Published online Aug 21, 2006. doi: 10.3748/wjg.v12.i31.5051
Revised: October 15, 2005
Accepted: October 26, 2005
Published online: August 21, 2006
AIM: To investigate the differentiation of rat bone marrow stem cells in liver after partial hepatectomy.
METHODS: Bone marrow cells were collected from the tibia of rat with partial hepatectomy, the medial and left hepatic lobes were excised. The bone marrow stem cells (Thy+CD3-CD45RA- cells) were enriched from the bone marrow cells by depleting red cells and fluorescence-activated cell sorting. The sorted bone marrow stem cells were labeled by PKH26-GL in vitro and autotransplanted by portal vein injection. After 2 wk, the transplanted bone marrow stem cells in liver were examined by the immunohistochemistry of albumin (hepatocyte-specific marker).
RESULTS: The bone marrow stem cells (Thy+CD3-CD45RA- cells) accounted for 2.8% of bone marrow cells without red cells. The labeling rate of 10 μM PKH26-GL on sorted bone marrow stem cells was about 95%. There were sporadic PKH26-GL-labeled cells among hepatocytes in liver tissue section, and some of the cells expressed albumin.
CONCLUSION: Rat bone marrow stem cells can differentiate into hepatocytes in regenerative environment and may participate in liver regeneration after partial hepatectomy.
- Citation: Zhan YT, Wang Y, Wei L, Liu B, Chen HS, Cong X, Fei R. Differentiation of rat bone marrow stem cells in liver after partial hepatectomy. World J Gastroenterol 2006; 12(31): 5051-5054
- URL: https://www.wjgnet.com/1007-9327/full/v12/i31/5051.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i31.5051
Recent studies indicate that mice and human bone marrow cells can differentiate into hepatocytes in normal liver and rat bone marrow cells can turn into hepatocytes in severely damaged liver with the suppression of hepatocyte proliferation[1-6]. However, whether rat bone marrow stem cells can differentiate into hepatocytes in liver after partial hepatectomy remains unclear. The aim of this study was to investigate the differentiation of rat bone marrow cells in regenerative environment after partial hepatectomy.
Male Sprague-Dawley rats weight 170-190 g were obtained from the Animal Center of Peking University People’s Hospital. They were allowed to have free access to standard laboratory chow and kept in a 12 h light/dark cycle.
Erythrolysin, phycoerythrin-conjugated mouse anti-rat CD45RA and phycoerythrin-conjugated mouse anti-rat CD3 were purchased from Becton Dickinson Inc. Fluorescein isothiocyanate-conjugated mouse anti-rat Thy-1.1 was obtained from Pharmingen Inc. PKH26-GL was purchased from Sigma Inc. Mouse anti- albumin antibody was obtained from Dako Inc. FITC-labeled anti- mouse IgG was supplied by Zhongshan Inc. RPMI 1640 was from Gibco Inc.
The rats were anesthetized with intraperitioneal injection of sodium pentobarbital (35 mg/kg body weight). Local skin was sterilized by routine method. Partial hepatectomy rat model was established by resecting medial and left liver lobes.
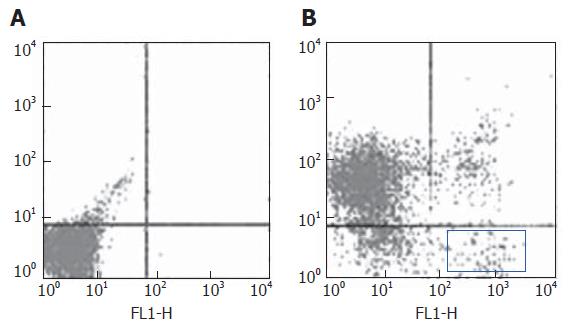
Under general anesthesia, bone marrow was aspirated from tibia with a syringe containing 1 mL heparin with an 18-gauge needle. The marrow cells were transferred to a sterile tube and mixed with 10 mL culture medium (RPM1640 supplemented with 10% fetal bovine serum, 100 μ/mL penicillin G and 100 μg/mL streptomycin). Red blood cells in bone marrow were depleted by erythrolysin. After washed three times with phosphate-buffered saline (PBS), the cells were incubated at 4°C for 30 min with fluorescein isothiocyanate-conjugated anti-Thy, phycoerythrin-conjugated anti-CD3 and phycoerythrin-conjugated anti-CD45RA. The cells were washed three times and resuspended in medium. Labeled cells were analyzed and separated with FASC-vantage (Becton Dickinson, San Jose, CA). Gating was based on Thy-positive, CD3-negtive and CD45RA-negtive.
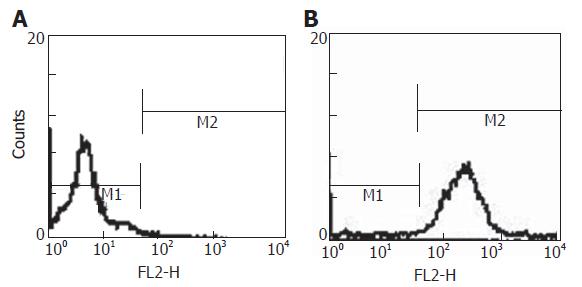
The sorted Thy+CD3-CD45RA- cells were labeled by 10 μM PKH26-GL. Labeling conditions employed were essentially as described by the manufacturer. Briefly, cells were suspended in Diluent C at a density of 2 × 106 cells/mL and mixed with an equal volume of the PHk26-GL dye in Diluent C to give a final dye concentration of 10 μmol/L. Labeling was carried out at room temperature for 3 min. The labeling reaction was terminated by the addition of an equal volume of fetal bovine serum. Labeled cells were diluted with culture medium containing serum and washed three times.
The PKH26-GL-labeled bone marrow stem cells were suspended in 300 μL of RPM1640 without serum at a concentration of 1 × 106 cells and autotransplanted by portal vein injection.
The liver tissue was obtained at two weeks after partial hepatectomy. Frozen sections were made and incubated overnight at 4°C in 50 μL anti-albumin antibody (1:50), then washed three times in PBS and incubated for 1 h at 37°C in 50 μL FITC-labeled second antibody. After washed three times with PBS, the slides were occluded by glycerin buffer and observed under confocal laser scanning microscope.
After erythrocytes were depleted in tibia bone marrow cells from rats after partial hepatectomy. Bone marrow stem cells were enriched by sorting the Thy+CD3-CD45RA- cells. The sorted cells accounted for about 2.8 % (Figure 1).
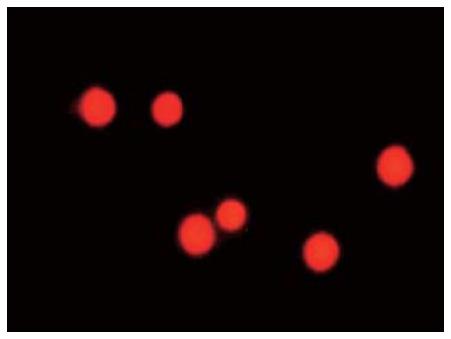
The Thy+CD3-CD45RA- cells were labeled with 10 μM PKH26-GL. The percentage of labeled cells was about 95% (Figure 2). The labeled Thy+CD3-CD45RA- cells were red under fluorescence microscope (Figure 3). PKH26-GL had no obvious effect on bone marrow cell viability. Trypan blue staining assay showed that the cell viability of PKH26-GL-labeled bone marrow cells was higher than 85%.
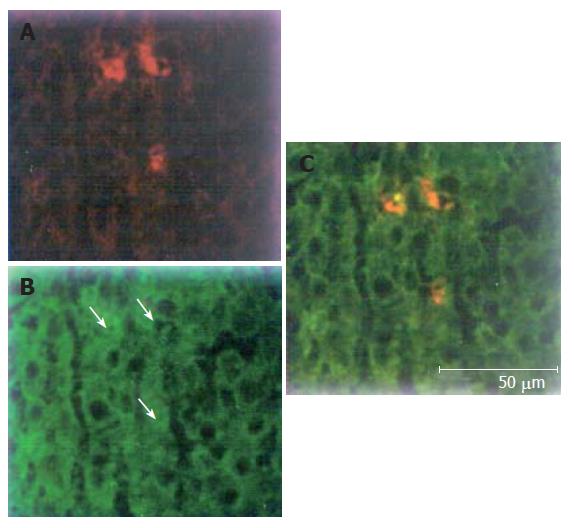
PKH26-Gl could emit red fluorescence at the wavelength of 567 nm, while FITC could emit green fluorescence at the wavelength of 494 nm. We tested liver tissue sections under confocal laser scanning microscope at the two wavelengths respectively. There were sporadic cells with red fluorescence among hepatocytes in liver sections (Figure 4A), suggesting that PKH26-GL- labeled bone marrow stem cells could migrate into liver. Hepatocytes showing green fluorescence suggested that hepatocytes expressed albumin (Figure 4B). Yellow was mixed with red and green. Yellow cells derived from PKH26-GL labeled in vitro bone marrow stem cells expressed albumin (Figure 4C). The findings indicated that bone marrow stem cells could differentiate into hepatocytes in regenerative hepatic environment after partial hepatectomy.
Seventy percent hepatectomy rat model is a classical animal model to study liver regeneration. The remaining hepatic lobes of the model rat can restore the liver mass at 2 wk after partial hepatectomy. It has been considered that the regeneration process is due to the proliferation of residual mature hepatocytes. Recently, important developments have been achieved in the field of stem cell study. Petersen et al[3] used 3 separate approaches to follow transplanted bone marrow cells and found an extrahepatic source for the liver cells. In a careful histological analysis of irradiated female mice that received male donor cells, the Y chromosome could be detected in some hepatocytes 2 to 6 mo after transplantation in the absence of any intentional liver injury[7]. In human female recipients of male bone marrow, some hepatocytes contain the Y chromosome[8]. These findings suggest that there is a linkage between bone marrow cells and liver. It is hypothesized that, similar to other organ systems, the liver has 3 levels of cells: “mature” hepatocyte, original tissue-determined stem cells represented in the adult organ by cells in the terminal bile ductules (canals of Hering), and a multipotent stem cells in the liver derived from circulating bone marrow stem cells[9]. However, whether circulating bone marrow stem cells can differentiate into hepatocytes in regenerative liver after partial hepatectomy remains unclear.
PKH26-GL shows little or no toxicity except for some phototoxicity following prolonged exposure of PKH26-GL labeled cells to excitation light and can be used to track lymphocyte migration for weeks to months[10,11]. It is the dye of choice for cell migration and proliferation studies. PKH26-GL has been used to in situ label mouse spleen cells and peripheral blood neutrophils, and is particularly effective in monitoring the in vivo homing and proliferation of haemopoietic stem cells[12-14]. Our study showed that 10 μmol/L PKH26-GL could effectively label rat bone marrow cells and has no obvious effect on cell viability. PKH26-GL-labeled bone marrow cells were autotransplanted in rats during partial hepatectomy. After 2 wk, there were PKH26-GL-labeled cells in liver, and the cells expressed hepatocyte-specific marker albumin. The result showed that bone marrow cells could differentiate into hepatocytes in regenerative hepatic environment. Bone marrow cells consist of white cells, erythrocyte, a few stem cells, etc. Only stem cells could differentiate into other type cells. As a result, the experiment indicated that circulating bone marrow stem cells could differentiate into hepatocytes in liver after partial hepatectomy and bone marrow stem cells might participate in hepatic regeneration. Fujii[15] examined the differentiation of mice bone marrow cells in liver regeneration after partial hepatectomy and found that bone marrow cells participate in liver sinusoid. They believe the bone marrow cells participation in liver regeneration after hepatectomy, where the majorities were committed to sinusoidal endothelial cells probably through endothelial progenitor cell mobilization.
It has been accepted that the hepatocyte regeneration process after partial hepatectomy is associated with the proliferation of remaining hepatocytes. We hold that the mechanism of hepatocyte regeneration after partial hepatectomy includes at least two ways. That is, the remaining mature hepatocytes proliferate and circulating bone marrow stem cells migrate into residual liver and differentiate into hepatocytes. With the development and application of stem cell technique, it is possible to obtain stem cells from bone marrow or blood and to make the stem cells proliferate in a great deal. Stem cell autotransplantation may become a new method to promote liver regeneration.
S- Editor Wang J L- Editor Wang XL E- Editor Bi L
| 1. | Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229-1234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1764] [Cited by in F6Publishing: 1845] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 2. | Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 779] [Cited by in F6Publishing: 737] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 3. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1795] [Cited by in F6Publishing: 1661] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 4. | Allen KJ, Cheah DM, Lee XL, Pettigrew-Buck NE, Vadolas J, Mercer JF, Ioannou PA, Williamson R. The potential of bone marrow stem cells to correct liver dysfunction in a mouse model of Wilson's disease. Cell Transplant. 2004;13:765-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Zhan YT, Wei L, Chen HS, Cong X, Fei R, Wang Y. [Differentiation of bone marrow stem cells in rat hepatic fibrogenesis environment]. Zhonghua Ganzangbing Zazhi. 2003;11:673-675. [PubMed] [Cited in This Article: ] |
| 6. | Liu ZC, Chang TM. Coencapsulation of hepatocytes and bone marrow stem cells: in vitro conversion of ammonia and in vivo lowering of bilirubin in hyperbilirubemia Gunn rats. Int J Artif Organs. 2003;26:491-497. [PubMed] [Cited in This Article: ] |
| 7. | Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 752] [Cited by in F6Publishing: 792] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 8. | Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 900] [Cited by in F6Publishing: 962] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 9. | Sell S. Heterogeneity and plasticity of hepatocyte lineage cells. Hepatology. 2001;33:738-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 283] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Wallace PK, Palmer LD, Perry-Lalley D, Bolton ES, Alexander RB, Horan PK, Yang JC, Muirhead KA. Mechanisms of adoptive immunotherapy: improved methods for in vivo tracking of tumor-infiltrating lymphocytes and lymphokine-activated killer cells. Cancer Res. 1993;53:2358-2367. [PubMed] [Cited in This Article: ] |
| 11. | Johnsson C, Festin R, Tufveson G, Tötterman TH. Ex vivo PKH26-labelling of lymphocytes for studies of cell migration in vivo. Scand J Immunol. 1997;45:511-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Hendrikx PJ, Martens CM, Hagenbeek A, Keij JF, Visser JW. Homing of fluorescently labeled murine hematopoietic stem cells. Exp Hematol. 1996;24:129-140. [PubMed] [Cited in This Article: ] |
| 13. | Khalaf AN, Wolff-Vorbeck G, Bross K, Kerp L, Petersen KG. In vivo labelling of the spleen with a red-fluorescent cell dye. J Immunol Methods. 1993;165:121-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Albertine KH, Gee MH. In vivo labeling of neutrophils using a fluorescent cell linker. J Leukoc Biol. 1996;59:631-638. [PubMed] [Cited in This Article: ] |
| 15. | Fujii H, Hirose T, Oe S, Yasuchika K, Azuma H, Fujikawa T, Nagao M, Yamaoka Y. Contribution of bone marrow cells to liver regeneration after partial hepatectomy in mice. J Hepatol. 2002;36:653-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |