Published online Mar 28, 2011. doi: 10.3748/wjg.v17.i12.1574
Revised: July 12, 2010
Accepted: July 19, 2010
Published online: March 28, 2011
AIM: To elucidate the effect and underlying mechanisms of omeprazole action on Mg2+ transport across the intestinal epithelium.
METHODS: Caco-2 monolayers were cultured in various dose omeprazole-containing media for 14 or 21 d before being inserted into a modified Ussing chamber apparatus to investigate the bi-directional Mg2+ transport and electrical parameters. Paracellular permeability of the monolayer was also observed by the dilution potential technique and a cation permeability study. An Arrhenius plot was performed to elucidate the activation energy of passive Mg2+ transport across the Caco-2 monolayers.
RESULTS: Both apical to basolateral and basolateral to apical passive Mg2+ fluxes of omeprazole-treated epithelium were decreased in a dose- and time-dependent manner. Omeprazole also decreased the paracellular cation selectivity and changed the paracellular selective permeability profile of Caco-2 epithelium to Li+, Na+, K+, Rb+, and Cs+ from series VII to series VI of the Eisenman sequence. The Arrhenius plot revealed the higher activation energy for passive Mg2+ transport in omeprazole-treated epithelium than that of control epithelium, indicating that omeprazole affected the paracellular channel of Caco-2 epithelium in such a way that Mg2+ movement was impeded.
CONCLUSION: Omeprazole decreased paracellular cation permeability and increased the activation energy for passive Mg2+ transport of Caco-2 monolayers that led to the suppression of passive Mg2+ absorption.
- Citation: Thongon N, Krishnamra N. Omeprazole decreases magnesium transport across Caco-2 monolayers. World J Gastroenterol 2011; 17(12): 1574-1583
- URL: https://www.wjgnet.com/1007-9327/full/v17/i12/1574.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i12.1574
Magnesium plays an important role in numerous biological functions. Mg2+ deficiency is associated with several diseases, e.g. Alzheimer’s disease[1], osteoporosis[2], and hypertension[3]. Therefore, its plasma level is tightly regulated within a narrow range (0.7-1.1 mmol/L) by intestinal absorption and renal excretion[4]. In human intestine, fractional Mg2+ absorption varies from 11% to 65% depending on the amount of Mg2+ intake[5]. Intestinal epithelium absorbs Mg2+via both saturable transcellular and non-saturable paracellular pathways. Transcellular Mg2+ transport is an active process that requires the activity of transient receptor potential melastatin 6 (TRPM6) and the basolateral Na+/Mg2+ exchanger[6,7]. On the other hand, paracellular Mg2+ transport is a passive mechanism and is implicated in about 90% of intestinal Mg2+ absorption[7]. The paracellular Mg2+ transport process is modulated by the tight junction proteins, i.e. Claudin-16 and Claudin-19[8].
Omeprazole is a common therapeutic tool for acid-peptic disorders. Its active sulphenamide form selectively and covalently interacts with the H+/K+-ATPase, particularly the extracellular cysteine 813, leading to potent inhibition of H+/K+-ATPase activity[9]. Previous reports demonstrated that prolonged omeprazole administration led to hypomagnesemia and hypomagnesuria in humans[10,11]. Withdrawal of omeprazole and intravenous Mg2+ replacement, but not high dose oral Mg2+ supplement, could normalize the plasma and urinary Mg2+ levels[10,12]. Renal Mg2+ handling was normal in patients with severe hypomagnesemia associated with long-term use of omeprazole[12-14]. This body of evidence suggested an inhibitory effect of omeprazole on intestinal Mg2+ absorption. However, the direct action of omeprazole on intestinal Mg2+ transport is still elusive. The present study, therefore, aimed to elucidate the effect of omeprazole as well as obtain information regarding possible mechanisms of omeprazole action on Mg2+ transport across the intestinal epithelium. This study employed a monolayer of Caco-2 cells which is a suitable in vitro model for studying intestinal transport of divalent cations, e.g. Ca2+[15] and Mg2+[16].
Caco-2 cells (ATCC No. HTB-37) were grown in Dulbecco’s modified Eagle medium (DMEM) (Sigma, St. Louis, MO, USA) supplemented with 15% fetal bovine serum (FBS-Gold) (PAA Laboratories GmbH, Pasching, Austria), 1% l-glutamine (Gibco, Grand Island, NY, USA), 1% non-essential amino acid (Sigma, St. Louis, MO, USA), and 1% antibiotic-antimycotic solution (Gibco, Grand Island, NY, USA) and maintained at a humidified atmosphere containing 5% CO2 at 37°C. The Caco-2 monolayers were developed by seeding cells (5.0 × 105 cells/cm2) onto permeable Snapwelltm inserts (12-mm diameter and 0.4-μm pore size polyester filter) (Corning, Corning, NY, USA). In the omeprazole-treated group, Caco-2 monolayers were grown in 200, 400, 600, 800, or 1000 ng/mL omeprazole (Calbiochem, San Diego, CA, USA) containing culture media. The culture medium was changed three times a week. On day 14 or 21 after seeding, the Snapwell was inserted into a modified Ussing chamber (1.13 cm2 exposed area).
In the Ussing chamber, the monolayer was equilibrated for 20 min in bathing solution at 37°C, pH 7.4, and osmolarity of 290-293 mmol/kg H2O[17]. To avoid the unstirred water layer and to maintain pH at 7.4, the bathing solution in each hemi-chamber was continuously gassed with humidified 5% CO2 in 95% O2. After equilibration, the apical or basolateral bathing solution was replaced with 2.5, 5, 10, 20, 40, or 80 mmol/L MgCl2-containing bathing solution, while the contralateral side was replaced with MgCl2-free bathing solution. At 1 and 2 h, 500 μL solution was collected from the side that contained MgCl2-free bathing solution and Mg2+ concentration was measured. Mg2+ flux (nmol/h per cm2) was calculated using Equation (Eq. 1):
Mg2+ flux = CMg/(t × S) (1)
Where CMg is Mg2+ concentration (nmol/L); t is time (h); and S is transport surface area (cm2).
To elucidate the involvement of solvent drag-induced mechanism on Mg2+ transport, 100 μmol/L phlorizin (Fluka Chemie AG, Buchs, Switzerland) and 100 μmol/L phloretin (Calbiochem, San Diego, CA, USA) were added to the apical and basolateral solution, respectively. Mg2+ transport was also observed at different temperatures (15, 25, or 35°C) and the results were presented as an Arrhernius plot[18] (Eq. 2):
ln(PMg) = (-Ea)/(RT) + ln(E) (2)
Where ln(PMg) is the natural logarithm of Mg2+ permeability (cm/s); Ea is activation energy (kJ/mol); R is gas constant; T is absolute temperature (273+˚C), E is pre-exponential factor. The temperature coefficient Q10 was determined as previously described[19].
The concentration of Mg2+ was determined by Xylidyl Blue (Sigma, St. Louis, MO, USA) colorimetric assay, modified from the method of Tang and Goodenough[20]. In brief, the sample solutions were spun at 1000 × g for 10 min and a 200 μL sample of the upper solution was collected. An aliquot was added to 100 μL water, gently mixed, and then 200 μL of 1.25 mmol/L EGTA was added to the assay tube. After mixing well, 500 μL of Xylidyl Blue solution (pH 10.5) was added to the assay tube. After 5 min of incubation at room temperature, the assay solution was subjected to colorimetric analysis using a spectrophotometer at 520 nm (model UV-2550; Shimadzu, Kyoto, Japan).
Trans-epithelium resistance (TER), potential difference (PD), and short-circuit current (ISC) were determined as previously described[21]. These electrical parameters were recorded after 20 min equilibration at 30 min intervals throughout the 2 h of Mg2+ flux study.
Absolute permeabilities of Na+ (PNa) and Cl- (PCl), as well as the relative permeability of Na+ to Cl- (PNa/PCl), of Caco-2 monolayers were obtained by the dilution potential technique as previously described[21]. The absolute permeability of group I alkaline metals (Li+, K+, Rb+, and Cs+), i.e. PLi, PK, PRb, and PCs was determined as previously described[21] using the same calculation as that used to obtain PNa.
The Mg2+ permeability (PMg) of Caco-2 monolayers was calculated using Eq. 3:
PMg = Mg2+ flux/∆CMg (3)
Where ∆CMg is the concentration difference of Mg2+ between the apical and basolateral solutions.
To estimate the kinetic values of the saturable active and non-saturable passive Mg2+ transport, the rate of apical to basolateral Mg2+ transport (MgA→B transport) was fitted to a modified Michaelis-Menten kinetic plus linear component as shown in Eq. 4:
MgA→B transport = (Vm × Cmg)/(Km + Cmg)+ mCmg (4)
Where Vm is the maximal rate of saturable MgA→B transport; Km is the rate constant of saturable MgA→B transport; m is the rate constant for non-saturable MgA→B transport; and CMg as mentioned above. This study was performed using a nonlinear regression program of GraphPad Prism version 5.0 for Window (GraphPad Software Inc., San Diego, CA, USA).
Results were expressed as means ± SE. Two sets of data were compared using the unpaired Student’s t-test. One-way analysis of variance (ANOVA) with Dunnett’s posttest was employed for multiple sets of data. The level of significance was P < 0.05. Linear regression and slope analysis were performed to obtain the basolateral to apical Mg2+ transport (MgB→A transport)-Mg concentration relationship. The curve of PMg-∆magnesium relationship was obtained using one phase exponential decay equation. All data were analyzed by GraphPad Prism (GraphPad Software Inc.).
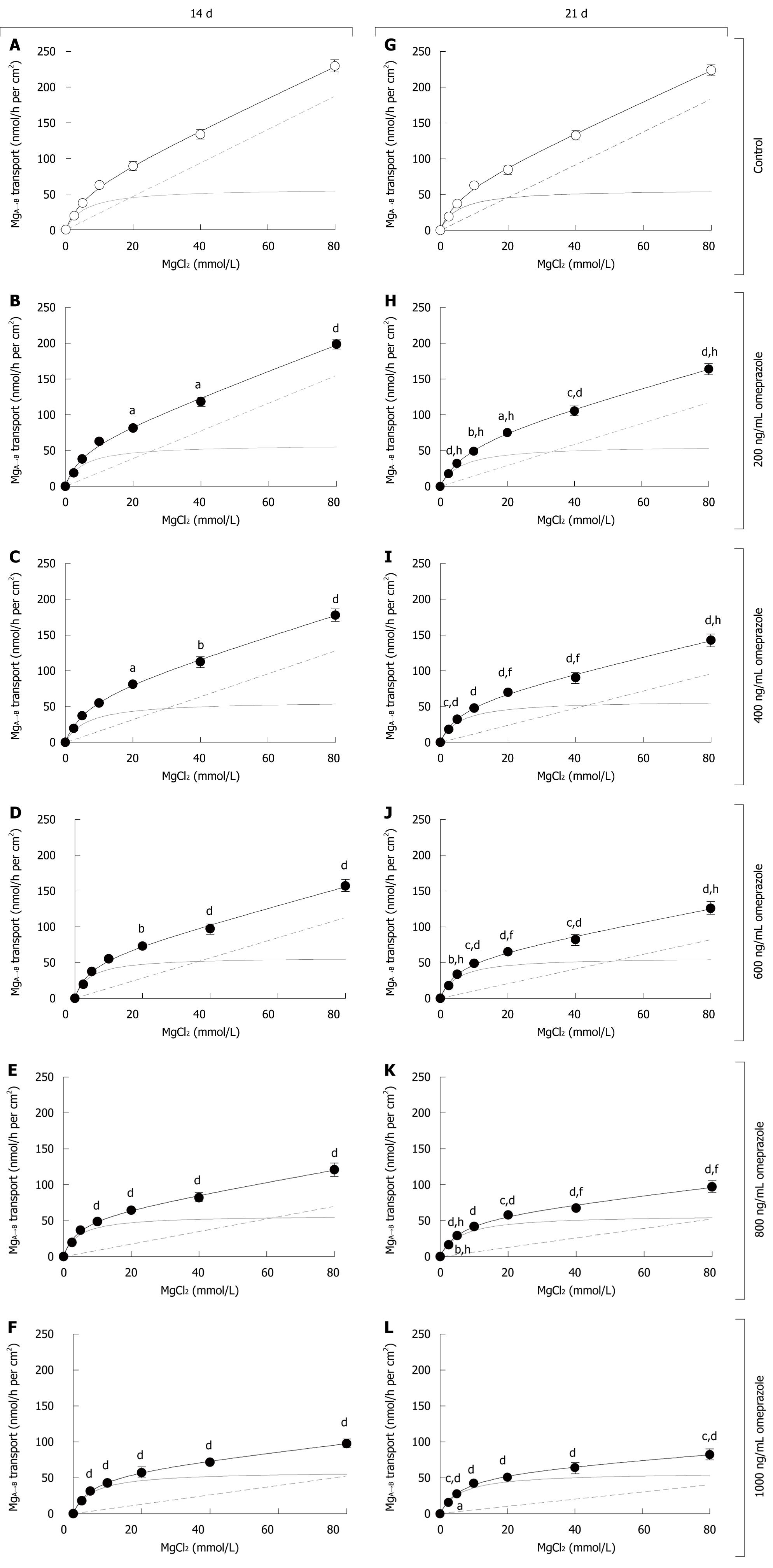
Omeprazole decreased MgA→B transport and PMgin both a dose- and time-dependent manner
As demonstrated in Figure 1, the MgA→B transport vs Mg2+ concentration plots of Caco-2 monolayers were curvilinear similar to that reported in humans[5]. After 14 d in the omeprazole-treated groups, MgA→B transport was inhibited when compared with its corresponding untreated group (Figures 1A-F). The level of inhibition progressively increased with higher concentrations of omeprazole. Omeprazole selectively decreased non-saturable MgA→B transport, but not the saturated component, as clearly demonstrated by the lower rate constant for non-saturable MgA→B transport (Table 1). For 21 d omeprazole-treated groups, the results were similar to those of the 14 d omeprazole-treatment (Figure 1G-L, Table 1). When the same omeprazole concentration was considered, 21 d-treated groups showed a significantly lower MgA→B transport than the 14 d-treated groups (Figure 1, Table 1). Therefore, omeprazole decreased MgA→B transport in a dose- and time-dependent manner. According to the MgA→B transport, omeprazole also decreased the apical to basolateral PMg in a dose- and time-dependent mechanism (Figure 2). Moreover, omeprazole significantly increased TER, but not PD or ISC, of Caco-2 monolayers (Table 2), indicating the lower paracellular permeability to ion transport.
| Vm (nmol/hper cm2) | Km (mmol/L) | m (× 10-3 cm/h) | |
| 14 d | |||
| Control | 57.22 ± 8.41 | 5.62 ± 1.83 | 2.18 ± 0.11 |
| Omeprazole treated (ng/mL) | |||
| 200 | 58.12 ± 6.19 | 4.55 ± 1.23 | 1.80 ± 0.08b |
| 400 | 62.82 ± 9.64 | 5.83 ± 2.22 | 1.54 ± 0.12b |
| 600 | 57.01 ± 7.49 | 4.48 ± 1.44 | 1.28 ± 0.09b |
| 800 | 59.35 ± 7.40 | 4.71 ± 1.40 | 0.83 ± 0.09b |
| 1000 | 58.84 ± 7.52 | 5.84 ± 1.59 | 0.52 ± 0.10b |
| 21 d | |||
| Control | 55.82 ± 8.02 | 5.09 ± 1.89 | 2.17 ± 0.10 |
| Omeprazole treated (ng/mL) | |||
| 200 | 57.81 ± 10.41 | 7.48 ± 2.24 | 1.26 ± 0.12bd |
| 400 | 53.47 ± 7.59 | 5.10 ± 1.87 | 1.18 ± 0.10bd |
| 600 | 53.12 ± 7.53 | 4.42 ± 1.68 | 0.96 ± 0.09bd |
| 800 | 57.65 ± 6.76 | 5.97 ± 1.51 | 0.53 ± 0.08bd |
| 1000 | 57.99 ± 7.72 | 6.09 ± 1.48 | 0.49 ± 0.07b |
| n | PD (mV) | ISC (mA/cm2) | TER (Ω.cm2) | |
| 14 d | ||||
| Control | 9 | 0.99 ± 0.12 | 3.09 ± 0.36 | 322.19 ± 6.37 |
| Omeprazole treated (ng/mL) | ||||
| 200 | 9 | 1.03 ± 0.54 | 2.52 ± 0.39 | 413.64 ± 12.95 |
| 400 | 9 | 0.98 ± 0.14 | 2.24 ± 0.29 | 433.23 ± 17.66 |
| 600 | 9 | 1.08 ± 0.15 | 2.29 ± 0.31 | 470.35 ± 23.87d |
| 800 | 9 | 1.09 ± 0.18 | 2.30 ± 0.41 | 483.22 ± 20.20d |
| 1000 | 9 | 1.20 ± 0.11 | 2.41 ± 0.20 | 502.88 ± 30.99d |
| 21 d | ||||
| Control | 9 | 1.00 ± 0.15 | 3.18 ± 0.47 | 314.05 ± 4.64 |
| Omeprazole treated (ng/mL) | ||||
| 200 | 9 | 1.26 ± 0.20 | 2.48 ± 0.32 | 485.09 ± 24.36b |
| 400 | 9 | 1.13 ± 0.19 | 2.20 ± 0.32 | 502.19 ± 27.47d |
| 600 | 9 | 1.06 ± 0.13 | 2.21 ± 0.34 | 500.33 ± 32.97d |
| 800 | 9 | 0.99 ± 0.19 | 1.97 ± 0.31 | 481.64 ± 25.48d |
| 1000 | 9 | 1.07 ± 0.18 | 2.06 ± 0.30 | 500.84 ± 26.61d |
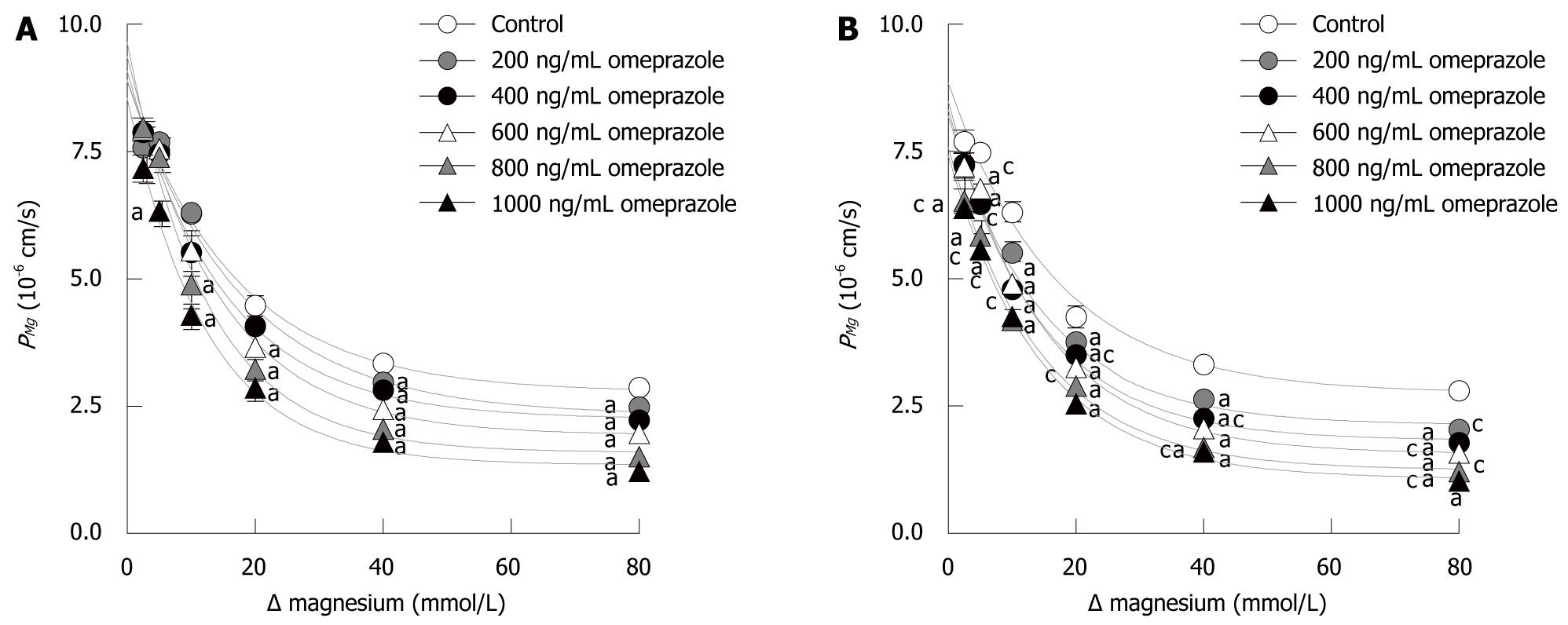
Since the MgB→A transport occurred solely through the paracellular pathway, the MgB→A transport vs Mg2+ concentration plot was linear (Figure 3A). Omeprazole significantly decreased the slope of the MgB→A transport-Mg2+ concentration plot. The slope progressively decreased with increased concentration of omeprazole (Figure 3A). In addition, omeprazole significantly suppressed the basolateral to apical PMg in a dose-dependent manner (Figure 3B). The collective results clearly showed that omeprazole suppressed paracellular passive Mg2+ transport across Caco-2 monolayers.
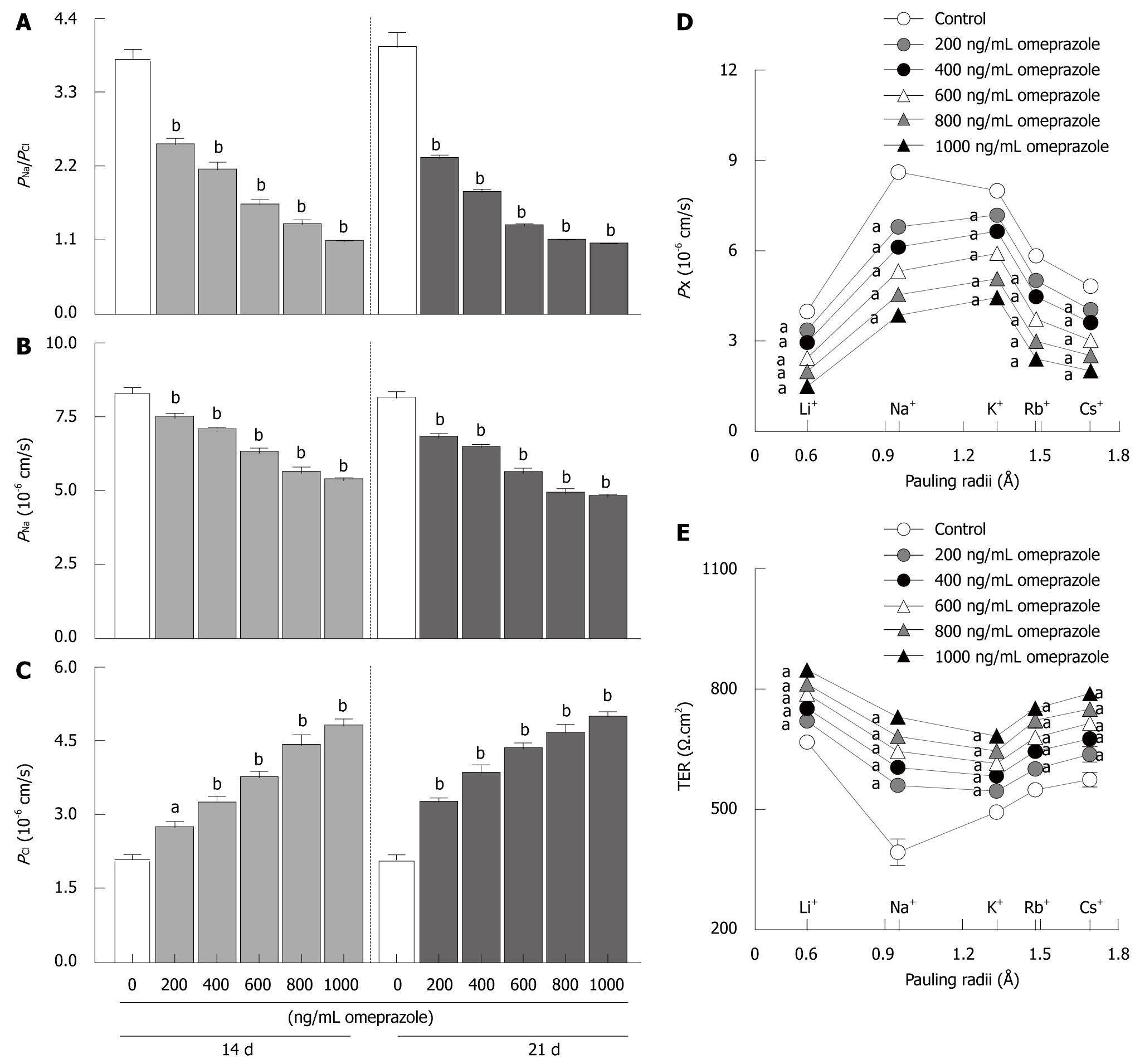
Similar to previous reports[21,22], Caco-2 monolayers showed high PNa/PCl (3.79 ± 0.15 in 14 d monolayers; 3.96 ± 0.22 in 21 d monolayers) from the higher PNa (8.28 ± 0.20 in 14 d monolayers; 8.11 ± 0.25 in 21 d monolayers) than PCl (2.08 ± 0.09 in 14 d monolayers; 2.07 ± 0.11 in 21 d monolayers) (Figure 4A-C). Therefore, the Caco-2 monolayer was a cation selective epithelium. In 14 d- as well as 21 d-omeprazole-treated groups, omeprazole significantly suppressed PNa/PCl and PNa but enhanced PCl in a dose-dependent manner (Figure 4A-C), indicating that omeprazole decreased cation selectivity of Caco-2 monolayers.
Moreover, the present study also examined the paracellular permeability to monovalent cations, i.e. Li+, Na+, K+, Rb+, and Cs+. In control conditions, Caco-2 monolayers showed the following selective sequence: PNa (8.62 ± 0.18) > PK (7.99 ± 0.19) > PRb (5.84 ± 0.08) > PCs (4.82 ± 0.07) > PLi (3.98 ± 0.12) (Figure 4D). Interestingly, 14 d-omeprazole (600 ng/mL)-exposed monolayers showed a different permeability sequence as follows: PK > PNa> PRb > PCs > PLi (Figure 4D). In addition, omeprazole also inhibited Caco-2 permeability to all of these monovalent cations in a dose-dependent manner.
In a parallel study, TER was simultaneously recorded when the monolayers were exposed to group I alkaline metals containing solution. In control conditions, Caco-2 monolayers showed the highest conductance (lowest TER) to Na+ (Figure 4E). The TER-Pauling radii relationship showed a V-shaped profile. Omeprazole-treated Caco-2 monolayers showed the lowest TER when the primary ion was K+ (Figure 4E). Omeprazole also changed the TER-Pauling radii graph to a U-shape relationship and increased TER in all groups.
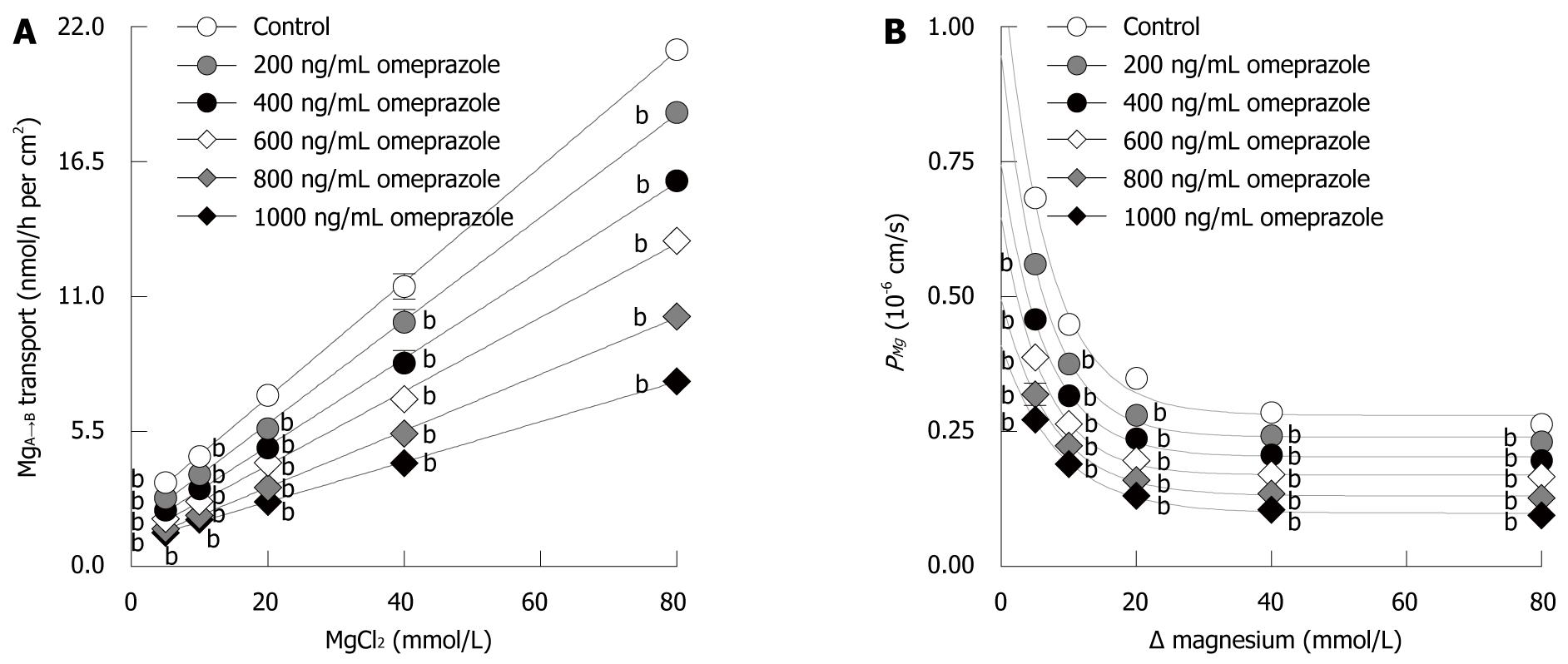
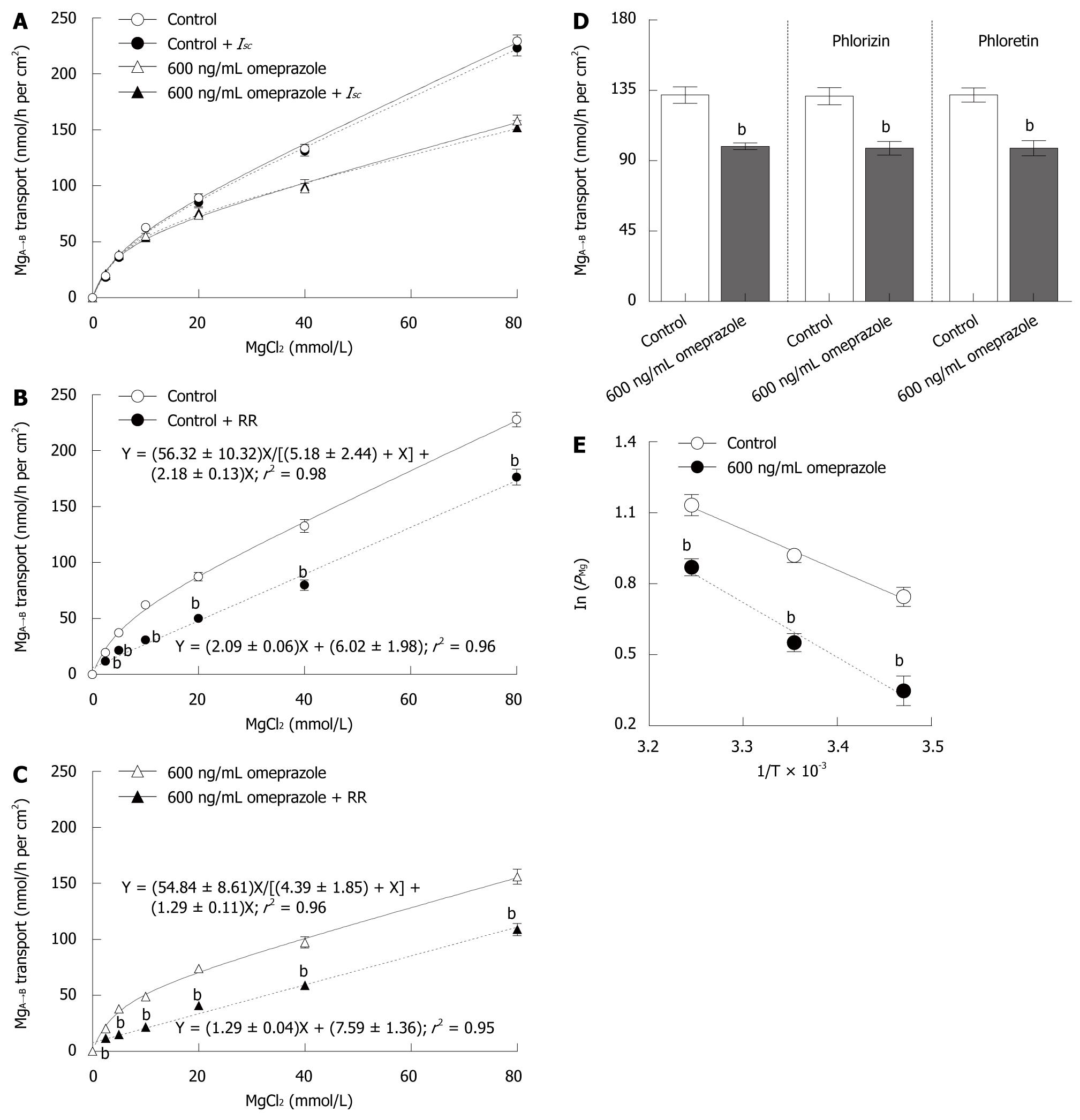
Theoretically, ions can move transversely across Caco-2 monolayer via four transport mechanisms, i.e. solvent drag-induced active, voltage dependent active, transcellular active, and paracellular passive transport. Therefore, the present experiment aimed to identify the relative involvement of each mechanism in Mg2+ transport across Caco-2 monolayers. Inhibitors of solvent drag-induced ion transport (phlorizin and phloretin) had no effect on MgA→B transport (40 mmol/L Mg2+ concentration gradient) in both control and omeprazole-treated monolayers (Figure 5D). In another set of experiments, Caco-2 monolayers received continuous application of Isc, simultaneously with the Mg2+ flux study, to nullify trans-epithelial PD and to abolish voltage dependent Mg2+ transport. The MgA→B transport in both control and omeprazole-treated monolayers were unaffected by Isc, (Figure 5A). The results indicated that solvent drag-induced and voltage dependent MgA→B transport were negligible.
Since transcellular Mg2+ transport required apical Mg2+ influx, inhibition of Mg2+ influx should abolish Mg2+ transport. When 20 μmol/L ruthenium red (RR), a TRPM6 inhibitor[23], was added to the apical solution, a linear relationship between MgA→B transport and Mg2+ concentration was observed (Figure 5B). The rate constant for non-saturable MgA→B transport of control monolayers (2.18 ± 0.13) was not different from the slope of MgA→B transport (2.09 ± 0.06) of RR-treated control monolayers. In parallel experiments, 14 d-600 ng/mL omeprazole-treated monolayers were bathed in bathing solution with or without 20 μmol/L RR (Figure 5C). Similar to control conditions, RR inhibited the saturable component, but not the non-saturable component, of MgA→B transport in omeprazole-treated monolayers. In RR-treated monolayers, the 14 d-omeprazole-treated group showed a less steep slope when compared with that of the control group (1.29 ± 0.04 vs 2.09 ± 0.06, P < 0.001, Figure 5B and C). Therefore, omeprazole suppressed the non-saturable passive Mg2+ transport across Caco-2 monolayers.
To elucidate the temperature dependent Mg2+ transport, Caco-2 monolayers were bathed in 40 mmol/L MgCl2 containing apical solution, while the basolateral solution had no MgCl2. As shown by the Arrhenius plot (Figure 5E), the ln(PMg) decreased in lower temperatures. The control monolayers showed Ea of 14.28 ± 1.19 kJ/mol and Q10 of 1.22 ± 0.04. Fourteen days of 600 ng/mL omeprazole exposure significantly suppressed Mg2+ transport and increased Ea (19.24 ± 1.98 kj/mol, P < 0.05), but not Q10 (1.31 ± 0.05), of Caco-2 monolayers.
The present study demonstrated the effect of omeprazole on Mg2+ transport across Caco-2 intestinal epithelium. Omeprazole-treated monolayers showed a dose- and time-dependent decrease in Mg2+ transport and PMg (Figures 1-3). Omeprazole selectively inhibited the non-saturable passive component, but not the saturable active component, of transepithelial Mg2+ transport (Table 1 and Figure 5). The paracellular cation selectivity of the monolayers was also reduced after prolonged exposure to omeprazole (Figure 4). Results of the Arrhenius plot (Figure 5) showed the higher Ea in the omeprazole-treated group, indicating impediment of the paracellular channel to Mg2+ movement.
In humans, intestinal Mg2+ absorption vs Mg2+ intake exhibited a curvilinear relationship[5] from the combination of saturable active and non-saturable passive absorption. Moreover, lower intestinal passive Mg2+ absorption as compared with passive Ca2+ absorption was also demonstrated5,24]. Similarly, in Caco-2 monolayers, a plot of MgA→B transport (representing Mg2+ absorption) against Mg2+ concentration (in apical solution) was also curvilinear (Figure 1) and MgA→B transport was lower than the apical to basolateral Ca2+ transport[21]. Therefore, the Caco-2 monolayer was a suitable in vitro model of intestinal Mg2+ absorption[16].
Several case reports demonstrated severe hypomagnesemia associated with prolonged omeprazole usage[10-14], suggesting that intestinal Mg2+ absorption, but not renal Mg2+ handling, was defective. On the other hand, short-term omeprazole administration had no effect on intestinal Mg2+ absorption[25] because its bioavailability was low and its half-life was short[9,26]. Therefore, the later development of hypomagnesemia was probably associated with the depletion of Mg2+ store in the human body[13]. The present study demonstrated an inhibitory effect of omeprazole on Mg2+ fluxes across 14 and 21 d-omeprazole-treated Caco-2 monolayers, suggesting that the intestinal Mg2+ flux defect could not be responsible for later development of hypomagnesemia in omeprazole use.
There are two transport mechanisms for Mg2+ absorption, i.e. transcellular active and paracellular passive transport, across the intestinal epithelium[7]. Previous reports suggested that omeprazole inhibited active intestinal Mg2+ absorption and TRPM6 activity because high dose oral Mg2+ supplement partially[13] and totally[14] resolved hypomagnesemia in prolonged omeprazole use. On the other hand, other reports showed different results i.e. high dose oral Mg2+ supplement, but not intravenous Mg2+ replacement and withdrawal of omeprazole, failed to normalize plasma and urinary Mg2+ levels[10,12]. The later evidence indicated that omeprazole inhibited passive Mg2+ absorption, which agreed with the present findings. In the present study, omeprazole inhibited the non-saturable passive, but not saturable active, Mg2+ transport across Caco-2 monolayers (Table 1). In addition, the role of transcellular active Mg2+ transport was examined using the TRPM6 inhibitor RR. Inhibition of TRPM6 in Caco-2 cells[27] abolished the saturable active Mg2+ transport and revealed the inhibition of non-saturable passive Mg2+ transport in omeprazole-treated monolayers (Figure 5B and C). Therefore, the paracellular passive Mg2+ absorption defect should be recognized in omeprazole usage.
Consistent with previous findings that the paracellular passive transport of cations, such as Na+, Cs+, H+, Ca2+, and Mg2+, was a temperature variance mechanism[18,20], the present Arrhenius plot (Figure 5E) showed the temperature-dependent Mg2+ transport. Since the temperature coefficient Q10 of passive ion diffusion through the open ion channel ranged from 1.2 to 1.4[28] and the paracellular pore of the tight junction behaved as the channel[20], therefore, the Q10 of control (1.22) and omeprazole treated (1.31) monolayers indicated that Mg2+ mainly moved through the paracellular channels of Caco-2 epithelium. The paracellular passive H+ transport occurred via the claudin-8 channel of MDCK II epithelium[18]. The paracellular claudin-8 channel was found to impede H+ transport by increasing the Ea[18]. Therefore, the higher Ea of omeprazole-treated Caco-2 epithelium suggested that the paracellular channel of Caco-2 epithelium impeded Mg2+ transport. In addition, the higher TER (Table 2) also indicated lower paracellular permeability. The present study supported a previous report by Hou et al[29], who demonstrated that the epithelium with higher TER showed lower passive Mg2+ transport.
The paracellular transport of Mg2+ was regulated by the paracellular charge selectivity, i.e. cation selectivity, of the tight junction[29,30]. Caco-2 epithelium was a cation selective epithelium (Figure 4A-C)[21,22] that favored the transport of cations through the paracellular pathway. Similar to a previous report[21], the paracellular selective permeability profile of Caco-2 monolayers to monovalent cations was Na+ > K+ > Rb+ > Cs+ > Li+ (Figure 4D) which was classified as series VII of the Eisenman sequence[31]. Series VII indicated the presence of moderate negative electrical field strength in the paracellular channel of Caco-2 epithelium. However, omeprazole changed the selective permeability profile to series VI of the Eisenman sequence (K+ > Na+ > Rb+ > Cs+ > Li+)[31]. Since series VI was characterized by lower negative electrical field strength than that of series VII[31], the paracellular cation selectivity was decreased when the monolayers were exposed to omeprazole (Figure 4A-C). Hou et al[29] also demonstrated lower paracellular Mg2+ transport due to lower paracellular cation selectivity of the epithelium. Thereby, omeprazole-induced suppression of paracellular cation selectivity led to the inhibition of paracellular Mg2+ transport across Caco-2 epithelium.
The present study demonstrated the inhibitory effect of omeprazole on passive Mg2+ transport which was consistent with previous reports[29,30,32-34]. The paracellular passive Mg2+ transport was mainly mediated by claudins at the tight junction[29,30], the distribution of which could be affected by the change in extracellular fluid pH. Inhibition of H+/K+-ATPase activity in Caco-2 cells[35] by omeprazole might decrease the extracellular H+ concentration, which in turn increased the sensitivity of the extracellular calcium sensing receptor (CaSR)[32,33], which was expressed in Caco-2 cells[36,37]. Ikari et al[34] clearly demonstrated that the activation of CaSR led to the translocation of claudin-16 from the tight junction into the cell, thus inhibiting paracellular Mg2+ transport. Therefore, omeprazole-inhibited passive Mg2+ transport appeared to involve the CaSR-tight junction-dependent mechanism.
In conclusion, omeprazole inhibited paracellular passive Mg2+ transport across Caco-2 epithelium in a dose- and time-dependent fashion. The inhibition of passive Mg2+ transport was due to the decrease in paracellular cation selectivity. The results from the present study provided evidence for the regulation of intestinal Mg2+ absorption. However, the underlying mechanism of omeprazole inhibiting passive Mg2+ transport requires further study.
Previously, it was widely believed that intestinal Mg2+ transport in humans depended on the amount of Mg2+ intake and was not tightly regulated by any hormones. Omeprazole, a common therapeutic drug for acid-peptic disorders, has been found to have effects on Mg2+ metabolism.
Several previous reports have demonstrated an association between severe hypomagnesemia and prolonged omeprazole usage in humans. Those patients had normal renal Mg2+ handling, suggesting that a defect in intestinal Mg2+ absorption may be responsible for hypomagnesemia. However, the direct action of omeprazole on intestinal Mg2+ absorption is unknown. In this manuscript, an inhibitory effect of omeprazole on intestinal passive Mg2+ absorption is demonstrated.
In this manuscript, the authors reported a direct inhibitory action of prolonged omeprazole treatment on paracellular passive Mg2+ absorption across the intestinal epithelium. This finding provides an explanation on how prolonged usage of omeprazole could lead to hypomagnesemia.
Acid-peptic disorders, e.g. gastro-oesophageal reflux disease, erosive oesophagitis, heartburn, and Barrett’s disease, are chronic diseases that require prolonged omeprazole administration. Therefore, plasma Mg2+ assessment should help prevent hypomagnesemia in these patients.
The paracellular charge selectivity is a property of epithelium that is selectively permeable to specific charged molecules, e.g. ions. This property is regulated by proteins of the tight junction, i.e. claudins. Alterations in claudin expression in the tight junction directly affect the charge selectivity and the paracellular ion transport across the epithelium.
This is an interesting paper investigating the inhibitory action of omeprazole on magnesium transepithelial transport at intestinal level. The study is well-done, the rationale is clear, the experimental design correct, and the results shown convincingly support the conclusions drawn.
Peer reviewer: Vittorio Ricci, MD, PhD, Department of Physiology, Human Physiology Section, University of Pavia Medical School, Via Forlanini 6, Pavia, 27100, Italy
S- Editor Shi ZF L- Editor Webster JR E- Editor Ma WH
| 1. | Durlach J. Magnesium depletion and pathogenesis of Alzheimer’s disease. Magnes Res. 1990;3:217-218. [Cited in This Article: ] |
| 2. | Rude RK, Gruber HE. Magnesium deficiency and osteoporosis: animal and human observations. J Nutr Biochem. 2004;15:710-716. [Cited in This Article: ] |
| 3. | Touyz RM. Role of magnesium in the pathogenesis of hypertension. Mol Aspects Med. 2003;24:107-136. [Cited in This Article: ] |
| 4. | Konrad M, Schlingmann KP, Gudermann T. Insights into the molecular nature of magnesium homeostasis. Am J Physiol Renal Physiol. 2004;286:F599-F605. [Cited in This Article: ] |
| 5. | Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Intestinal absorption of magnesium from food and supplements. J Clin Invest. 1991;88:396-402. [Cited in This Article: ] |
| 6. | Schlingmann KP, Waldegger S, Konrad M, Chubanov V, Gudermann T. TRPM6 and TRPM7--Gatekeepers of human magnesium metabolism. Biochim Biophys Acta. 2007;1772:813-821. [Cited in This Article: ] |
| 7. | Quamme GA. Recent developments in intestinal magnesium absorption. Curr Opin Gastroenterol. 2008;24:230-235. [Cited in This Article: ] |
| 8. | Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest. 2008;118:619-628. [Cited in This Article: ] |
| 9. | Olbe L, Carlsson E, Lindberg P. A proton-pump inhibitor expedition: the case histories of omeprazole and esomeprazole. Nat Rev Drug Discov. 2003;2:132-139. [Cited in This Article: ] |
| 10. | Epstein M, McGrath S, Law F. Proton-pump inhibitors and hypomagnesemic hypoparathyroidism. N Engl J Med. 2006;355:1834-1836. [Cited in This Article: ] |
| 11. | Hoorn EJ, van der Hoek J, de Man RA, Kuipers EJ, Bolwerk C, Zietse R. A case series of proton pump inhibitor-induced hypomagnesemia. Am J Kidney Dis. 2010;56:112-116. [Cited in This Article: ] |
| 12. | Shabajee N, Lamb EJ, Sturgess I, Sumathipala RW. Omeprazole and refractory hypomagnesaemia. BMJ. 2008;337:a425. [Cited in This Article: ] |
| 13. | Cundy T, Dissanayake A. Severe hypomagnesaemia in long-term users of proton-pump inhibitors. Clin Endocrinol (Oxf). 2008;69:338-341. [Cited in This Article: ] |
| 14. | Broeren MA, Geerdink EA, Vader HL, van den Wall Bake AW. Hypomagnesemia induced by several proton-pump inhibitors. Ann Intern Med. 2009;151:755-756. [Cited in This Article: ] |
| 15. | Jantarajit W, Thongon N, Pandaranandaka J, Teerapornpuntakit J, Krishnamra N, Charoenphandhu N. Prolactin-stimulated transepithelial calcium transport in duodenum and Caco-2 monolayer are mediated by the phosphoinositide 3-kinase pathway. Am J Physiol Endocrinol Metab. 2007;293:E372-E384. [Cited in This Article: ] |
| 16. | Ekmekcioglu C, Ekmekcioglu A, Marktl W. Magnesium transport from aqueous solutions across Caco-2 cells--an experimental model for intestinal bioavailability studies. Physiological considerations and recommendations. Magnes Res. 2000;13:93-102. [Cited in This Article: ] |
| 17. | Thongon N, Nakkrasae LI, Thongbunchoo J, Krishnamra N, Charoenphandhu N. Enhancement of calcium transport in Caco-2 monolayer through PKCzeta-dependent Cav1.3-mediated transcellular and rectifying paracellular pathways by prolactin. Am J Physiol Cell Physiol. 2009;296:C1373-C1382. [Cited in This Article: ] |
| 18. | Angelow S, Kim KJ, Yu AS. Claudin-8 modulates paracellular permeability to acidic and basic ions in MDCK II cells. J Physiol. 2006;571:15-26. [Cited in This Article: ] |
| 19. | Winkler JP, Cherry RS, Schlesinger WH. The Q10 relationship of microbial respiration in a temperate forest soil. Soil Biol Biochem. 1996;28:1067-1072. [Cited in This Article: ] |
| 20. | Tang VW, Goodenough DA. Paracellular ion channel at the tight junction. Biophys J. 2003;84:1660-1673. [Cited in This Article: ] |
| 21. | Thongon N, Nakkrasae LI, Thongbunchoo J, Krishnamra N, Charoenphandhu N. Prolactin stimulates transepithelial calcium transport and modulates paracellular permselectivity in Caco-2 monolayer: mediation by PKC and ROCK pathways. Am J Physiol Cell Physiol. 2008;294:C1158-C1168. [Cited in This Article: ] |
| 22. | Carr G, Haslam IS, Simmons NL. Voltage dependence of transepithelial guanidine permeation across Caco-2 epithelia allows determination of the paracellular flux component. Pharm Res. 2006;23:540-548. [Cited in This Article: ] |
| 23. | Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19-25. [Cited in This Article: ] |
| 24. | Roth P, Werner E. Intestinal absorption of magnesium in man. Int J Appl Radiat Isot. 1979;30:523-526. [Cited in This Article: ] |
| 25. | Serfaty-Lacrosniere C, Wood RJ, Voytko D, Saltzman JR, Pedrosa M, Sepe TE, Russell RR. Hypochlorhydria from short-term omeprazole treatment does not inhibit intestinal absorption of calcium, phosphorus, magnesium or zinc from food in humans. J Am Coll Nutr. 1995;14:364-368. [Cited in This Article: ] |
| 26. | Boparai V, Rajagopalan J, Triadafilopoulos G. Guide to the use of proton pump inhibitors in adult patients. Drugs. 2008;68:925-947. [Cited in This Article: ] |
| 27. | Flach CF, Qadri F, Bhuiyan TR, Alam NH, Jennische E, Holmgren J, Lönnroth I. Differential expression of intestinal membrane transporters in cholera patients. FEBS Lett. 2007;581:3183-3188. [Cited in This Article: ] |
| 28. | Hille B. Ion channels of Excitable Membranes. Massachusetts: Sinauer Associates 2001; . [Cited in This Article: ] |
| 29. | Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci. 2005;118:5109-5118. [Cited in This Article: ] |
| 30. | Shan Q, Himmerkus N, Hou J, Goodenough DA, Bleich M. Insights into driving forces and paracellular permeability from claudin-16 knockdown mouse. Ann N Y Acad Sci. 2009;1165:148-151. [Cited in This Article: ] |
| 31. | Eisenman G, Horn R. Ionic selectivity revisited: the role of kinetic and equilibrium processes in ion permeation through channels. J Membr Biol. 1983;76:197-225. [Cited in This Article: ] |
| 32. | Quinn SJ, Bai M, Brown EM. pH Sensing by the calcium-sensing receptor. J Biol Chem. 2004;279:37241-37249. [Cited in This Article: ] |
| 33. | Doroszewicz J, Waldegger P, Jeck N, Seyberth H, Waldegger S. pH dependence of extracellular calcium sensing receptor activity determined by a novel technique. Kidney Int. 2005;67:187-192. [Cited in This Article: ] |
| 34. | Ikari A, Okude C, Sawada H, Sasaki Y, Yamazaki Y, Sugatani J, Degawa M, Miwa M. Activation of a polyvalent cation-sensing receptor decreases magnesium transport via claudin-16. Biochim Biophys Acta. 2008;1778:283-290. [Cited in This Article: ] |
| 35. | Abrahamse SL, Bindels RJ, van Os CH. The colon carcinoma cell line Caco-2 contains an H+/K+-ATPase that contributes to intracellular pH regulation. Pflugers Arch. 1992;421:591-597. [Cited in This Article: ] |
| 36. | Davies SL, Gibbons CE, Steward MC, Ward DT. Extracellular calcium- and magnesium-mediated regulation of passive calcium transport across Caco-2 monolayers. Biochim Biophys Acta. 2008;1778:2318-2324. [Cited in This Article: ] |
| 37. | Gama L, Baxendale-Cox LM, Breitwieser GE. Ca2+-sensing receptors in intestinal epithelium. Am J Physiol. 1997;273:C1168-C1175. [Cited in This Article: ] |