Published online Apr 7, 2011. doi: 10.3748/wjg.v17.i13.1732
Revised: December 1, 2010
Accepted: December 8, 2010
Published online: April 7, 2011
AIM: To evaluate the feasibility, reproducibility and efficacy of a new tissue anchoring device in a porcine survival model.
METHODS: Gastrotomies were performed using a needle-knife and balloon dilator in 10 female Yorkshire pigs weighing 30-35 kg. Gastric closure was attempted using a new tissue anchoring device. The tightness of the closure was confirmed by means of air insufflation and the ability to maintain gastric distension with stability in peritoneal pressure measured with a Veress needle. All animals were monitored daily for signs of peritonitis and sepsis over 14 d. During necropsy, the peritoneal cavity and the gastric access site were examined.
RESULTS: Transgastric access, closure and 14 d survival was achieved in all pigs. The mean closure time was 18.1 ± 19.2 min and a mean of 2.1 ± 1 devices were used. Supplementary clips were necessary in 2 cases. The closure time was progressively reduced (24.8 ± 13.9 min in the first 5 pigs vs 11.4 ± 5.9 min in the last 5, P = NS). At necropsy, the gastric access site was correctly closed in all cases with all brace-bars present. One device was misplaced in the mesocolon. Minimal adhesions were observed in 3 pigs and signs of mild peritonitis and adhesions in one.
CONCLUSIONS: The use of this new tissue anchoring device in porcine stomachs is feasible, reproducible and effective and requires a short learning curve.
- Citation: Guarner-Argente C, Córdova H, Martínez-Pallí G, Navarro-Ripoll R, Rodríguez-d’Jesús A, Miguel CR, Beltrán M, Fernández-Esparrach G. Gastrotomy closure with a new tissue anchoring device: A porcine survival study. World J Gastroenterol 2011; 17(13): 1732-1738
- URL: https://www.wjgnet.com/1007-9327/full/v17/i13/1732.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i13.1732
Natural orifice transluminal endoscopic surgery (NOTES) has changed the approach to the peritoneum in the last few years[1-5]. This novel technique permits access to the peritoneal organs through the mouth, rectosigmoid or vagina with diagnostic and therapeutic purposes. Numerous hybrid NOTES procedures (combining NOTES with laparoscopy) have been described in the last five years[6-9], but it was not until 2007 that the first pure NOTES procedures in humans were reported[10-13]. Although the transluminal approach holds great potential, secure access site closure remains a critical issue[14]. In recent cases and series, endoscopic closure is substituted by use of rigid instruments, using the transvaginal access in almost all cases. However, this approach excludes the male population.
Considering the safety of laparoscopy, studies are mandatory to evaluate secure and reproducible closure methods in NOTES procedures[15]. Several closure techniques have been tested[16], including clips[1,17-19], septal occluders[20], T-tags[21-23], more complex suturing devices[24-26], and linear endoscopic staplers[27]. T-tags have been tested recently to treat gastrogastric fistulas in humans[28]. However, most of these devices are time consuming and often difficult to implement endoscopically and the current data do not allow definitive conclusions regarding the different options[16,29].
The aim of this study was to assess the feasibility, reproducibility and efficacy of a new tissue anchoring device as a gastric suture system in a porcine survival model.
A total of ten female Yorkshire pigs weighing 30-35 kg were included in the study. Animals underwent a 3-d quarantine and acclimation period. During this period of time, veterinary personnel evaluated each animal to ensure baseline health. Animals were fed the same diet and had unlimited access to water. The study was conducted at the University of Barcelona Medical School’s animal facilities. The protocol was approved by the University of Barcelona’s Animal Ethics Committee.
Animals fasted from solids 24 h prior to the procedure. All procedures were performed with pigs under general anesthesia with endotracheal intubation and mechanical ventilation.
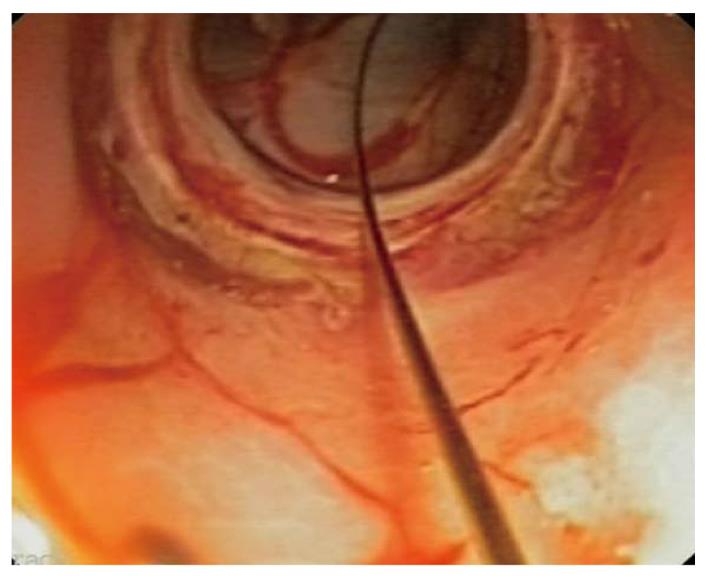
A non sterile endoscope (GIF 160, Olympus Medical Systems, Europe, Hamburg, Germany) was first inserted through the pig’s mouth and the esophagus and stomach were inspected. Afterwards, gastric lavage was performed with water until the stomach was free of solid particles. An iodated solution followed by an antibiotic suspension (ceftriaxone 1 g/300 mL saline solution) was instilled and the antibiotic solution was left in the stomach for 10 min. From this point on, all the instruments used were sterile or high level disinfected. With a regular endoscope, an overtube was inserted and a double channel gastroscope (GIF 2T160, Olympus Medical Systems, Europe, Hamburg, Germany) was used until the end of the peritoneoscopy. By external palpation, the anterior gastric wall was selected to perform the gastric access. A 5 mm incision was made with a needle-knife (KD-V451M, Olympus Europe, Hamburg, Germany) and it was subsequently dilated with an 18 mm balloon (CRE wire-guided balloon, Boston Scientific Microvasive, Natick, MA) (Figure 1). Then, the scope was passed through the gastric wall for a 30 min peritoneoscopy.
A Veress needle was placed at the lower left quadrant of the abdomen to control intraperitoneal pressure. To avoid respiratory compromise and impaired venous return, intraperitoneal pressures were monitored and maintained below 15 mm H2O. Pneumoperitoneum was maintained with CO2 insufflation through the scope.
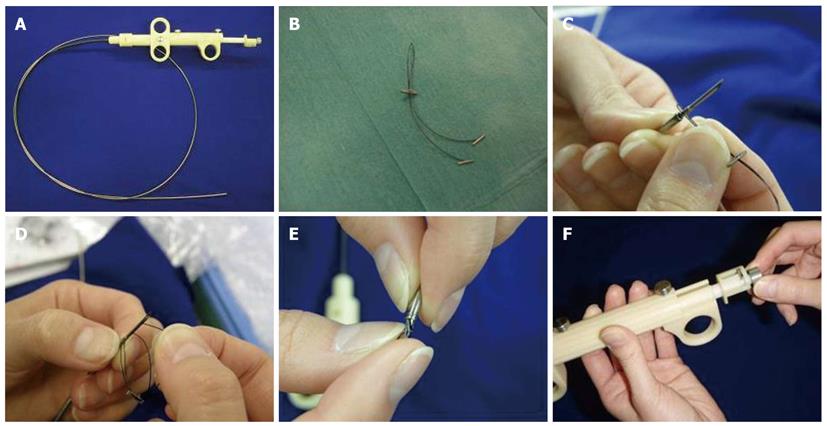
The tissue-anchoring device prototype is called brace-bar (Olympus Medical Systems, Europe, Hamburg, Germany) and is an evolution of a former prototype[21]. It consists of a single 18-gauge flexible needle catheter (Figure 2A), and a bifurcated nylon thread (“Y” shaped) with 3 small tags (2 regular tags fixed at both bifurcated distal ends and the other tag stopper at the single proximal end, which will be used for tightening) (Figure 2B). The tag stopper is movable and can be slid forward for cinching of the other tissue-anchoring tags. The proximal end of the thread is fixed to the needle with a small metallic guide (Figure 2C). Before deployment, the device has to be extracorporally loaded inside the needle catheter. The two distal tags are consecutively inserted into the needle (Figure 2D) and, finally, the needle is pulled back into the sheath inserting also the stopper tag (Figure 2E). Once inside the gastric cavity, the needle is pushed forward and the device is ready for use. The pusher button (Figure 2F) allows release of one tag at each side of the incision and the suture is tightened by pressing the tag Stopper with the needle sheath. Finally, the suture is released by extracting the metallic guide that fixed it to the needle.
The tightness of the closure was confirmed by means of air insufflation and the ability to maintain gastric distension with stability in peritoneal pressure measured with the Veress needle.
A single channel scope (Olympus GIG 160) was only used for the suture. Endoclips were added when the sutures were not placed at the middle of the incision and one of the sides seemed not completely sealed.
Water was immediately allowed and food was allowed after 24 h. All animals received intravenous ceftriaxone 1 g daily for 3 d and they were monitored daily for signs of peritonitis and sepsis during the next 14 d. Weight was controlled prior to surgery and necropsy. During necropsy, the peritoneal cavity and the gastric access site were examined for signs of peritonitis (exudates, abscesses) or other complications.
Data were expressed as mean ± SD or range. Results were analyzed using the χ2 test with Yates correction and Fisher exact test for qualitative variables and Mann-Whitney test for quantitative parameters. A P value < 0.05 was considered statistically significant. Statistical analysis was performed with SPSS Statistical Package (version 17.0, SPSS Inc., Chicago, IL).
All transgastric accesses were achieved with no difficulties and a mean time of 5.7 ± 3.6 min (range 2-14). No major complications occurred. Minor complications included an accidental injury to the anterior abdominal wall and 4 minor bleedings during the use of the needle-knife. A 30 min peritoneoscopy was possible in all animals.
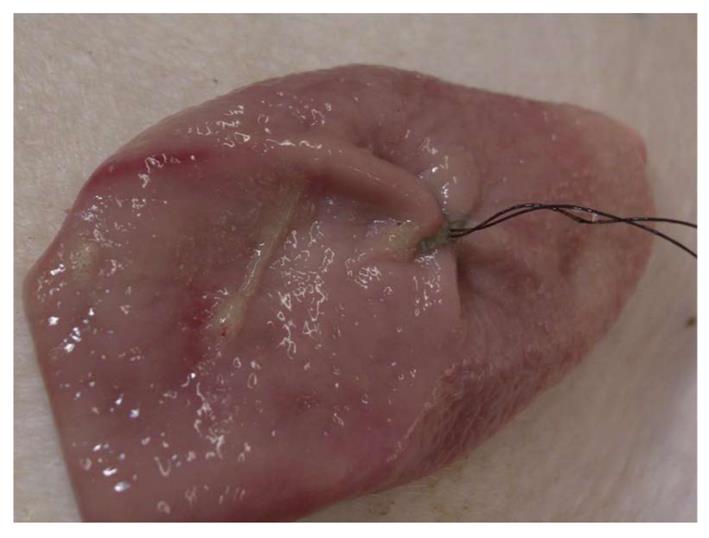
The brace-bar was used in all cases and closure was easily achieved in 18.1 ± 19.2 min. This time was reduced to 11.4 ± 5.9 min when considering only the last 5 cases and was less than 9 min in the last 3 cases, whereas it was 24.8 ± 13.9 min in the first 5 (P = 0.1). Details of all procedures are described in Table 1. A total of 21 sets of sutures (mean 2.1 ± 1, range 1-4) were used to achieve closure but only 15 (mean 1.5 ± 0.5, range 1-2) could be completely tightened. Therefore, in 5 cases, the incision was closed with only 1 suture (Figure 3), but in two of them 1 and 3 clips respectively, were added.
| Case | Number ofbrace-bars used | Number of brace-barscorrectly placed | Cause ofMisplaced brace-bar | Adjunctive method | Closure time(min) | Endoscopic view |
| 1 | 2 | 2 | Omental patch | 8 | Correct | |
| 2 | 1 | 1 | 3 endoclips | 42 | Correct | |
| 3 | 4 | 2 | 1 tag detached 1 brace-bar not tightened | None | 36 | Correct |
| 4 | 2 | 2 | None | 19 | Correct | |
| 5 | 2 | 1 | 1 tag detached | 1 endoclip | 19 | Correct |
| 6 | 3 | 2 | 1 tag detached | None | 15 | Correct |
| 7 | 3 | 1 | 2 tags detached | None | 20 | Correct |
| 8 | 2 | 2 | None | 9 | Correct | |
| 9 | 1 | 1 | None | 7 | Correct | |
| 10 | 1 | 1 | None | 6 | Correct |
In total, 6 sutures (29%) were ineffectively positioned. In 5 attempts one of the tags did not stay attached to the mucosa either immediately after the tag release or when tightening the suture. The remaining failure was caused by a thread rupture after steady tightening.
Immediately after gastrotomy closure, all brace-bars seemed well positioned and gastric distension with air was possible in all cases without changes in intraperitoneal pressure, suggesting the closure was correct (mean peritoneal pressure before and after the closure: 14.3 ± 3.3 mmHg, range 3-15).
The mean procedure time, including gastric access creation, peritoneoscopy and gastrotomy suture, was 63.7 ± 18.2 min.
All the pigs completed the 14 d follow-up period. They had a weight gain of 3.3 ± 3.2 kg. At necropsy, the gastric access site was completely closed in all cases and all brace-bars were present (Table 2). The tags were usually attached at the gastric serosa (n = 15) or inside the gastric wall (n = 14). In the first case, 1 tag was misplaced inside the mesocolon. In this case, an omental patch had been added to the suture pulling the omentum inside the stomach through the incision. Minimal adhesions were observed in 3 pigs and signs of mild peritonitis and adhesions in one.
| Case | Weight gain (kg) | Abdominal cavity | Incision | Location of tags |
| 1 | 6.85 | Minimal adhesions | Totally closed | 1 tag at mesocolon 3 tags at gastric serosa 2 tag stoppers at gastric mucosa |
| 2 | 6.90 | Normal | Totally closed | 2 tags at gastric serosa. 1 tag stopper at gastric mucosa |
| 3 | 5.00 | Minimal adhesions | Totally closed | 4 tags at gastric serosa 2 tag stoppers at gastric mucosa |
| 4 | 3.70 | Normal | Totally closed | 4 tags inside gastric wall 2 tag stoppers at gastric mucosa |
| 5 | 1.90 | Normal | Totally closed | 1 tag at gastric serosa 1 tag inside gastric wall 1 tag stopper at gastric mucosa |
| 6 | 3.74 | Small clot | Totally closed | 3 tags at gastric serosa 1 tag inside gastric wall 1 tag stopper at gastric mucosa |
| 7 | 0.90 | Normal | Totally closed | 1 tag at gastric serosa 1 tag at gastric wall 1 tag stopper at gastric mucosa |
| 8 | 0.00 | Normal | Totally closed | 1 tag at gastric serosa 3 tags at gastric wall 2 tag stoppers at gastric mucosa |
| 9 | -2.78 | Fibrin exudates Minimal adhesions | Totally closed | 2 tags at gastric wall 1 tag stopper at gastric mucosa |
| 10 | 6.42 | Minimal adhesions | Totally closed | 2 tags at gastric wall 1 tag stopper at gastric mucosa |
NOTES holds great appeal as a less invasive alternative to laparoscopic surgery. As NOTES heads toward human trials, it is essential that the creation and closure of transluminal incisions be performed in a safe, rapid, and reproducible manner[14-16,28].
In this study, we assessed the feasibility of a new generation tissue anchoring device with relatively good results. Moreover, it turned out to be easy and intuitive to use and the time for placement was short and progressively reduced. It was not necessary to use complementary clips when we gained experience with the system. One of the advantages of this device (and the main difference with the former prototype used by Sumiyama et al[22]) is that it can be used with a single channel endoscope. The same needle catheter is used for releasing the tags and tightening them later without need for a different forceps grasper, and this makes the procedure shorter. Furthermore, the depth of the needle insertion is limited to 20 mm and this might decrease the risk of complications.
However, we still found some problems with the device: the needle had to be loaded extracorporally after each set of tissue anchors was applied and this prevented sequential stitching. Moreover, we observed a dysfunction of the needle after several attempts which could explain the high rate of sutures being ineffectively positioned because the tags could not be released deeply enough. We think that pre-charged and non-reusable devices might improve the procedure time and security. On the other hand, we did not drop any tags in the peritoneal cavity and, since each pair of tags is attached to a thread, we think that the possibility of dropping one in the peritoneum is extremely low.
The possibility of an inadvertent injury of organs and structures outside of the gut wall has been described as a possible limitation of T-tag based systems[30,31]. Sumiyama et al[22] produced 12 gastric perforations in 6 pigs that were closed with 48 tissue anchor sets and three of the 24 used in the anterior gastric wall (12.5%) penetrated surrounding organs (2 penetrated the liver and 1 the anterior abdominal wall). However, as mentioned above, with the new brace-bar prototype the depth of the needle insertion is limited to 20 mm and this fact was crucial in the low incidence of surrounding structure injuries in our series (1 tag out of 21 sets, 4.8%). This was the first case and we tried to perform an omental patch pulling the omentum through the incision. During this maneuver, the mesocolon was probably unsafely moved towards the gastric wall causing this complication. In the remaining cases in which the omental patch was not attempted no lesions occurred at the adjacent structures. The importance of the depth of the needle to avoid complications has been demonstrated very recently by Park et al[32] These authors performed needle punctures of 1-1.5 mm using a different anchor-based endoscopic system (the TAS o tissue apposition system) and they did not have any adjacent organ penetration with a 100% of closure effectiveness.
Although the ex-vivo study of Voermans et al[33] suggested that t-tag based methods do not permit the serosa to serosa approach and leaking pressure is lower than with other devices, the surviving pigs showed a good postoperative course. This fact could be explained because physiological intraluminal pressures are much lower than pressures obtained in acute bursting tests and, therefore, might not be a necessary objective test for viscerotomy closure[34]. From a clinical standpoint, the critical test for a gastric closure is animal survival without clinical signs of leakage or complications.
Previous studies have demonstrated that peritoneal contamination occurs when using transgastric access. A conservative interpretation of these findings is that the current “aseptic” technique may require further refinement, as suggested by Rolanda et al[18] and Ryou et al[35]. In fact, we had some difficulties in completely cleaning some of the stomachs and it was very common to notice residual liquid near the incision. Only one pig did not have a satisfactory recovery and the necropsy showed the presence of fibrin exudates in the upper abdomen. Although the incision site was seen completely sealed at necropsy, we cannot totally exclude the possibility that an initial suture failure occurred but it could be also related with a potential contamination of the abdominal cavity by the stomach content. Because in this case we used only one brace-bar, we now consider it prudent to use two devices to ensure a safer closure.
The present study has some limitations: first, the number of cases is low and we did not include a control group. Second, the 14 d survival period might be short to evaluate late complications. Finally, the use of complementary clips in two cases might modify the results of the study.
In conclusion, the use of a brace-bar in a gastric porcine model is easy, fast, and reproducible after a short learning curve and permits the use of a single channel endoscope. We believe this tissue anchoring system holds tremendous potential as a suturing method for both iatrogenic and intentional perforations of the gastric wall. Unfortunately, it is still far from a safe application in humans. Further studies and more technological improvements are still mandatory before expanding its use to humans.
Natural orifice transluminal endoscopic surgery (NOTES) has changed the approach to the peritoneum in the last few years. Secure closure of the gastrotomy access is one of the most important issues for the development of NOTES. However, most new suturing devices are time consuming and often difficult to implement endoscopically.
T-tag based systems have already been used with variable results. The possibility of an inadvertent injury of organs and structures outside of the gut wall has been described as a possible limitation of these devices. In this study, the authors demonstrate the usefulness and safety of a new tissue-anchoring-device prototype.
One of the advantages of this device (and the main difference with the former prototype) is that it can be used with a single channel endoscope. On the other hand, the same needle catheter is used for releasing the tags and tightening them later without need for a different forceps grasper, and this makes the procedure shorter. Furthermore, the depth of the needle insertion is limited to 20 mm and this might decrease the risk of complications.
Because the use of the brace-bar is easy, fast, and reproducible after a short learning curve, we believe this tissue anchoring system holds tremendous potential as a suturing method for both iatrogenic and intentional perforations of the gastric wall.
Natural orifice transluminal endoscopic surgery permits access to the peritoneal organs without the need of skin incisions. Tissue-anchoring devices are endoscopic suturing devices based on a nylon thread and a small tag at the distal end that are deployed within the gastric wall. When two or more of them are tight together, the margins of the incision approach and the incision is sealed.
This is well written and succinct with appropriate interpretation and caution in the discussion.
Peer reviewer: John K Marshall, MD, Associate Professor of Medicine, Division of Gastroenterology (4W8), McMaster University Medical Centre, 1200 Main Street West, Hamilton, Ontario L8N 3Z5, Canada
S- Editor Tian L L- Editor O’Neill M E- Editor Ma WH
| 1. | Kalloo AN, Singh VK, Jagannath SB, Niiyama H, Hill SL, Vaughn CA, Magee CA, Kantsevoy SV. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117. [Cited in This Article: ] |
| 2. | Kantsevoy SV, Jagannath SB, Niiyama H, Chung SS, Cotton PB, Gostout CJ, Hawes RH, Pasricha PJ, Magee CA, Vaughn CA. Endoscopic gastrojejunostomy with survival in a porcine model. Gastrointest Endosc. 2005;62:287-292. [Cited in This Article: ] |
| 3. | Merrifield BF, Wagh MS, Thompson CC. Peroral transgastric organ resection: a feasibility study in pigs. Gastrointest Endosc. 2006;63:693-697. [Cited in This Article: ] |
| 4. | Jagannath SB, Kantsevoy SV, Vaughn CA, Chung SS, Cotton PB, Gostout CJ, Hawes RH, Pasricha PJ, Scorpio DG, Magee CA. Peroral transgastric endoscopic ligation of fallopian tubes with long-term survival in a porcine model. Gastrointest Endosc. 2005;61:449-453. [Cited in This Article: ] |
| 5. | Wagh MS, Merrifield BF, Thompson CC. Survival studies after endoscopic transgastric oophorectomy and tubectomy in a porcine model. Gastrointest Endosc. 2006;63:473-478. [Cited in This Article: ] |
| 6. | Marescaux J, Dallemagne B, Perretta S, Wattiez A, Mutter D, Coumaros D. Surgery without scars: report of transluminal cholecystectomy in a human being. Arch Surg. 2007;142:823-826; discussion 826-827. [Cited in This Article: ] |
| 7. | Zornig C, Emmermann A, von Waldenfels HA, Mofid H. Laparoscopic cholecystectomy without visible scar: combined transvaginal and transumbilical approach. Endoscopy. 2007;39:913-915. [Cited in This Article: ] |
| 8. | Zorrón R, Filgueiras M, Maggioni LC, Pombo L, Lopes Carvalho G, Lacerda Oliveira A. NOTES. Transvaginal cholecystectomy: report of the first case. Surg Innov. 2007;14:279-283. [Cited in This Article: ] |
| 9. | Lacy AM, Delgado S, Rojas OA, Ibarzabal A, Fernandez-Esparrach G, Taura P. Hybrid vaginal MA-NOS sleeve gastrectomy: technical note on the procedure in a patient. Surg Endosc. 2009;23:1130-1137. [Cited in This Article: ] |
| 10. | Marks JM, Ponsky JL, Pearl JP, McGee MF. PEG “Rescue”: a practical NOTES technique. Surg Endosc. 2007;21:816-819. [Cited in This Article: ] |
| 11. | Rao GV, Reddy DN, Banerjee R. NOTES: human experience. Gastrointest Endosc Clin N Am. 2008;18:361-370; x. [Cited in This Article: ] |
| 12. | Bernhardt J, Gerber B, Schober HC, Kähler G, Ludwig K. NOTES--case report of a unidirectional flexible appendectomy. Int J Colorectal Dis. 2008;23:547-550. [Cited in This Article: ] |
| 13. | Voermans RP, Sheppard B, van Berge Henegouwen MI, Fockens P, Faigel DO. Comparison of Transgastric NOTES and laparoscopic peritoneoscopy for detection of peritoneal metastases. Ann Surg. 2009;250:255-259. [Cited in This Article: ] |
| 14. | ASGE/SAGES Working Group on Natural Orifice Translumenal Endoscopic Surgery White Paper October 2005. Gastrointest Endosc. 2006;63:199-203. [Cited in This Article: ] |
| 15. | Flora ED, Wilson TG, Martin IJ, O'Rourke NA, Maddern GJ. A review of natural orifice translumenal endoscopic surgery (NOTES) for intra-abdominal surgery: experimental models, techniques, and applicability to the clinical setting. Ann Surg. 2008;247:583-602. [Cited in This Article: ] |
| 16. | Teoh AY, Chiu PW, Ng EK. Current developments in natural orifices transluminal endoscopic surgery: an evidence-based review. World J Gastroenterol. 2010;16:4792-4799. [Cited in This Article: ] |
| 17. | Schurr MO, Arezzo A, Ho CN, Anhoeck G, Buess G, Di Lorenzo N. The OTSC clip for endoscopic organ closure in NOTES: device and technique. Minim Invasive Ther Allied Technol. 2008;17:262-266. [Cited in This Article: ] |
| 18. | Rolanda C, Lima E, Silva D, Moreira I, Pêgo JM, Macedo G, Correia-Pinto J. In vivo assessment of gastrotomy closure with over-the-scope clips in an experimental model for varicocelectomy (with video). Gastrointest Endosc. 2009;70:1137-1145. [Cited in This Article: ] |
| 19. | Arezzo A, Kratt T, Schurr MO, Morino M. Laparoscopic-assisted transgastric cholecystectomy and secure endoscopic closure of the transgastric defect in a survival porcine model. Endoscopy. 2009;41:767-772. [Cited in This Article: ] |
| 20. | Perretta S, Sereno S, Forgione A, Dallemagne B, Coumaros D, Boosfeld C, Moll C, Marescaux J. A new method to close the gastrotomy by using a cardiac septal occluder: long-term survival study in a porcine model. Gastrointest Endosc. 2007;66:809-813. [Cited in This Article: ] |
| 21. | Bergström M, Swain P, Park PO. Early clinical experience with a new flexible endoscopic suturing method for natural orifice transluminal endoscopic surgery and intraluminal endosurgery (with videos). Gastrointest Endosc. 2008;67:528-533. [Cited in This Article: ] |
| 22. | Sumiyama K, Gostout CJ, Rajan E, Bakken TA, Deters JL, Knipschield MA. Endoscopic full-thickness closure of large gastric perforations by use of tissue anchors. Gastrointest Endosc. 2007;65:134-139. [Cited in This Article: ] |
| 23. | Dray X, Gabrielson KL, Buscaglia JM, Shin EJ, Giday SA, Surti VC, Assumpcao L, Marohn MR, Magno P, Pipitone LJ. Air and fluid leak tests after NOTES procedures: a pilot study in a live porcine model (with videos). Gastrointest Endosc. 2008;68:513-519. [Cited in This Article: ] |
| 24. | Chiu PW, Lau JY, Ng EK, Lam CC, Hui M, To KF, Sung JJ, Chung SS. Closure of a gastrotomy after transgastric tubal ligation by using the Eagle Claw VII: a survival experiment in a porcine model (with video). Gastrointest Endosc. 2008;68:554-559. [Cited in This Article: ] |
| 25. | McGee MF, Marks JM, Onders RP, Chak A, Jin J, Williams CP, Schomisch SJ, Ponsky JL. Complete endoscopic closure of gastrotomy after natural orifice translumenal endoscopic surgery using the NDO Plicator. Surg Endosc. 2008;22:214-220. [Cited in This Article: ] |
| 26. | Bardaro SJ, Swanström L. Development of advanced endoscopes for Natural Orifice Transluminal Endoscopic Surgery (NOTES). Minim Invasive Ther Allied Technol. 2006;15:378-383. [Cited in This Article: ] |
| 27. | Magno P, Giday SA, Dray X, Chung SS, Cotton PB, Gostout CJ, Hawes RH, Kalloo AN, Pasricha PJ, White JJ. A new stapler-based full-thickness transgastric access closure: results from an animal pilot trial. Endoscopy. 2007;39:876-880. [Cited in This Article: ] |
| 28. | Spaun GO, Martinec DV, Kennedy TJ, Swanström LL. Endoscopic closure of gastrogastric fistulas by using a tissue apposition system (with videos). Gastrointest Endosc. 2010;71:606-611. [Cited in This Article: ] |
| 29. | Arezzo A, Morino M. Endoscopic closure of gastric access in perspective NOTES: an update on techniques and technologies. Surg Endosc. 2010;24:298-303. [Cited in This Article: ] |
| 30. | Ikeda K, Fritscher-Ravens A, Mosse CA, Mills T, Tajiri H, Swain CP. Endoscopic full-thickness resection with sutured closure in a porcine model. Gastrointest Endosc. 2005;62:122-129. [Cited in This Article: ] |
| 31. | Kantsevoy SV. Endoscopic full-thickness resection: new minimally invasive therapeutic alternative for GI-tract lesions. Gastrointest Endosc. 2006;64:90-91. [Cited in This Article: ] |
| 32. | Park PO, Bergström M, Rothstein R, Swain P, Ahmed I, Gomez G, Raju GS. Endoscopic sutured closure of a gastric natural orifice transluminal endoscopic surgery access gastrotomy compared with open surgical closure in a porcine model. A randomized, multicenter controlled trial. Endoscopy. 2010;42:311-317. [Cited in This Article: ] |
| 33. | Voermans RP, Worm AM, van Berge Henegouwen MI, Breedveld P, Bemelman WA, Fockens P. In vitro comparison and evaluation of seven gastric closure modalities for natural orifice transluminal endoscopic surgery (NOTES). Endoscopy. 2008;40:595-601. [Cited in This Article: ] |
| 34. | Trunzo JA, Schomisch SJ, Mishra T, Andrews J, Ponsky JL, Marks JM. The validity of busting pressure as an objective measure in Natural Orifice Translumenal Endsocopic Surgery (NOTES) closure testing. Gastrointest Endosc. 2009;69:AB169. [Cited in This Article: ] |
| 35. | Ryou M, Fong DG, Pai RD, Tavakkolizadeh A, Rattner DW, Thompson CC. Dual-port distal pancreatectomy using a prototype endoscope and endoscopic stapler: a natural orifice transluminal endoscopic surgery (NOTES) survival study in a porcine model. Endoscopy. 2007;39:881-887. [Cited in This Article: ] |