Published online Mar 14, 2011. doi: 10.3748/wjg.v17.i10.1368
Revised: December 1, 2010
Accepted: December 8, 2010
Published online: March 14, 2011
AIM: To investigate the endoscopic hemostasis for gastrointestinal bleeding due to Dieulafoy’s lesion.
METHODS: One hundred and seven patients with gastrointestinal bleeding due to Dieulafoy’s lesion were treated with three endoscopic hemostasis methods: aethoxysklerol injection (46 cases), endoscopic hemoclip hemostasis (31 cases), and a combination of hemoclip hemostasis with aethoxysklerol injection (30 cases).
RESULTS: The rates of successful hemostasis using the three methods were 71.7% (33/46), 77.4% (24/31) and 96.7% (29/30), respectively, with significant differences between the methods (P < 0.05). Among those who had unsuccessful treatment with aethoxysklerol injection, 13 were treated with hemoclip hemostasis and 4 underwent surgical operation; 9 cases were successful in the injection therapy. Among the cases with unsuccessful treatment with hemoclip hemostasis, 7 were treated with injection of aethoxysklerol and 3 cases underwent surgical operation; 4 cases were successful in the treatment with hemoclip hemostasis. Only 1 case had unsuccessful treatment with a combined therapy of hemoclip hemostasis and aethoxysklerol injection, and surgery was then performed. No serious complications of perforation occurred in the patients whose bleeding was treated with the endoscopic hemostasis, and no re-bleeding was found during a 1-year follow-up.
CONCLUSION: The combined therapy of hemoclip hemostasis with aethoxysklerol injection is the most effective method for gastrointestinal bleeding due to Dieulafoy’s lesion.
- Citation: Cui J, Huang LY, Liu YX, Song B, Yi LZ, Xu N, Zhang B, Wu CR. Efficacy of endoscopic therapy for gastrointestinal bleeding from Dieulafoy’s lesion. World J Gastroenterol 2011; 17(10): 1368-1372
- URL: https://www.wjgnet.com/1007-9327/full/v17/i10/1368.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i10.1368
Dieulafoy’s lesion was first reported by Gallad in 1884, and a systemic description was made by Dieulafoy in 1903. It is also called Dieulafoy’s vascular malformation[1]. Patients with gastrointestinal bleeding due to Dieulafoy’s lesion in our hospital were treated with the endoscopic hemostasis, and a satisfactory therapeutic efficacy was achieved.
One hundred and seven patients with gastrointestinal bleeding due to Dieulafoy’s lesion were enrolled in this study. There were 70 males and 37 females, with a mean age of 54 years. Clinical presentations were repeated hematemesis, a dark or bloody stool, the amount of blood loss of 800-2000 mL, shock occurring in some patients, and hemoglobin 46-80 g/L. No obvious epigastric discomfort and abdominal pain were noted, and there was no history of peptic ulcer or heredity disease.
(1) The bottom of the ulcer had exposed vessels (most were dilated small arteries); (2) The ulcer was superficial and small, and the diameter was less than 0.5 cm; (3) Spurting blood from the ulcer was observed in the active phase, and if bleeding stopped, a black blood scab was observed with endoscopic examination and was easily misdiagnosed; (4) Dieulafoy’s lesion occurred mostly in elderly people, possibly because of a small vascular malformation or arteriosclerosis; and (5) Dieulafoy’s lesion was mostly located at the gastric body, or at the juncture of the gastric fundus and the gastric body[2-4].
Emergent endoscopic examination was performed for all the patients. Blood clotting was observed and they were flushed with saline. Fresh blood clotting covered the mucosa in 42 cases (39.3%), blood spurting from a small artery occurred in 32 cases (29.9%), ruptured vessels occurred in 33 cases (30.8%), and no obvious hyperemia was observed in the surrounding mucosa. Lesions were found in the following areas: the upper part of the gastric corpus (36 cases, 33.6%), surrounding the cardia (28 cases, 26.2%), the gastric fundus (18 cases, 16.8%), the middle of the gastric body (5 cases, 4.7%), the lower part of the gastric body (4 cases, 3.7%), the gastric antrum (3 cases, 2.8%), the duodenum (2 cases, 1.9%), the descending part of the duodenum (2 cases, 1.9%), the rectum and colon (6 cases, 5.6%), and the esophagus (3 cases, 2.8%).
Patients were randomly divided into 3 groups according to the three types of endoscopic therapy used.
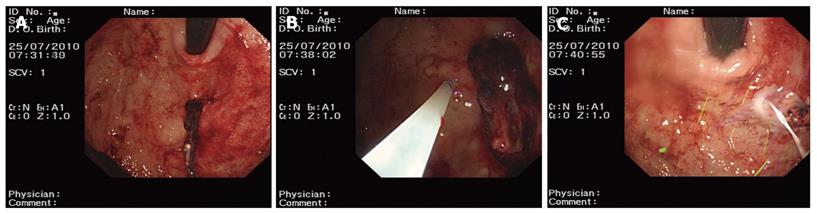
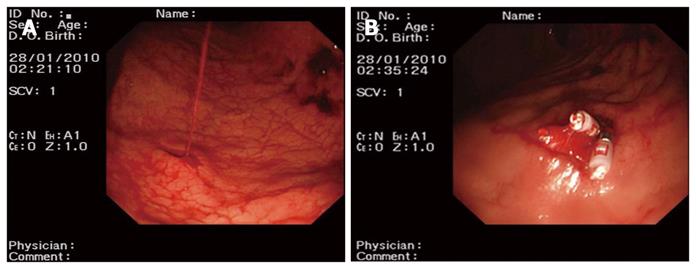
Group one: patients received aethoxysklerol injection (n = 46 cases). A concentration of 1% aethoxysklerol was injected around the blood vessels beneath the mucosa and 0.5 mL was used for each injected site. Three to 6 sites were injected and the amount of aethoxysklerol used was 3-5 mL. Aethoxysklerol was injected into the ruptured site of the blood vessel (Figure 1).
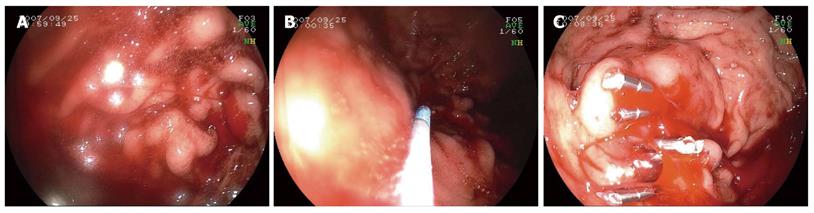
Group two: patients were treated with endoscopic hemoclip hemostasis (n = 31 cases). We used an Olympus HX-5LR-1 and Olympus clip HX-600-135 for bleeding arteries. Hemoclip hemostasis was performed until the vessel stopped bleeding (Figure 2).
Group three: patients received a combined therapy of hemoclip hemostasis and aethoxysklerol injection (n = 30 cases). For bleeding arteries, hemoclip hemostasis was used until the bleeding stopped. A concentration of 1% aethoxysklerol was injected around the blood vessel beneath the mucosa, and 0.5 mL was used for each injected site. Three to 6 sites were injected and the amount of aethoxysklerol used was 3-5 mL (Figure 3).
The criteria for determining successful endoscopic hemostasis were as follows. (1) Endoscopic demonstration: blood spurting or capillary hemorrhage stopped, and the endoscopic field of view became clear; (2) Clinical manifestations: there was no hematemesis or dark stool after treatment, blood pressure rose to a normal range and was stable, and pulse rate decreased and strengthened.
Criteria for determining unsuccessful endoscopic hemostasis were as follows: (1) hematemesis and/or dark stool occurred 48 h after endoscopic treatment; (2) hemoglobin was decreased by more than 20 g/L; (3) there was evidence of hypovolemic shock; and (4) there was manifestation of bleeding and blood transfusion was necessary. Re-bleeding was confirmed at the original site as demonstrated by endoscopic examination.
Rates of successful hemostasis and perforation among the groups were compared using the χ2 test (SPSS 10.0). P < 0.05 was considered as a significant difference.
One hundred and seven patients received endoscopic hemostasis in this study. Eight cases had unsuccessful treatment with endoscopic hemostasis and underwent a surgical operation. No serious complications such as perforation occurred in the 99 cases whose bleeding was treated with endoscopic hemostasis, and no re-bleeding was found during a 1-year follow-up.
The success rate of hemostasis by aethoxysklerol injection was 71.7% (33/46). Re-bleeding occurred in 13 cases in a short period after treatment (less than 48 h). For the re-bleeding cases, the hemoclip hemostasis was used; 9 cases were successfully treated and 4 cases whose bleeding was not controlled received a surgical operation.
The success rate of hemostasis by endoscopic hemoclip hemostasis was 77.4% (24/31). Re-bleeding occurred in 7 cases in a short period afters treatment (less than 48 h) and was treated with aethoxysklerol injection. Four cases were successfully treated and 3 unsuccessful cases were treated surgically.
The successful rate of hemostasis by the combined therapy of hemoclip hemostasis with aethoxysklerol injection was 96.7% (29/30). Re-bleeding occurred in 1 case in a short period after treatment (less than 48 h), and he was treated by surgical operation.
Dieulafoy’s lesion is a type of rare congenital vascular malformation, and it is also called “Dieulafoy’s ulcer” or “constant diameter artery bleeding”. The lesion can occur in any part of the gastrointestinal tract, such as the esophagus, colon, and small intestine. However, it often occurs in the lesser curvature of the stomach 6 cm from the cardioesophageal junction[5-8]. While Dieulafoy’s lesion has no specific symptoms and is not easily diagnosed, the emergent endoscopic examination is an effective means for its diagnosis. The endoscopic presentations of Dieulafoy’s lesion are as follows: (1) a superficial notch in the gastric mucosa, blood vessels in the mucosa, and coagulum on its surface; (2) a focal defect of lesser curvature of stomach mucosa complicated with active bleeding; (3) small arteries can protrude on the mucosa and active bleeding can occasionally be detected; and (4) occasionally, blood permeation can be detected from the mucosa, and is often detected when bleeding[9-12].
The bleeding of Dieulafoy’s lesion can be unexpected, with no obvious cause. Patients often do not have other bleeding diseases such as peptic ulcers and cirrhosis, since the ruptured vessel is an artery with a constant diameter. If the bleeding is serious, patients often complain of hematemesis or hematemesis accompanied by a dark stool[13-16]. The pathogenesis of Dieulafoy’s lesion is considered as a congenital vascular malformation. Normally, the diameter of the gastrointestinal artery gradually decreases to 0.12-0.2 mm, branching to the mucosa. However, the diameter of the artery in a Dieulafoy’s lesion does not decrease; it is abnormally dilated and its diameter is 0.4-4 mm. This constant arterial diameter leads to the pathology of Dieulafoy’s lesion. The abnormally dilated artery travels under the mucosa. The mucosa in Dieulafoy’s lesion is compressed and becomes ischemic, atrophied and thin. A small ulcer can form with the decay of digestive juice and friction from chyme, and if the small artery is exposed, it can rupture and bleed. The artery is often the branch of the left gastric artery; therefore, the bleeding lesion is often located at the lesser curvature of the stomach 6 cm from the cardioesophageal junction[17-19]. When a small artery bleeds, blood pressure decreases, and vasoconstriction and thrombosis occur in the bleeding artery. Bleeding can temporarily stop and the artery is invisible when bleeding stops. It might not be detec by endoscopic examination, even with a surgical operation. If blood pressure rises to normal or the blood clot is shed, serious bleeding can reoccur.
Generally, it is considered that medical treatment plays a minor role in the treatment of Dieulafoy’s lesion; endoscopic therapy and surgery are often performed. The use of pituitrin and somatostatin causes bowel vessels to contract and blood flow to decrease, and these drugs are used for endoscopic therapy and surgical operations. The Dieulafoy’s lesion before the 1980’s was mainly treated with surgical operations. Since Boron first successfully treated 3 cases of Dieulafoy’s lesion in 1987 with the endoscopic therapy[20], an increasing number of doctors have treated this lesion with endoscopic therapy. The death rate decreased from 60%-70% to 20%. Endoscopic treatment has the advantages of being simple in operation, which is easily replicated, is microtraumatic, safe and useful, and it can be performed during the examination. Methods used include injection beneath the mucosa, electric coagulation, laser, heat probe, microwaves, ligation and hemoclips. The efficacy was reported to be more than 80%[21-28].
Three types of endoscopic therapy were used in this study for the treatment of bleeding due to Dieulafoy’s lesion. Aethoxysklerol injection therapy can result in sclerosis and emphraxis to prevent re-bleeding. Local tissue edema occurred and the surrounding pressure of the bleeding site increased when aethoxysklerol was injected. The artery was compressed and thrombosis occurred in the vessel. When aethoxysklerol was injected around the vessel, edema and inflammation rapidly occurred, with fibroblast proliferation. Forty-six cases received this type of therapy and the rate of successful hemostasis was 71.7% (33/46). We also treated patients with endoscopic hemoclip hemostasis. The mechanism of hemoclip hemostasis is similar to that of surgical vascular suturing. In our study, 31 cases received this type of therapy, and the rate of successful hemostasis was 77.4% (24/31). The third method we used in this study was a combined therapy of hemoclip hemostasis with injection of aethoxysklerol. Thirty cases received this therapy, and the rate of successful hemostasis was 96.7% (29/30). We found that this combined therapy was the most effective method for stopping bleeding from a Dieulafoy’s lesion, and it could effectively reduce the re-bleeding rate as well.
Hemoclip hemostasis is widely used for non-variceal active bleeding, and it is indicated for Dieulafoy’s lesion[29,30]. Our study used the following procedure. A clip was assembled on the delivery device. When a bleeding lesion was detected, the delivery device was inserted into the endoscopic working channel to push it to the anterior extremity of the endoscope. The clip was stretched out of the endoscope, and then the clip was stretched open to the largest width (1.2 cm). The direction of the clip could be adjusted with the rotating device on the delivery system. The stretched clip was collimated to the lesion, the gliding lug on the device was pushed back, and the clip was locked. The clip was then released and the delivery device was pulled out. One clip was used for ordinary lesions, and for larger lesions, 2-3 clips were needed. We believe that the key points for successful clipping are as follows: (1) the lesion needs to be directly observed; (2) the lesion and its surrounding tissue should be fully exposed, and the angle of the clip and bleeding site should be in a range of 45-90°; and (3) the depth of the clip should be considered. The optimal depth is where the exposed vessel and deep tissue are able to be clipped. The clip should not be superficial, and if it is superficial, the clip can come off in a short period, and then re-bleeding is inevitable. The clip often releases automatically in 1-3 wk. It is mixed with food debris and the stool, and it is then eliminated from the body. This method is considered as microtraumatic. If it is not successful at the first time, it can be replicated. Even if it fails, it can mark the bleeding site, thereby making the surgical operation easier, and avoiding blind resection. This method is effective for the treatment of Dieulafoy’s lesion, and it is increasingly recognized by doctors. We found that if aethoxysklerol was injected after clipping, the re-bleeding rate in Dieulafoy’s lesion was effectively decreased.
Generally, it is considered that medical treatment plays a minor role in the treatment of bleeding from Dieulafoy’s lesion. Endoscopic therapy and surgical operations are the preferred treatments for this lesion. In recent years, an increasing number of doctors have treated bleeding from a Dieulafoy’s lesion with endoscopic therapy, and the death rate has decreased. The purpose of this study was to explore a new endoscopic method for the treatment of bleeding due to Dieulafoy’s lesion.
Patients with gastrointestinal bleeding due to Dieulafoy’s lesion were treated with the endoscopic hemostasis. Three methods were used: aethoxysklerol injection, endoscopic hemoclip hemostasis, and a combination of hemoclip hemostasis with aethoxysklerol injection.
The authors concluded that a combined therapy of hemoclip hemostasis with aethoxysklerol injection is the most effective method for gastrointestinal bleeding due to Dieulafoy’s lesion.
A combined therapy of hemoclip hemostasis with injection of aethoxysklerol, can be applied successfully in the treatment of bleeding due to a Dieulafoy’s lesion.
Dieulafoy’s lesion: Dieulafoy’s lesion is a type of rare congenital vascular malformation, and it is also called “Dieulafoy’s ulcer” or “constant diameter artery bleeding”. It was first reported in 1898 by a French surgeon named Dieulafoy. Endoscopic hemostasis: A kind of endoscopic method to control bleeding.
Since the nature of bleeding from Dieulafoy’s lesion poses several technical difficulties in its identification and its nonsurgical treatment, several endoscopic modalities have been used, but the treatment of choice is still unclear. Therefore, comparison of the efficacy and safety among two single hemostatic methods and a combined method applied to more than 100 patients provided useful results. In addition, the technical details given in the text may be helpful for endoscopists for the diagnosis and treatment of bleeding from a Dieulafoy’s lesion.
Peer reviewer: George H Sakorafas, MD, PhD, Consultant Surgeon, Department of Surgery, 251 Hellenic Air Force Hospital, 3, P. Kanellopoulou Av., 115 25 Athens, Greece
S- Editor Sun H L- Editor Ma JY E- Editor Ma WH
| 1. | Sai Prasad TR, Lim KH, Lim KH, Yap TL. Bleeding jejunal Dieulafoy pseudopolyp: capsule endoscopic detection and laparoscopic-assisted resection. J Laparoendosc Adv Surg Tech A. 2007;17:509-512. [Cited in This Article: ] |
| 2. | Ujiki MB, Adler A, Swanstrom LL, Diwan TS, Hansen PD. Emergent pancreaticoduodenectomy for Dieulafoy lesion of the duodenum. Am Surg. 2010;76:656-657. [Cited in This Article: ] |
| 3. | Garg R. Bleeding from a gastric Dieulafoy lesion. Emerg Med J. 2007;24:520. [Cited in This Article: ] |
| 4. | Apiratpracha W, Ho JK, Powell JJ, Yoshida EM. Acute lower gastrointestinal bleeding from a dieulafoy lesion proximal to the anorectal junction post-orthotopic liver transplant. World J Gastroenterol. 2006;12:7547-7548. [Cited in This Article: ] |
| 5. | Nga ME, Buhari SA, Iau PT, Raju GC. Jejunal Dieulafoy lesion with massive lower intestinal bleeding. Int J Colorectal Dis. 2007;22:1417-1418. [Cited in This Article: ] |
| 6. | Alshumrani G, Almuaikeel M. Angiographic findings and endovascular embolization in Dieulafoy disease: a case report and literature review. Diagn Interv Radiol. 2006;12:151-154. [Cited in This Article: ] |
| 7. | Jain R, Chetty R. Dieulafoy disease of the colon. Arch Pathol Lab Med. 2009;133:1865-1867. [Cited in This Article: ] |
| 8. | Pathan NF, El-Fanek H. A 70-year-old man with episodes of upper gastrointestinal bleeding. Dieulafoy lesion/malformation. Arch Pathol Lab Med. 2006;130:e27-e29. [Cited in This Article: ] |
| 9. | Scudiere JR, Cimbaluk D, Jakate S. A 74-year-old man with fatal gastrointestinal bleeding. Ruptured Dieulafoy lesion or caliber-persistent artery. Arch Pathol Lab Med. 2006;130:223-224. [Cited in This Article: ] |
| 10. | Dharia T, Tang SJ, Lara L. Bleeding sigmoid colonic Dieulafoy lesion (with video). Gastrointest Endosc. 2009;70:1028-1029. [Cited in This Article: ] |
| 11. | Hokama A, Takeshima Y, Toyoda A, Yonamine Y, Tomiyama R, Kinjo F, Nishimaki T, Saito A. Images of interest. Gastrointestinal: rectal Dieulafoy lesion. J Gastroenterol Hepatol. 2005;20:1303. [Cited in This Article: ] |
| 12. | Marangoni G, Cresswell AB, Faraj W, Shaikh H, Bowles MJ. An uncommon cause of life-threatening gastrointestinal bleeding: 2 synchronous Dieulafoy lesions. J Pediatr Surg. 2009;44:441-443. [Cited in This Article: ] |
| 13. | Njeru M, Seifi A, Salam Z, Ognibene L. Dieulafoy lesion: a rare cause of gastrointestinal bleeding. South Med J. 2009;102:336-337. [Cited in This Article: ] |
| 14. | Seya T, Tanaka N, Yokoi K, Shinji S, Oaki Y, Tajiri T. Life-threatening bleeding from gastrointestinal stromal tumor of the stomach. J Nippon Med Sch. 2008;75:306-311. [Cited in This Article: ] |
| 15. | Chen YY, Yen HH. Massive bleeding from a rectal dieulafoy lesion: combined multidetector-row CT diagnosis and endoscopic therapy. Surg Laparosc Endosc Percutan Tech. 2008;18:398-399. [Cited in This Article: ] |
| 16. | Wade AD, Kothari SN. Dieulafoy lesion after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2009;5:135-136. [Cited in This Article: ] |
| 17. | Katsinelos P, Paroutoglou G, Pilpilidis I, Tsolkas P, Papagiannis A, Kapelidis P, Trakateli C, Iliadis A, Georgiadou E, Kamperis E. Double Dieulafoy-like lesion in the stomach. Surg Endosc. 2003;17:1324. [Cited in This Article: ] |
| 18. | Soon MS, Chen YY, Yen HH. Extravasation of sclerosant after injection of N-butyl-2-cyanoacrylate for a bleeding gastric Dieulafoy lesion. Endoscopy. 2008;40 Suppl 2:E51-E52. [Cited in This Article: ] |
| 19. | Vats HS, Wengert TJ, Torbey CF. Gastrointestinal stromal tumor with Dieulafoy lesion: a novel association. Clin Med Res. 2006;4:228-229. [Cited in This Article: ] |
| 20. | Boron B, Mobarhan S. Endoscopic treatment of Dieulafoy hemorrhage. J Clin Gastroenterol. 1987;9:518-520. [Cited in This Article: ] |
| 21. | Yilmaz M, Ozütemiz O, Karasu Z, Ersöz G, Günsar F, Batur Y, Aydin A, Tekesin O, Yönetci N, Ilter T. Endoscopic injection therapy of bleeding Dieulafoy lesion of the stomach. Hepatogastroenterology. 2005;52:1622-1625. [Cited in This Article: ] |
| 22. | Kim HK, Kim JS, Son HS, Park YW, Chae HS, Cho YS. Endoscopic band ligation for the treatment of rectal Dieulafoy lesions: risks and disadvantages. Endoscopy. 2007;39:924-925. [Cited in This Article: ] |
| 23. | Yen HH, Chen YY. Endoscopic band ligation for Dieulafoy lesions: disadvantages and risks. Endoscopy. 2006;38:651. [Cited in This Article: ] |
| 24. | Linhares MM, Filho BH, Schraibman V, Goitia-Durán MB, Grande JC, Sato NY, Lourenço LG, Lopes-Filho GD. Dieulafoy lesion: endoscopic and surgical management. Surg Laparosc Endosc Percutan Tech. 2006;16:1-3. [Cited in This Article: ] |
| 25. | Valera JM, Pino RQ, Poniachik J, Gil LC, O’Brien M, Sáenz R, Quigley EM. Endoscopic band ligation of bleeding dieulafoy lesions: the best therapeutic strategy. Endoscopy. 2006;38:193-194. [Cited in This Article: ] |
| 26. | Xavier S. Band ligation of Dieulafoy lesions. Indian J Gastroenterol. 2005;24:114-115. [Cited in This Article: ] |
| 27. | Nunoo-Mensah JW, Alkari B, Murphy GJ, Watson AJ. Rectal Dieulafoy lesions. J Am Coll Surg. 2008;206:388-389. [Cited in This Article: ] |
| 28. | Ruiz-Tovar J, Díe-Trill J, López-Quindós P, Rey A, López-Hervás P, Devesa JM. Massive low gastrointestinal bleeding due to a Dieulafoy rectal lesion. Colorectal Dis. 2008;10:624-625. [Cited in This Article: ] |
| 29. | Casella G, Bonforte G, Corso R, Buda CA, Corti G, Cambareri AR, Magrì F, Baldini V. Rectal bleeding by Dieulafoy-like lesion: successful endoscopic treatment. G Chir. 2005;26:415-418. [Cited in This Article: ] |
| 30. | Lim W, Kim TO, Park SB, Rhee HR, Park JH, Bae JH, Jung HR, Kim MR, lee N, Lee SM. Endoscopic treatment of dieulafoy lesions and risk factors for rebleeding. Korean J Intern Med. 2009;24:318-322. [Cited in This Article: ] |