Published online Mar 14, 2010. doi: 10.3748/wjg.v16.i10.1239
Revised: December 21, 2009
Accepted: December 28, 2009
Published online: March 14, 2010
AIM: To review the clinical course and the management of pseudoaneurysms post-pancreaticoduodenectomy.
METHODS: Medical records of 907 patients who underwent pancreaticoduodenectomies from January 1995 to May 2007 were evaluated retrospectively. The clinical course, management strategy, and outcome of ruptured pseudoaneurysms cases were analyzed.
RESULTS: Twenty-seven (3.0%) of 907 cases had post-operative hemorrhage from ruptured pseudoaneurysms. Pancreatic fistula was evident in 12 (44%) cases. Sentinel bleeding appeared in 21 (77.8%) cases. Of the 27 cases, 11 (41%) cases demonstrated bleeding pseudoaneurysm of the ligated gastroduodenal artery, 8 (30%) of the right, proper, common hepatic artery, 2 (7%) of the right gastric artery, and 4 (15%) of the peripancreatic arteries. The remaining two patients died due to sudden-onset massive hemorrhage and pseudoaneurysm rupture was suspected. Emergent operation was performed on 2 cases directly without angiography. Angiography was attempted in 23 cases. Eighteen (78.2%) cases succeeded to hemostasis; the five failed cases were explored. After embolization of the hepatic artery, five cases developed liver abscesses or infarction and a single case of hepatic failure expired. Gastroduodenal artery embolization with common hepatic artery stent insertion was performed to enhance hepatic artery flow in a single case and was successfully controlled.
CONCLUSION: Bleeding pseudoaneurysms are among the most serious and fatal complications following pancreaticoduodenectomy. Diagnostic angiography has been preferred over endoscopy and is rapidly becoming the standard therapeutic treatment for bleeding pseudoaneurysms.
- Citation: Lee HG, Heo JS, Choi SH, Choi DW. Management of bleeding from pseudoaneurysms following pancreaticoduodenectomy. World J Gastroenterol 2010; 16(10): 1239-1244
- URL: https://www.wjgnet.com/1007-9327/full/v16/i10/1239.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i10.1239
Major pancreatic surgery has advanced greatly in the last two decades with a dramatic decrease in its mortality rate. In the case of pancreaticoduodenectomy, several recent studies have reported mortality rates of less than 5%, with a few reporting mortality rates of less than 1%[1,2]. Despite the reduction in mortality, the morbidity remains high at 40%. Some of the complications following major pancreatic surgery include pancreatic fistula, hemorrhage, delayed gastric emptying, and intra-abdominal abscess. Hemorrhage is one of the main complications following pancreaticoduodenectomy. Its incidence is anywhere between 1.5% and 15%[1,3,4]. Hemorrhage on postoperative day #1 is defined as early, while hemorrhage after postoperative day #1 is termed late or delayed[3,5,6]. Early-onset hemorrhage is usually due to poor hemostasis at the operation site. Delayed hemorrhage occurs with pancreatico-enterostomy leakage and pseudoaneurysm formation, intra-abdominal sepsis or abscess, or marginal ulcer[1,3]. Without prior knowledge and experience, delayed hemorrhage is hard to diagnose and manage. Hence, it is crucial to understand the clinical features and etiology in anticipation and treatment of such delayed bleeding. Delayed hemorrhage, especially from a ruptured pseudoaneurysm, is a rare but rapidly progressing and potentially life threatening complication following pancreaticoduodenectomies. It has been widely studied, yet each study has reported different findings on its incidence, location, and clinical presentation. Recently, positive results have been achieved with angiographic intervention of ruptured pseudoaneurysms and a shift in management strategy from laparotomy to this less invasive alternative has occurred. The purpose of this study was to analyze data on delayed hemorrhage after pancreaticoduodenenctomy due to pseudoaneurysm rupture. The clinical features, management strategy, and outcome of delayed hemorrhage from ruptured pseudoaneurysms were evaluated.
Medical records of 907 patients who received pancreaticoduodenectomies between January 1995 and May 2007 were analyzed retrospectively. Based on the definition of postpancreatectomy hemorrhage from the International Study Group of Pancreatic Surgery (ISGPS), we suggest a definition that includes two times of onset: early hemorrhage, occurring in the first 24 h postoperatively, meaning 24 h after the end of the index operation, and late or delayed hemorrhage, occurring more than 24 h postoperatively. The severity of bleeding can be differentiated into two categories based on the amount of blood loss or transfusion requirements: (a) mild (no clinical impairment and transfusion requirements), or (b) severe (more than 4 or 6 units of packed cells transfused within 24 h; a decrease in hemoglobin of more than 4 g/dL or need for relaparotomy or interventional angiographic therapy due to severe blood loss). Evidence of bleeding in the surgical drains or GI tract 6 to 24 h prior to delayed massive hemorrhage was termed ‘sentinel bleeding’. Pancreatic fistula or leakage was identified by the character of the drained fluid in the surgical drain. A definition of pancreatic fistula is a drain output of any measurable volume of fluid on or after postoperative day 3 with an amylase content greater than 3 times the serum amylase activity. Determination of the bleeding focus and management strategy were based on endoscopy, computed tomography (CT), angiography, and the details of the initial surgery. Medical records were analyzed to determine the effectiveness of the treatment provided and to review prognosis. Preceding complications were also evaluated.
Nine hundred and five patients received pancreaticoduodenectomies [pancreatic duct to jejunal mucosa anastomosis (445 patients), invaginating pancreaticojejunostomy (454 patients), pancreaticogastrostomy (8 patients)]. Total frequency of pancreatic fistula was 43% (389 patients). Thirty-one (3.4%) of 907 patients who received pancreaticoduodenectomies were diagnosed with delayed massive hemorrhage. Twenty-seven [pancreatic cancer (11 patients), bile duct cancer (9 patients), ampulla of vater cancer (7 patients)] of 31 patients were diagnosed with hemorrhage from a ruptured pseudoaneurysm. Twenty-three of the 27 patients were diagnosed by angiography and 2 were diagnosed by re-operation. The remaining 2 patients died due to sudden-onset massive hemorrhage and considering their clinical features, rupture of pseudoaneurysm is suspected in both cases. In total, taking into account both confirmed and suspected ruptured pseudoaneurysms, there were 27 patients with an incidence of 3.0%. Eleven patients died (mortality rate of 1.2%) following pancreaticoduodenectomy. Only 6 of the 11 patient deaths were related to ruptured pseudoaneurysm. Sentinel bleeding was evident in 21 of the 27 cases (prevalence of 77.8%). Reviewing the clinical features of the first episode of bleeding from ruptured pseudoaneurysms, sentinel bleeding appeared in the surgical drains in 11 cases (41%), GI bleeding appeared in 8 cases (30%), and sentinel bleeding and GI bleeding were observed in 2 cases (7%). The clinical features of the ruptured pseudoaneurysms are shown in Table 1.
| Initial sign or symptom | n (%) |
| Intraabdominal drain bleeding | 11 (41) |
| Gastrointestinal (GI) bleeding | 8 (30) |
| Intraabdominal drain bleeding with GI bleeding | 2 (7) |
| Hypotension | 2 (7) |
| Abdominal distension | 2 (7) |
| Syncope | 1 (4) |
| Arrest | 1 (4) |
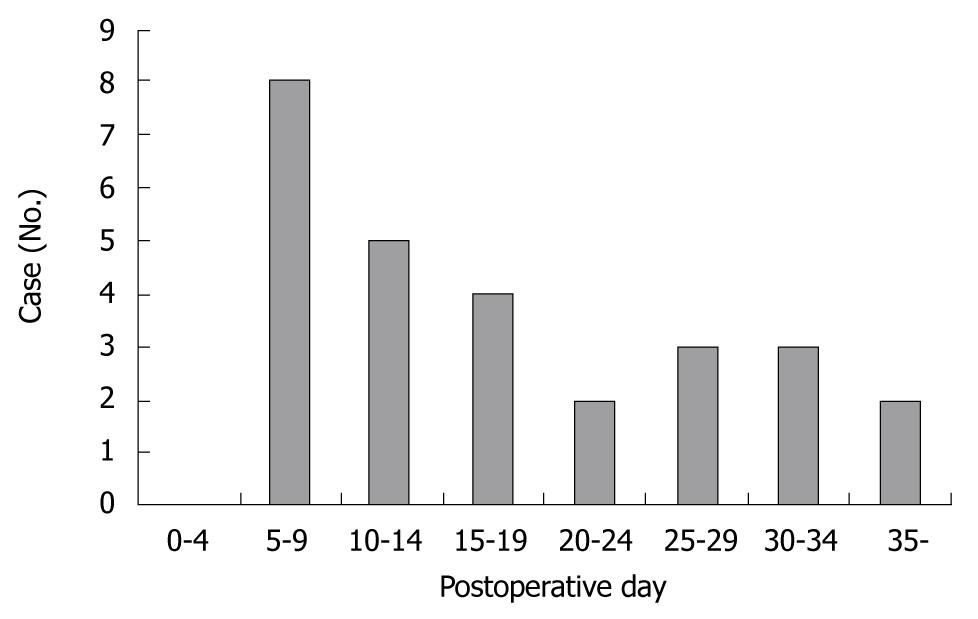
Twenty-seven cases of first and recurrent hemorrhage were investigated to determine the exact onset of bleeding. Cases were evenly distributed after surgery, with most of the bleeding occurring between day 5 and 9 post surgery. The onset of hemorrhage from ruptured pseudoaneurysms is outlined in Figure 1. Table 2 is a list showing the number of cases for each preceding complication of ruptured pseudoaneurysm. Pancreaticojejunostomy anastomotic leakage was the most common complication to occur before hemorrhage [12 cases (44%): pancreatic cancer (7 cases), bile duct cancer (4 cases), ampulla of vater cancer (1 case)]. Other complications include hepaticojejunostomy anastomotic leakage, wound dehiscence and infection, and intra-abdominal abscess formation. Most of the bleeding occurred from vessels around the pancreaticojejunostomy. Eleven patients (40.7%) experienced bleeding from the gastroduodenal artery, 4 patients (14.8%) from the right hepatic artery, 2 patients (7.4%) each from the hepatic artery proper, common hepatic artery and the right gastric artery, and 4 patients (14.8%) from the peripancreatic artery. The bleeding sites of the ruptured pseudoaneurysms are presented in Table 3.
| Complication | n (%) |
| Pancreatico-enterostomy leakage | 12 (44) |
| Wound dehiscence or infection | 3 (11) |
| Hepaticojejunostomy leakage | 1 (4) |
| Abdominal abscess | 1 (4) |
| No evidence of complication | 10 (37) |
| Site | n (%) |
| Gastroduodenal artery | 11 (42) |
| Right hepatic artery | 4 (15) |
| Proper hepatic artery | 2 (7) |
| Common hepatic artery | 2 (7) |
| Right gastric artery | 2 (7) |
| Left hepatic artery | 1 (4) |
| Superior mesenteric artery | 1 (4) |
| Pancreatic branch of splenic artery | 1 (4) |
| Inferior pancreaticoduodenal artery | 1 (4) |
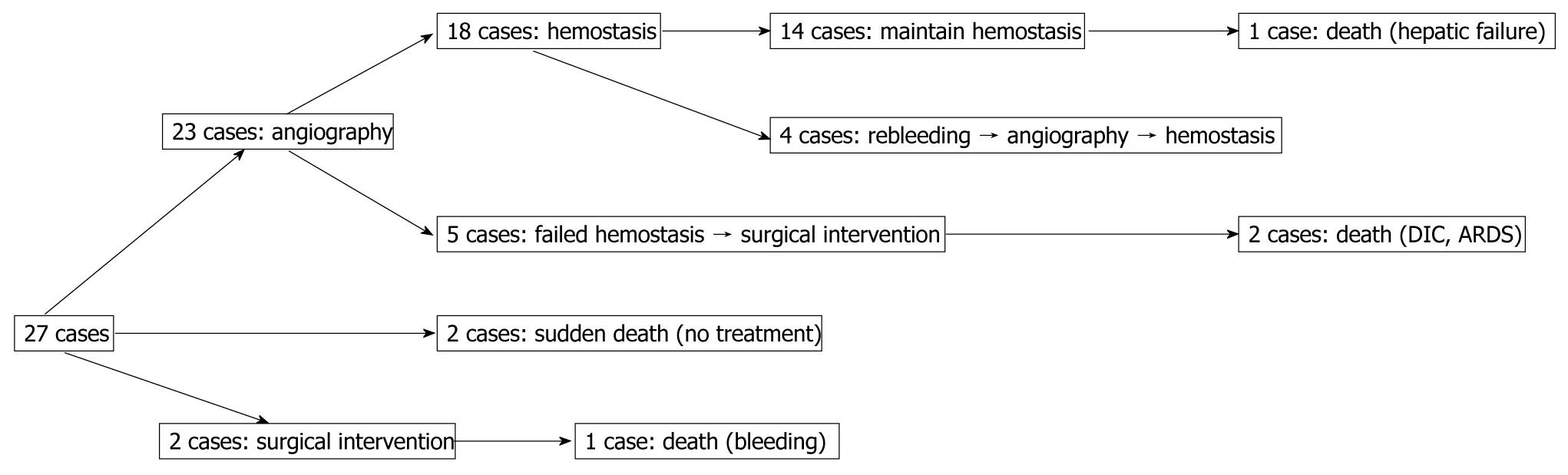
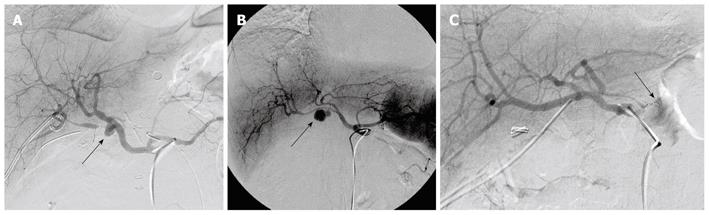
Reviewing the treatment strategy of the 27 patients with ruptured pseudoaneurysms, 23 patients were managed by angiography, 2 patients had surgical intervention, and 2 patients with rapidly escalating hemorrhage could not be intervened and died. Of 23 cases of angiography, hemostasis was achieved in 18 patients. In the other 5 patients, hemostasis could not be achieved and they required surgery; 2 for failed attempts at coil embolization of the bleeding vessel and 3 for clinically continuous bleeding and hemodynamic instability despite successful coiling. Of these latter 3 patients, 2 died. Continuing hemorrhage due to disseminated intravascular coagulation (DIC) after total pancreatectomy was responsible for one death and the other death was due to the development of pulmonary complications namely adult respiratory distress syndrome (ARDS) and pneumonia after becoming hemodynamically stable. Of 18 cases with hemostasis, 14 maintained their hemostatic state. However, 1 of 14 cases died of hepatic failure after common hepatic artery embolization. Four cases presented with recurrent hemorrhage from ruptures of new pseudoaneurysms. They were successfully treated by another embolization. Evaluating the outcome of 23 angiography cases, 18 cases displayed functional/therapeutic success (success rate of 78.2%), that is, achievement of a hemostatic state by embolization. The outcome of the above mentioned patients is summarized in Figure 2. Figure 3A-C are angiographs of the ruptured pseudoaneurysms of different vessels.
Of the 2 patients who underwent emergent surgery without angiography, 1 recovered without further complications but the other patient died due to rebleeding from an unknown focus.
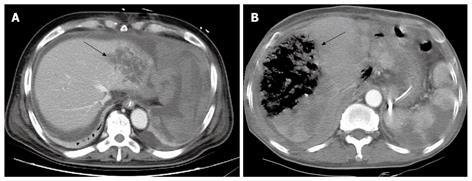
Eight patients underwent embolization of the right proper common hepatic artery. All 8 patients had normal portal blood flow and this procedure was only considered after thorough review by the radiologist and surgeon. There was a single case of hepatic failure, 2 cases where liver abscesses were detected on CT scans, and 3 cases of liver infarction without hepatic failure. In the remaining 2 cases, no radiological abnormalities were detected. Figure 4A and B are abdominal CT scans showing liver abscess and liver infarction, respectively, which developed after embolization of the common hepatic artery. The patient who died of hepatic failure was a 63-year-old male who underwent a pancreaticoduodenectomy with left lateral sectionectomy for pancreatic head cancer with liver metastases. Following surgery, the patient showed signs of pancreaticojejunostomy anastomotic leakage. Thirty days post operation, common hepatic artery embolization was performed to manage bleeding of a ruptured pseudoaneurysm. However, the patient passed away on day 41 post surgery.
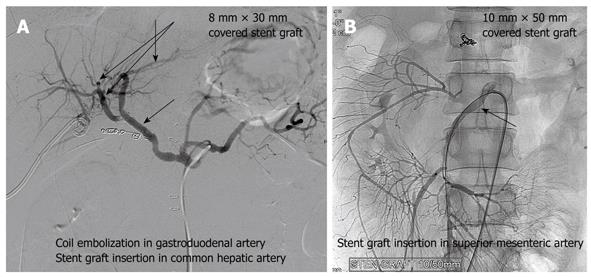
Stent grafts were placed for pseudoaneurysms to maintain liver or visceral blood flow. Stent grafting was successfully attempted in 2 of our cases for hemorrhagic pseudoaneurysm of the gastroduodenal and superior mesenteric artery. For gastroduodenal artery pseudoaneurysm, embolization of the gastroduodenal artery with a short stump was performed with stent grafting (8 mm × 30 mm size) of the common hepatic artery (Figure 5A). In the other case, with the first episode of early bleeding, hemostasis was achieved by laparotomy without any intervention by angiography. This patient presented with recurrent hemorrhage from the rupture of a new pseudoaneurysm of the superior mesenteric artery. Embolization of the superior mesenteric artery results in severe ischemia of the end-organ and therefore needs careful consideration. Stent grafting (10 mm × 50 mm size) was also performed for recurrent bleeding (Figure 5B). Considering the risk of bleeding, anti-coagulant therapy was not administered and occlusion of the vessel by thrombus was shown on CT angiography taken 3 d later. This patient currently requires continuous total parenteral nutrition for GI malabsorption.
The mortality rate for hemorrhage of ruptured pseudoaneurysms was high at 22% (6/27 patients). As mentioned previously, 2 patients died due to rapidly developing hemorrhage of sudden onset. In these cases, no surgical or other intervening treatment could be applied in time. One patient died of continuing hemorrhage due to disseminated intravascular coagulation and another due to hepatic failure after common hepatic artery embolization. One patient died of acute respiratory distress syndrome and pneumonia after becoming hemodynamically stable. Another death was due to recurrent hemorrhage from an unknown focus.
Hemorrhage after pancreaticoduodenectomy is one of the major life-threatening complications of this procedure and is responsible for 0.4%-5% of its mortality. According to numerous studies, mortality rates can range from 14%-58%[1,4]. A ruptured pseudoaneurysm is among the most serious and life threatening causes of post pancreaticoduodenectomy hemorrhage. Pseudoaneurysmal bleeding refers to disrupted vascular wall integrity which becomes continuous with pulsatile hematoma[7]. Many pseudoaneurysms develop secondary to pancreatitis in association with pseudocyst formation. The etiology of pseudoaneurysm formation after pancreaticoduodenectomies is unclear. Erosion of the arterial wall by trypsin and elastase, in the case of pancreaticojejunostomy site leakage, has been suggested as one of the causes. Furthermore, infections of post-operative hematomas remaining in the abdominal cavity and radiotherapy prior to surgery have both been associated with the development of pseudoaneurysms. According to other authors, preceding sepsis due to anastomotic leakage is evident in the majority of patients who present with hemorrhage from pseudoaneurysms (8%-25% of cases result in anastomotic leak after pancreaticoduodenectomies)[7,8]. In our study, pancreaticojejunostomy anastomotic leak (44%) was the most common cause of subsequent complications. CT was performed on postoperative day 7 in all patients. Patients with pancreaticojejunostomy leaks are closely monitored and CT scans are taken immediately if there is sentinel bleeding and they are hemodynamically stable.
Sentinel bleeding was first described in 1989 by Shankar and russell[9]. They defined it as gastrointestinal (intraluminal) or intra-abdominal (placed drain) hemorrhage which occurs 6 h to 10 d prior to massive hemorrhage. Currently, different authors use different definitions for this term[1,3,10,11]. Sentinel bleeding is evident in most cases of massive hemorrhage and it starts off as an intermittent or small-volume hemorrhage. Koukoutsis et al[3] reported that sentinel bleeding was evident in 57.1% of cases of post-operative hemorrhage 7 d after surgery, but they reached the conclusion that sentinel bleeding has no statistically significant association with post-operative mortality. In the study by Sato et al[8], sentinel bleeding was noted in 10 patients with massive arterial hemorrhage out of 81 pancreaticoduodenectomy patients and only one case of sentinel bleeding was not followed by massive hemorrhage. In our study, sentinel bleeding was evident in 21 of 27 cases (prevalence of 77.8%). It is important to adequately manage sentinel bleeding as soon as possible to prevent massive hemorrhage because sentinel bleeding is an important preceding sign of massive hemorrhage.
Current studies have suggested that angiography should be the treatment of choice for delayed massive hemorrhage after pancreaticoduodenectomies[5,8,12]. They also argue that the therapeutic value of endoscopy is limited and should only be attempted if the precise location of the bleeding ulcer is obvious. Nevertheless, it is inappropriate to conclude that angiography is always more sophisticated than therapeutic endoscopy as their suitability varies on a case-by-case basis. Not all delayed massive hemorrhages are due to ruptured pseudoaneurysms. Furthermore, hemorrhage from ruptured pseudoaneurysms cannot be completely ruled out based on negative findings on angiography[10]. Initial intermittent bleeding of ruptured pseudoaneurysms is hard to detect by angiography, which is only sensitive to bleeding greater than 2 mL per minute[6,10]. In contrast, bleeding foci are hard to find using endoscopy in massive intraluminal hemorrhages[5,8]. Location of the bleeding focus by endoscopy does not completely exclude bleeding from ruptured pseudoaneurysms elsewhere. Choi et al[5] stated that diagnostic endoscopy delays treatment in hemodynamically unstable patients and more often than not, intra-luminal hemorrhage is due to erosion of pseudoaneurysms. de Castro et al[1] recommended CT scanning for patients with sentinel bleeding who are hemodynamically stable. In the case where the bleeding focus cannot be identified by this method, or if the patient is hemodynamically unstable, angiography is suggested. Therefore, diagnosis and treatment strategies should be decided on a case-by-case basis. Angiography should be considered, with the understanding that most cases of hemorrhage from ruptured pseudoaneurysms present with preceding sepsis due to anastomotic leakage.
Transcatheter arterial embolization has been proven by many authors to be an efficacious and minimally invasive alternative to open surgery[1,7,8]. It has a success rate of 67%-100%, morbidity rate of 14%-25%, and mortality rate of 0%-14%. Thirty-seven percent of patients in these studies required re-embolization either for rebleeding or recanalization of the vessels[1,7,8]. When the bleeding focus cannot be identified by conventional investigation and hemostasis cannot be achieved by therapeutic angiography or endoscopy, emergency laparotomy is necessary for delayed massive hemorrhage[1,7]. Thorough examination of the ligated vessel stumps should be carried out. In more difficult cases, inspection of the anastomoses for suture line bleeding and bleeding of ruptured vessel stumps into the GI lumen is required[6]. In other words, emergency laparotomy is rarely required for hemorrhagic pseudoaneurysms and therapeutic angiography has become the standard treatment strategy.
Stent grafting was successfully performed in 2 of our cases for hemorrhagic pseudoaneurysms. This procedure can be used as an alternative or in addition to embolization[13]. This technique has the advantage of providing continued perfusion to the end-organ and, therefore, obviates the risk of occlusion and ischemia often seen with embolization. On the other hand, potential complications do occur and include stent occlusion, stent deformation or kinking, and exclusion of branch vessels. In our study, a stent graft was used for a pseudoaneurysm of the common hepatic artery and superior mesenteric artery to maintain the visceral and liver blood flow. Embolization of the superior mesenteric artery results in severe ischemia of the end-organ and therefore needs careful consideration. Stent grafting has the advantage of maintaining the peripheral blood flow and hence could be considered an alternative to embolization. However, it is a technically difficult procedure and requires adaptation to different-sized vessels. The limitation and the role of the interventions used to manage ruptured pseudoaneurysms need to be studied further.
Various surgeons have developed different surgical techniques in an attempt to prevent pseudoaneurysm formation. Turrini et al[11] suggested leaving 1 cm of the gastroduodenal artery stump to minimize direct contact of pancreatic juice with adjacent vessels. Koukoutsis et al[3] suggested spreading an omental flap behind the pancreaticojejunostomy site. We leave behind a long segment (5-10 mm) of the gastroduodenal artery stump to minimize gastroduodenal artery intimal injury with Hemolok® clipping.
Bleeding pseudoaneurysms are a rare cause of delayed hemorrhage but these delayed hemorrhages are among the most serious and fatal complications following pancreaticoduodenectomy. A decade ago, diagnostic or therapeutic endoscopy followed by laparotomy was the treatment strategy for delayed massive hemorrhage. However, in recent years, diagnostic angiography has been preferred over endoscopy and is rapidly becoming the standard therapeutic treatment for bleeding pseudoaneurysms. Fundamentally, however, the patient’s clinical history and presentation should be taken into consideration when planning an appropriate management strategy that is tailored to that patient.
Delayed hemorrhage, especially from a ruptured pseudoaneurysm, is a rare but rapidly progressing and potentially life threatening complication following pancreaticoduodenectomies.
To review the clinical course and the outcome of pseudoaneurysms post-pancreaticoduodenectomy.
It has been widely studied, yet each study has reported different findings on its incidence, location, and clinical presentation. The purpose of this study was to analyze data on delayed hemorrhage after pancreaticoduodenenctomy due to pseudoaneurysm rupture.
Delayed massive hemorrhage following pancreaticoduodenectomies should be ruled out regardless of its association with rupture of pseudoaneurysms. In this study, arterial embolization was shown to be an effective and safe way to manage and control bleeding pseudoaneurysms.
Well written article by very experienced authors in the field of pancreatic surgery.
Peer reviewers: Tadatoshi Takayama, Professor, Department of Digestive Surgery, Nihon University School of Medicine, 30-1 Oyaguchikami-machi, Itabashi-ku, Tokyo 173-8610, Japan; Massimo Falconi, MD, Chirurgia B, Department of Anesthesiological and Surgical Sciences Policlinico GB Rossi, Piazzale LA Scuro, 37134 Verona, Italy
S- Editor Tian L L- Editor O’Neill M E- Editor Ma WH
| 1. | de Castro SM, Busch OR, Gouma DJ. Management of bleeding and leakage after pancreatic surgery. Best Pract Res Clin Gastroenterol. 2004;18:847-864. [Cited in This Article: ] |
| 2. | Choi JJ, Choi H, Shin DS, Song IS, Bae JS. Risk factors for the pancreatic leakage after pancreaticoduodenectomy. Korean J Hepato Pancreat Surg. 2005;9:225-232. [Cited in This Article: ] |
| 3. | Koukoutsis I, Bellagamba R, Morris-Stiff G, Wickremesekera S, Coldham C, Wigmore SJ, Mayer AD, Mirza DF, Buckels JA, Bramhall SR. Haemorrhage following pancreaticoduodenectomy: risk factors and the importance of sentinel bleed. Dig Surg. 2006;23:224-228. [Cited in This Article: ] |
| 4. | Rumstadt B, Schwab M, Korth P, Samman M, Trede M. Hemorrhage after pancreatoduodenectomy. Ann Surg. 1998;227:236-241. [Cited in This Article: ] |
| 5. | Choi SH, Moon HJ, Heo JS, Joh JW, Kim YI. Delayed hemorrhage after pancreaticoduodenectomy. J Am Coll Surg. 2004;199:186-191. [Cited in This Article: ] |
| 6. | van Berge Henegouwen MI, Allema JH, van Gulik TM, Verbeek PC, Obertop H, Gouma DJ. Delayed massive haemorrhage after pancreatic and biliary surgery. Br J Surg. 1995;82:1527-1531. [Cited in This Article: ] |
| 7. | Otah E, Cushin BJ, Rozenblit GN, Neff R, Otah KE, Cooperman AM. Visceral artery pseudoaneurysms following pancreatoduodenectomy. Arch Surg. 2002;137:55-59. [Cited in This Article: ] |
| 8. | Sato N, Yamaguchi K, Shimizu S, Morisaki T, Yokohata K, Chijiiwa K, Tanaka M. Coil embolization of bleeding visceral pseudoaneurysms following pancreatectomy: the importance of early angiography. Arch Surg. 1998;133:1099-1102. [Cited in This Article: ] |
| 9. | Shankar S, Russell RC. Haemorrhage in pancreatic disease. Br J Surg. 1989;76:863-866. [Cited in This Article: ] |
| 10. | de Castro SM, Kuhlmann KF, Busch OR, van Delden OM, Laméris JS, van Gulik TM, Obertop H, Gouma DJ. Delayed massive hemorrhage after pancreatic and biliary surgery: embolization or surgery? Ann Surg. 2005;241:85-91. [Cited in This Article: ] |
| 11. | Turrini O, Moutardier V, Guiramand J, Lelong B, Bories E, Sannini A, Magnin V, Viret F, Blache JL, Giovannini M. Hemorrhage after duodenopancreatectomy: impact of neoadjuvant radiochemotherapy and experience with sentinel bleeding. World J Surg. 2005;29:212-216. [Cited in This Article: ] |
| 12. | Okuno A, Miyazaki M, Ito H, Ambiru S, Yoshidome H, Shimizu H, Nakagawa K, Shimizu Y, Nukui Y, Nakajima N. Nonsurgical management of ruptured pseudoaneurysm in patients with hepatobiliary pancreatic diseases. Am J Gastroenterol. 2001;96:1067-1071. [Cited in This Article: ] |