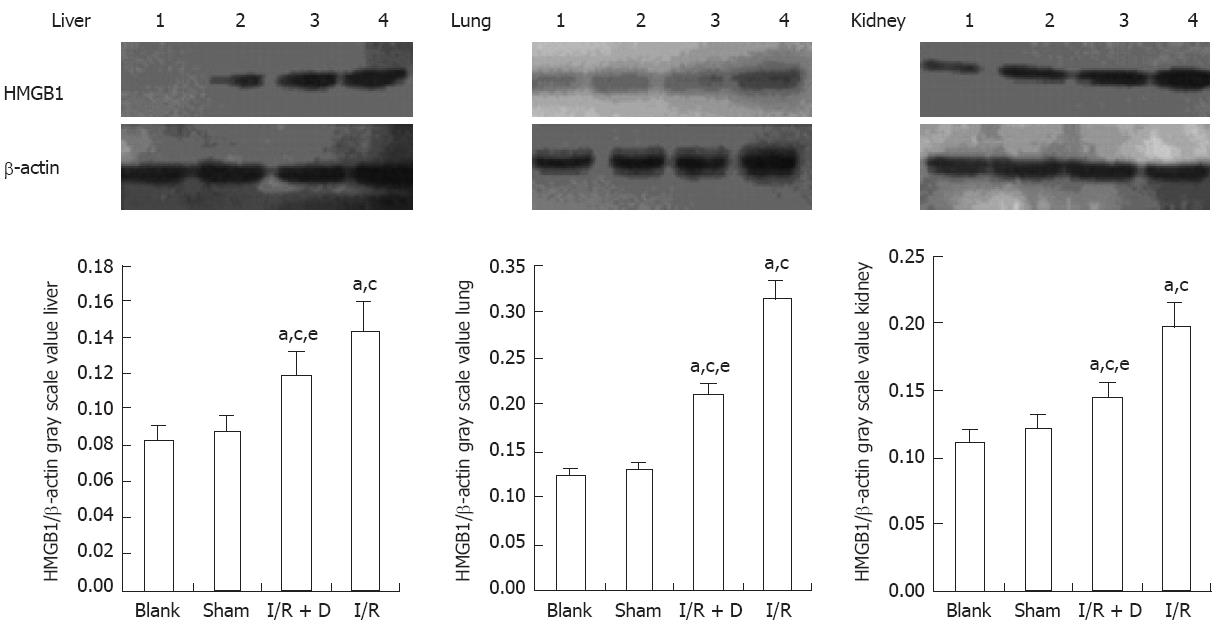
The expression of HMGB1 levels in the jejunum and ileum was significantly higher (P < 0.05) in the I/R and I/R + D groups than in the blank and sham groups (which corresponded to the immunohistochemistry data) and HMGB1 expression was significantly lower in the I/R + D group than in the I/R group (P < 0.05) (Figure 2). Furthermore, the expression of HMGB1 protein levels in the liver, kidney, and lung was significantly higher in the I/R and I/R + D groups than in the blank and sham groups (P < 0.05), and levels in all three organs in the I/R + D group were significantly lower than those in the I/R group (P < 0.05) (Figure 3). Together, these data showed that rats in the I/R + D group had significantly less injury than those of the I/R group, suggesting that blocking the “gut-lymph” pathway may attenuate the increase in HMGB1 levels incurred by I/R, and consequently decrease distant tissue injury.
Intestinal I/R injury can lead to severe intestinal damage and increased intestinal permeability. This study showed that intestinal I/R causes intestinal morphological changes, such as intestinal mucosal injury, visible erosion, necrosis, interstitial congestion in the lamina propria of villi top, edema, inflammation, and mucosa and submucosa hemorrhage. In the jejunum and ileum, slight staining was observed at the top of microvillus in the sham group, and both the top villi and the basement membrane were stained in most areas in the I/R group. Moreover, we found that injury in the I/R + D group was less than that of the I/R group. Our previous study in rats showed that the intestinal permeability increased and bacteria translocated after I/R injury, and that the mucosal thickness and villus height were significantly lower than in the control group[29].
Recent studies have shown that the key mechanism of intestinal I/R injury that leads to a systemic inflammatory response and injury of distant tissues and organs may be mediated by several gut-derived cytokines released in the circulatory system through the “gut-lymph” pathway, which causes downstream injurious effects. The “gut-lymph” pathway theory is based on several observations. Firstly, lymph drainage before hemorrhagic shock can prevent organ dysfunction caused by the shock[8,30]; and secondly, injecting normal animals with the lymph from animals undergoing hemorrhagic shock can lead to a variety of pathological injuries[12], such as increased permeability, alveolar cell apoptosis, and accumulated neutrophils in the lung.
In this study, our results demonstrated a change in intestinal morphology and an increase in endotoxin, HMGB1, and cytokine serum levels in rats with intestinal I/R injury. Importantly, we found that intestinal lymph drainage could significantly reduce the symptoms of I/R injury. The endotoxin, HMGB1, and cytokine serum levels in the I/R + D group were significantly reduced compared to the I/R group. Cavriani et al[15] observed that lymphatic ligation changed the serum levels of IL-1β and IL-10, and alleviated the inflammatory response and lung injury in rats. Our previous study also indicated that intestinal lymph duct ligation could effectively reduce intestinal permeability and inflammatory cytokine and endotoxin levels in the circulation in rats after I/R injury[14,29]. Another study[31] showed that D-lactate could be detected in the circulation during ischemia for 5 min in I/R rats, and as time progressed, the degree of intestinal mucosa damage increased. Therefore, the D-lactate level could be an early indicator of mucosa damage and permeability changes. The results reported in this study are consistent with previous findings. These studies suggest that both lymph ligation and drainage can block the “gut-lymph” pathway and exert a protective effect on intestinal barrier function.
HMGB1 is the endogenous ligand of TLR4, and the HMGB1-TLR4 combination is an important step in I/R injury[32]. HMGB1, which was initially found to be a late lethal inflammatory factor in sepsis, was recently considered to be an inflammatory cytokine in ALI and hepatic injury. In the early stages, HMGB1 levels increases immediately and continue to slowly increase as reperfusion time is extended. Moreover, an anti-HMGB1 antibody was able to alleviate the inflammation response, as well as significantly reduce myeloperoxidase, IL-1β, and IL-6 expression levels, and especially decrease the accumulation of neutrophils and pulmonary edema[23,33]. A recent study[4] found no bacteria or endotoxin in the circulation in rats with some trauma, surgery-induced SIRS, distant organ injury, and the administration of a TLR4 blocker significantly reduced inflammation and tissue damage. Therefore, it may not be endotoxin, but rather the endogenous ligand that activates TLR4.
During I/R injury, the intestinal epithelial cells and macrophages synthesize and release TLR4 endogenous ligands, which bind to TLR4. TLR4 then activates signaling pathways, such as nuclear factor-κB, and regulates the synthesis of proteins and enzymes that promote the synthesis and release of a variety of cytokines. This results in the subsequent injury of the distant organs[19,23,27]. This study showed that serum levels of HMGB1 were significantly higher in the I/R and I/R + D groups than in the blank and sham groups, and that HMGB1 in the I/R + D group was significantly lower than in the I/R group. These results were consistent with HMGB1 protein expression in the intestine, liver, kidney, and lung as determined by western blot. The immunohistochemistry analysis of HMGB1 in the liver, kidney, and lung also showed that the distant organs were damaged during intestinal I/R injury. Yang et al[34] and Liu et al[35] found that treatment of transient ischemic rats with a neutralizing anti-HMGB1 antibody could reduce IL-6 mRNA and TNF-α mRNA on the surface of intestinal mucosa, attenuate injury, and improve the survival rate. This indicates that HMGB1 released during the early stage may be the factor that promotes intestinal injury and a systemic inflammatory response. In this study, we observed an improvement in morphology, and the serum levels of endotoxin, inflammatory cytokines, and HMGB1 in the I/R + D group were significantly lower than in the I/R group. This could be due to the low blood flow caused by ischemia and hypoxia when organs were subjected to perfusion. It could also be due to the fact that factors such as inflammatory cytokines diffuse throughout the entire body through reperfused blood[7,36]. The degree of the injury in the liver, kidney, and lung, as well as the expression of HMGB1 in the I/R group, were significantly greater than in the I/R + D group (P < 0.05). This demonstrates that HMGB1 participates in the occurrence and development of the injury during intestinal I/R, and that blocking the “gut-lymph” pathway can effectively reduce the injury of the intestinal barrier function and the levels of systemic inflammatory cytokines, as well as attenuate the stimulation of HMGB1 on intestine and distant organs.
In conclusion, intestinal injury after I/R can stimulate the release of HMGB1, endotoxin, and inflammatory cytokines, which may be related to intestinal barrier dysfunction and distant organ injury. Intestinal lymph drainage may improve the morphology and function of the intestine, reduce the levels of cytokines and endotoxin in circulation, and attenuate the injury of distant organs by blocking the “gut-lymph” pathway; providing a reference for clinical treatment.
We sincerely thank Mr. Dong Zhang, BS for his assistance with animal care and our technician De-Tian Wang for help with pathology. We also express our thanks to Yun-Fei Xu, MMC and Xi-Zeng Cui, MMC.
Intestinal ischemia/reperfusion (I/R) injury is the “motor” of systemic inflammatory response syndrome (SIRS), acute respiratory distress syndrome (ARDS), and multiple organ dysfunction syndrome (MODS). The ligating lymph duct in a variety of species after hemorrhagic shock can prevent distant organ injury. However, the composition of lymph that is responsible for distant organ injury remains unknown.
Recent studies proposed that mesenteric lymph-induced distant organ injury in intestinal I/R was directly mediated by gut-derived endogenous ligands, one of which is the high mobility group protein box 1 (HMGB1). HMGB1 acts as a late mediator of lethal sepsis and an early mediator of inflammation and necrosis following I/R injury. The research hot spot is whether HMGB1 in the intestinal mesenteric lymph after I/R mediates distant organ injury.
This study is a continuation of previous study. The authors hypothesized that the HMGB1 and toll-like receptor 4 (TLR4) combination plays an important role in the distant organ injury caused by intestinal I/R injury. The purpose of this study was to determine the impact of intestinal I/R injury and lymph drainage on the intestine and distant organs in rats, and to clarify whether HMGB1 in the intestinal mesenteric lymph after I/R mediates distant organ injury.
The study results suggest that intestinal injury after I/R stimulate the release of HMGB1, endotoxin, and inflammatory cytokines, and that lymph drainage could block the “gut-lymph” pathway, improve intestinal barrier function, reduce cytokine and endotoxin, and attenuate the injury of distant organs incurred by intestinal I/R; providing a reference for future clinical practice.
Intestinal I/R injury: Intestinal I/R injury is the “motor” of SIRS, ARDS and MODS, and can be associated with severe trauma, acute necrotizing pancreatitis, major surgery, extensive burns, and other stresses; HMGB1: HMGB1 is one of the endogenous ligands which can be recognized by and combine with TLR4, and is also the key factor of inflammation of aseptic injury.
This is a good descriptive study in which the authors have analyzed the impact of intestinal I/R injury and lymph drainage on distant organs in rats. The authors conclude that lymph drainage could block the “gut-lymph” pathway, improve intestinal barrier function, reduce cytokine and endotoxin, and attenuate the injury of distant organs in intestinal I/R lesions. The experiments were well designed, and the results were clearly demonstrated and analyzed. The techniques are appropriate and the conclusions are supported by the data presented.
Peer reviewer: Marcel CC Machado, MD, PhD, Professor, Department of Gastroenterology, University of Sao Paulo, R Sr. Arnaldo 455 sala 3218, Sao Paulo 01246903, Brazil
S- Editor Wen LL L- Editor Rutherford A E- Editor Li JY
| 1. | Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the "motor" of critical illness. Shock. 2007;28:384-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 328] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 2. | Leaphart CL, Tepas JJ. The gut is a motor of organ system dysfunction. Surgery. 2007;141:563-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 3. | Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1-10. [PubMed] [Cited in This Article: ] |
| 4. | Marshall JC. Sepsis: rethinking the approach to clinical research. J Leukoc Biol. 2008;83:471-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Deitch EA. Gut lymph and lymphatics: a source of factors leading to organ injury and dysfunction. Ann N Y Acad Sci. 2010;1207 Suppl 1:E103-E111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Fanous MY, Phillips AJ, Windsor JA. Mesenteric lymph: the bridge to future management of critical illness. JOP. 2007;8:374-399. [PubMed] [Cited in This Article: ] |
| 7. | Damle SS, Moore EE, Nydam TL, Banerjee M, Gamboni-Robertson F, Su X, Banerjee A. Postshock mesenteric lymph induces endothelial NF-kappaB activation. J Surg Res. 2007;143:136-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Watkins AC, Caputo FJ, Badami C, Barlos D, Xu da Z, Lu Q, Feketeova E, Deitch EA. Mesenteric lymph duct ligation attenuates lung injury and neutrophil activation after intraperitoneal injection of endotoxin in rats. J Trauma. 2008;64:126-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Jordan JR, Moore EE, Sarin EL, Damle SS, Kashuk SB, Silliman CC, Banerjee A. Arachidonic acid in postshock mesenteric lymph induces pulmonary synthesis of leukotriene B4. J Appl Physiol. 2008;104:1161-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Feinman R, Deitch EA, Aris V, Chu HB, Abungu B, Caputo FJ, Galante A, Xu D, Lu Q, Colorado I. Molecular signatures of trauma-hemorrhagic shock-induced lung injury: hemorrhage- and injury-associated genes. Shock. 2007;28:360-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 11. | Harward TR, Brooks DL, Flynn TC, Seeger JM. Multiple organ dysfunction after mesenteric artery revascularization. J Vasc Surg. 1993;18:459-467; discussion 467-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 98] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Senthil M, Watkins A, Barlos D, Xu DZ, Lu Q, Abungu B, Caputo F, Feinman R, Deitch EA. Intravenous injection of trauma-hemorrhagic shock mesenteric lymph causes lung injury that is dependent upon activation of the inducible nitric oxide synthase pathway. Ann Surg. 2007;246:822-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Wohlauer MV, Moore EE, Harr J, Eun J, Fragoso M, Banerjee A, Silliman CC. Cross-transfusion of postshock mesenteric lymph provokes acute lung injury. J Surg Res. 2011;170:314-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | He GZ, Dong LG, Cui XY, Chen XF, Shu H. Effect of mesenteric lymphatic duct ligation on the system inflammation during the intestinal ischemia-reperfusion. Zhonghua Weichang Waike Zazhi. 2008;11:469-471. [PubMed] [Cited in This Article: ] |
| 15. | Cavriani G, Domingos HV, Oliveira-Filho RM, Sudo-Hayashi LS, Vargaftig BB, de Lima WT. Lymphatic thoracic duct ligation modulates the serum levels of IL-1beta and IL-10 after intestinal ischemia/reperfusion in rats with the involvement of tumor necrosis factor alpha and nitric oxide. Shock. 2007;27:209-213. [PubMed] [Cited in This Article: ] |
| 16. | Adams CA, Xu DZ, Lu Q, Deitch EA. Factors larger than 100 kd in post-hemorrhagic shock mesenteric lymph are toxic for endothelial cells. Surgery. 2001;129:351-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Kaiser VL, Sifri ZC, Dikdan GS, Berezina T, Zaets S, Lu Q, Xu DZ, Deitch EA. Trauma-hemorrhagic shock mesenteric lymph from rat contains a modified form of albumin that is implicated in endothelial cell toxicity. Shock. 2005;23:417-425. [PubMed] [Cited in This Article: ] |
| 18. | Gonzalez RJ, Moore EE, Biffl WL, Ciesla DJ, Silliman CC. The lipid fraction of post-hemorrhagic shock mesenteric lymph (PHSML) inhibits neutrophil apoptosis and enhances cytotoxic potential. Shock. 2000;14:404-408. [PubMed] [Cited in This Article: ] |
| 19. | Kim JB, Lim CM, Yu YM, Lee JK. Induction and subcellular localization of high-mobility group box-1 (HMGB1) in the postischemic rat brain. J Neurosci Res. 2008;86:1125-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Kaczorowski DJ, Mollen KP, Edmonds R, Billiar TR. Early events in the recognition of danger signals after tissue injury. J Leukoc Biol. 2008;83:546-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Levy RM, Mollen KP, Prince JM, Kaczorowski DJ, Vallabhaneni R, Liu S, Tracey KJ, Lotze MT, Hackam DJ, Fink MP. Systemic inflammation and remote organ injury following trauma require HMGB1. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1538-R1544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 23. | Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135-1143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 845] [Cited by in F6Publishing: 903] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 24. | Ao L, Zou N, Cleveland JC, Fullerton DA, Meng X. Myocardial TLR4 is a determinant of neutrophil infiltration after global myocardial ischemia: mediating KC and MCP-1 expression induced by extracellular HSC70. Am J Physiol Heart Circ Physiol. 2009;297:H21-H28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Pulskens WP, Teske GJ, Butter LM, Roelofs JJ, van der Poll T, Florquin S, Leemans JC. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS One. 2008;3:e3596. [PubMed] [Cited in This Article: ] |
| 26. | Hayakawa K, Mishima K, Irie K, Hazekawa M, Mishima S, Fujioka M, Orito K, Egashira N, Katsurabayashi S, Takasaki K. Cannabidiol prevents a post-ischemic injury progressively induced by cerebral ischemia via a high-mobility group box1-inhibiting mechanism. Neuropharmacology. 2008;55:1280-1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Evankovich J, Cho SW, Zhang R, Cardinal J, Dhupar R, Zhang L, Klune JR, Zlotnicki J, Billiar T, Tsung A. High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J Biol Chem. 2010;285:39888-39897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 28. | Liu A, Dirsch O, Fang H, Sun J, Jin H, Dong W, Dahmen U. HMGB1 in ischemic and non-ischemic liver after selective warm ischemia/reperfusion in rat. Histochem Cell Biol. 2011;135:443-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | He GZ, Cui XY, Dong LG, Shu H, Chen XF. Impact of ligation of the mesenteric lymphatic duct on gut barrier function after intestinal ischemia/reperfusion in rat. Zhongguo Linchuang Yingyang Zazhi. 2007;15:364-367. [Cited in This Article: ] |
| 30. | Senthil M, Brown M, Xu DZ, Lu Q, Feketeova E, Deitch EA. Gut-lymph hypothesis of systemic inflammatory response syndrome/multiple-organ dysfunction syndrome: validating studies in a porcine model. J Trauma. 2006;60:958-965; discussion 965-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Murray MJ, Barbose JJ, Cobb CF. Serum D(-)-lactate levels as a predictor of acute intestinal ischemia in a rat model. J Surg Res. 1993;54:507-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 83] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847-2859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 610] [Cited by in F6Publishing: 647] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 33. | Shimamoto A, Pohlman TH, Shomura S, Tarukawa T, Takao M, Shimpo H. Toll-like receptor 4 mediates lung ischemia-reperfusion injury. Ann Thorac Surg. 2006;82:2017-2023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Yang R, Harada T, Mollen KP, Prince JM, Levy RM, Englert JA, Gallowitsch-Puerta M, Yang L, Yang H, Tracey KJ. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med. 2006;12:105-114. [PubMed] [Cited in This Article: ] |
| 35. | Liu K, Mori S, Takahashi HK, Tomono Y, Wake H, Kanke T, Sato Y, Hiraga N, Adachi N, Yoshino T. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J. 2007;21:3904-3916. [PubMed] [Cited in This Article: ] |
| 36. | Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913-2923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 455] [Cited by in F6Publishing: 494] [Article Influence: 29.1] [Reference Citation Analysis (0)] |