INTRODUCTION
Liver fibrosis is a dynamic process resulting in excessive deposition of extracellular matrix (ECM) components. It is a multifunctional process that involves hepatic stellate cell (HSC) and Kupffer cells (KCs), cytokines, chemokines and growth factors and results from a disruption of homeostatic mechanisms that maintain the liver ecosystem[1–3]. The fibrogenic cascade can be divided into the following steps[1–3]: (1) activation of HSC and KCs; (2) migration and proliferation of HSCs; (3) synthesis and deposition of ECM components; (4) remodeling of scar tissue; (5) wound contraction; (6) apoptosis of HSCs.
In this manuscript, we briefly review the liver fibrosis process and current aspects of the Renin angiotensin system (RAS) and further discuss the putative role of Angiotensin (Ang)-(1-7) in controlling hepatic injury.
LIVER FIBROSIS PROCESS
The HSC is the main cell type responsible for excessive deposition of connective tissue components, including type I collagen, in response to liver injury[1–6]. HSCs, also called lipid storage cells, lipocytes or Ito’s cells, are found in the space of Disse among endothelial cells and hepatocytes. These cells represent approximately one third of the non-parenchymatous cell population or 15% of the total number of hepatic cells[2–4]. The main function of HSCs is to metabolize vitamin A, which is intracellularly reserved as cytoplasmatic fat bodies, mainly as retinol esters[4]. Such cells also contain a small amount of triglycerides, phospholipids, cholesterol and free fatty acids. Moreover, they produce cytokines, growth factors and inflammatory mediators[4–6]. The activation of HSCs is a process not fully understood that involves the depletion of vitamin A storage and the lowering of retinol chains[24]. There are also important morphological and functional changes in the activated HSC, which include the increase in the expression of myogenic and neurogenic proteins and the subsequent change into highly contractile fibroblasts[2–4]. HSCs, despite being firmly adhered to hepatic endothelial cells, also play a pivotal role in the regulation of portal pressure. Besides that, due to the expression of multiple actin and muscular and non-muscular myosin types, when the HSC transforms itself into a myofibroblast, it acquires the ability to contract scar tissue and fibrous septa[1–3].
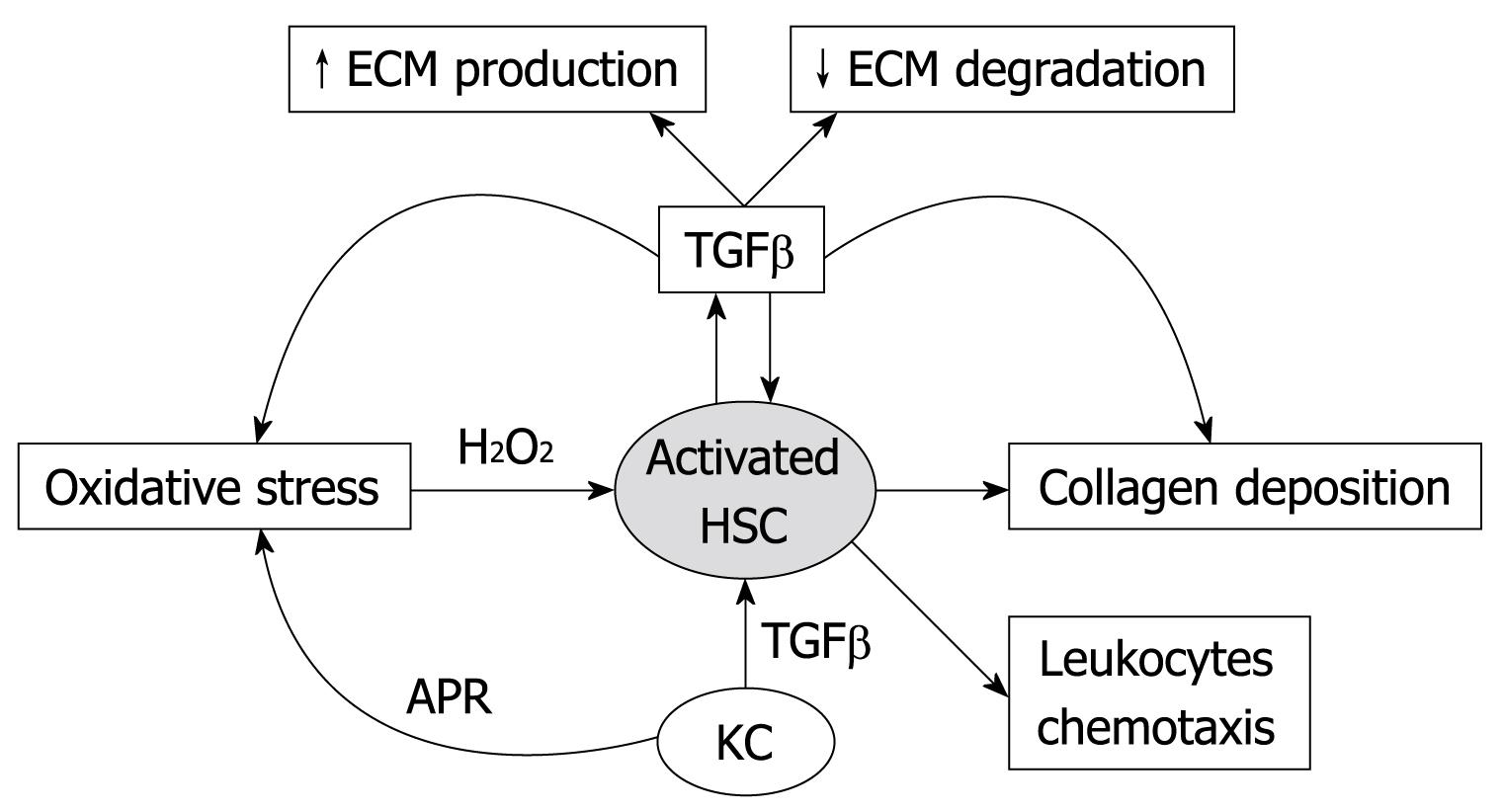
Formal pathogenesis of fibrosis is initiated by parenchymal cell destruction (necrosis rather than apoptosis) due to multiple injurious agents and mechanisms followed by inflammation, which in turn activates “resting” HSCs[1–3]. The HSCs lose their lipid droplets, proliferate, migrate to the third zone of the hepatic lobule, modify themselves in order to acquire a myofibroblast-like phenotype and start producing collagen types I, III and IV and laminin[2–4]. It is generally accepted that α actin expression reflects the activation of HSC[4]. Activated HSCs migrate to injured sites and proliferate in response to numerous pro-fibrogenic mediators, among which transforming growth factor β-1 (TGFβ-1) and platelet-derived growth factor are considered the most effective ones[78]. HSCs are the main cells responsible for ECM production in the liver. The ECM serves as a storage site for cytokines and growth factors, and thus, tissue injury could induce their release[24]. This event may be responsible for providing the initial signal for tissue repair, prior to the activation of cells within the liver and/or the arrival of inflammatory cells (Figure 1).
Figure 1 Diagram of activated hepatic stellate cell actions and interactions in liver fibrosis process.
HSC: Hepatic stellate cell; KC: Kupffer cell; APR: Acute phase response; ECM: Extracellular matrix; TGFβ: Transforming growth factor-β.
Activated HSCs express and secrete matrix molecules, cytokines and chemokines, matrix metalloproteinases (MMPs) and their respective tissue inhibitors of metalloproteinases (TIMPs)[1–4]. Thus, HSCs participate pathophysiologically both in fibrogenesis and fibrolysis, i.e. enzymatic dissolution of the ECM and, thus, in tissue remodeling. According to this hypothesis, fibrosis is conditionally reversible, based on the fact that HSCs produce multiple MMPs, which degrade interstitial and basement membrane collagens[9–11]. Thus, when the fibrogenic stimulus is singular, or multiple stimuli have not induced a state in which the excess deposition of ECM components is accompanied by a distortion of liver architecture, the process may be completely reversible[259–11]. At this stage of liver fibrosis, interruption of the fibrogenic stimulus results in MMPs secretion[11]. However, when the fibrogenic process is already associated with formation of connective tissue septa, distortion of liver architecture and formation of vascular shunts, fibrosis becomes irreversible, unless one finds the means to stimulate production and activation of MMPs, to down-regulate the expression of TIMPs, and to inhibit the production of collagen[259–13].
Another cell line involved in liver fibrogenic cascade is the KC. KCs are highly mobile macrophages that are attached to the endothelium[1–3]. Physiologically, KCs produce the immunosuppressive cytokine, interleukin (IL)-10, that prevent HSC proliferation and/or collagen synthesis[124]. These cells are activated by engulfment of apoptotic hepatocytes; this leads to removal of dead cells from the liver. Furthermore, activated KCs secrete inflammatory cytokines, linking apoptosis in the liver to inflammation. Once there is hepatic injury, activated KCs possess autocrine and paracrine loops that induce the expression of TGFβ, which, in turn, promotes HSC and hepatocyte proliferation and/or chemotaxis of inflammatory cells and HSCs[1241415]. In these conditions, circulating levels of cytokines and chemokines, such as tumor necrosis factor-α (TNFα), IL-6, IL-1, IL-8, colony stimulating factor, monocyte chemotactic protein and leukotrienes, are increased. These elevated mediators have a key role in recruiting neutrophils and monocytes into the lesion site and exert an important regulatory effect on the expression of collagen genes by HSCs[1–3]. Indeed, KCs have a pivotal role in the activation of HSCs and/or in increasing their capacity to produce ECM components during hepatic diseases[1–3].
Among all cytokines and growth factors, the TGFβ superfamily exerts important functions during the development, differentiation and tissue remodeling[1415]. TGFβ1 strongly modulates cell proliferation by increasing ECM proteins and proteases inhibitors and by diminishing several metalloproteases expression[1–312–15]. In normal hepatic tissue, TGFβ-1 and TGFβ-2 mRNA are predominantly expressed by KCs, while TGFβ-3 is detected in stellate cells. During fibrogenesis, TGFβ-2 and TGFβ-3 expression are diminished, while TGFβ-1 expression is significantly increased among stellate and endothelial cells[1–312–15]. This cytokine is a potent inhibitor of hepatocyte proliferation, but it is also capable of regulating hepatocyte growth during its regeneration. TGFβ induces the formation of oxygen reactive species, which is involved in HSC activation and in the augmentation of mRNA expression for collagen I[1–312–15].
Depending on the magnitude of the injury, the host’s response may be local and/or systemic. When the events take place in the liver, the response is restricted to HSCs and KC activation or results in recruiting inflammatory cells that, along with KCs, produce cytokines and growth factors necessary to the healing process[2312–15]. When the injury takes a larger extent and local events cannot control it, there is a systemic response, common to every other inflammatory process, regardless of the causal agent. This systemic reaction corresponds to the acute phase response, characterized by the increased production of TNFα, IL-6, IL-1, oncostatin M and acute phase proteins[123]. Although these changes are intended to limit the tissue injury, elevated cytokine expression, mostly IL-6, may contribute to hepatic fibrosis by enhancing ECM deposition, collagen I content and fibronectin genetic transcription, as well as by stimulating other fibrogenic cytokines (such as TGFβ), and by amplifying TIMP production[1–311–15].
There are other factors involved in the ECM remodeling process, which include MMPs and TIMPs. MMPs 1 and 13, also known as collagenases 1 and 3, respectively, are the main secreted neutral proteinases capable of initiating degradation of collagen types I, III and V[11]. The individual contribution of MMPs in ECM degradation within the normal liver and during hepatic fibrogenesis remains unclear. HSCs express the MMP 1 mRNA gene, but the enzyme levels are not increased in patients with fibrosis. On the other hand, MMP 13 expression is augmented at early phases of liver fibrosis, preceding the increase of collagen I production[1213]. Among other MMPs, types 2 and 3 have their expression elevated in HSCs during intermediate and initial phases, respectively, of carbon tetrachloride induced hepatic fibrosis[3101213]. MMP 9, also called gelatinase B, can degrade collagen IV, gelatin and laminin, thus facilitating cellular migration through basement membranes[31213]. There are also TIMPs that regulate enzyme activity and play a role in different models of hepatic fibrosis[5]. Summing up, the hepatic tissue remodeling process is highly complex, resulting from the balance between collagen degradation and synthesis (Figure 1).
The understanding of the HSC activation pathways and the approach of molecular biology have provided new strategies to hepatic antifibrotic therapy[1–4]. Many of these strategies are based on the inhibition of collagen deposition and/or inactivation of the HSCs. Experimental studies in which the treatment is provided simultaneously and/or during the course of fibrosis induction have been successful[129–11]. Such approaches may include: (1) healing the primary disease in order to prevent the injury[12]; (2) reducing inflammation or the host’s response to avoid HSC activation (interferon-α, ursodesoxycholic acid, corticosteroids and TNF-α antagonists)[129]; (3) direct inhibition of HSC activation (antioxidants-vitamin E and interferon γ-endothelin receptor antagonists)[129]; (4) neutralizing the HSCs proliferative, fibrogenic, contractile and/or pro-inflammatory response [angiotensin converting enzyme (ACE) inhibitors, angiotensin II type 1 receptor (AT1) antagonists, Ang-(1-7) receptor Mas agonists, proteases inhibitors, hepatocyte growth factor, tyrosine kinase inhibitors, endothelin receptor antagonists][9–11]; (5) stimulating HSC apoptosis (gliotoxin, Fas ligands)[9–11]; (6) increasing scar matrix degradation by stimulating proteases producer cells and by providing such proteases (TGFβ antagonist, MMP tissue inhibitors)[9–11].
Nevertheless, liver fibrosis in humans is a silent disease. Many patients are diagnosed in an advanced phase when fibrous septa and hepatic architecture distortion already exist. Thus, the development of new treatments focusing on the removal of fibrous septa and promoting hepatic tissue regeneration becomes essential.
CURRENT ASPECTS ON THE RAS
The RAS is classically conceived as a hormonal cascade responsible for controlling cardiovascular, renal and adrenal functions that regulate hydro-electrolytic balance and blood pressure through Ang II actions[16].
Recent advances in cellular and molecular biology, as well as physiological and pharmacological approaches, have generated new concepts through the identification of new peptides, enzymes, receptors and biological actions. Additionally, tissue RAS has been characterized in different organs and systems, in which significant interactions between receptors, mediators and metabolic pathways have been discovered[17–21]. In this field, some of the latest advances should be mentioned: (1) the characterization of other biologically active RAS fragments, besides Ang II, such as Ang III, Ang IV and Ang-(1-7)[17–21]; (2) the discovery of a new enzyme, a homolog to the ACE, called ACE2[2223], which is the main enzyme responsible for the conversion of Ang II into Ang-(1-7)[2425]; (3) the identification of the G protein-coupled receptor Mas, a functional receptor for Ang-(1-7)[26]. These discoveries have contributed to the understanding of RAS in normal physiology and in pathological conditions[19–21].
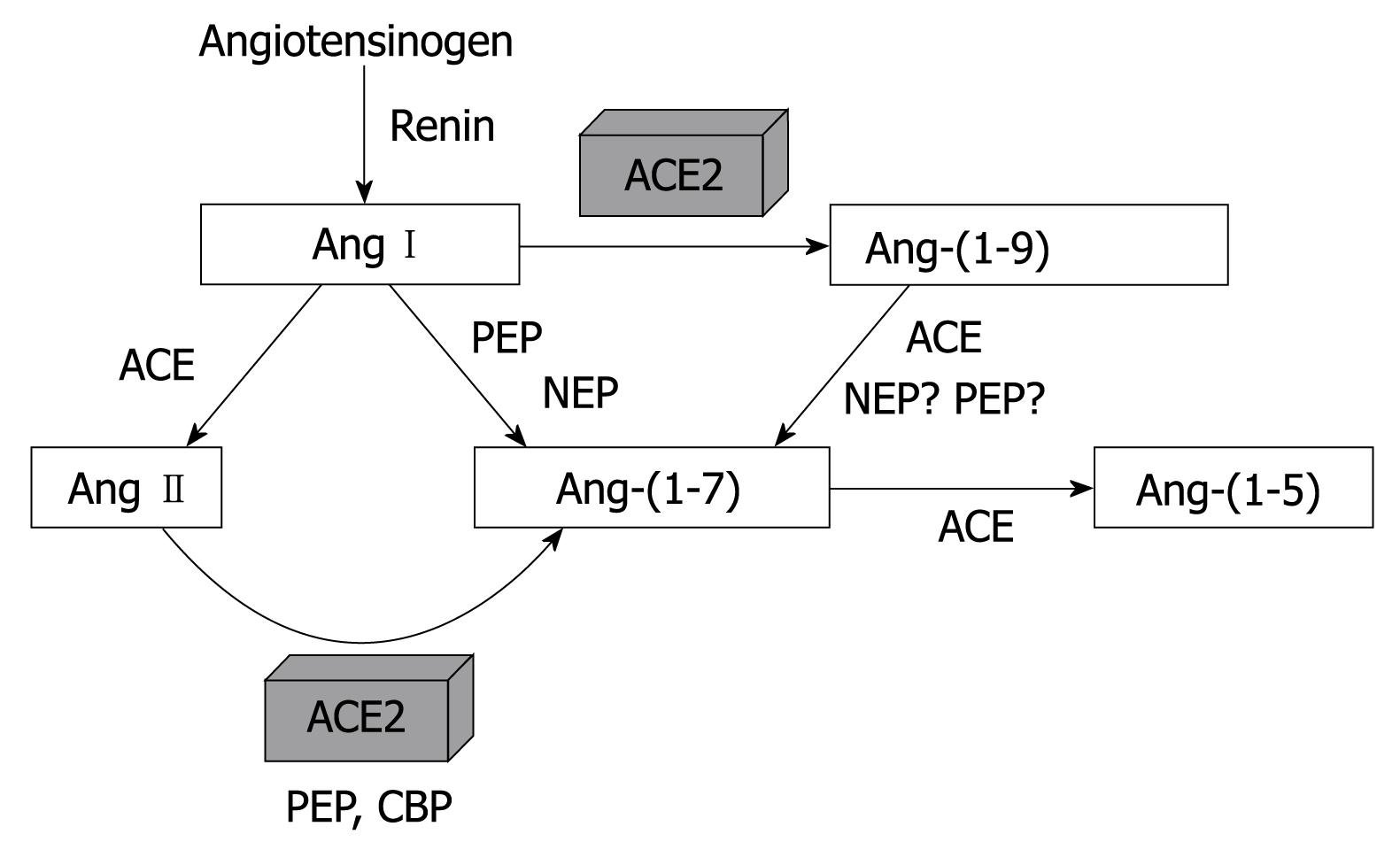
Among RAS mediators, Ang-(1-7) is of particular interest due to its selectivity, which is attributed to the absence of phenylalanine (Phe) in the C-terminal position, which is critical for the binding of Ang II to AT1 receptors[27]. Several enzymatic routes may be involved in Ang-(1-7) formation, either directly from Ang I or from Ang II through tissue peptidase actions, including neutral endopeptidase, oligopeptidase, prolyl-carboxypeptidase, prolyl-endopeptidase and ACE2[2428]. The recent discovery of ACE2 has provided an important pathway for production of Ang-(1-7), probably by exerting an important role in tissue peptide formation[19–21]. Figure 2 shows the main pathways for Ang-(1-7) metabolism.
Figure 2 View of the main metabolic pathways of Ang II and Ang-(1-7).
ACE: Angiotensin converting enzyme; ACE2: Angiotensin converting enzyme 2; PEP: Prolyl-endopeptidase; NEP: Neutral endopeptidase; CBP: Carboxypeptidase.
Once it is formed, Ang-(1-7) is rapidly hydrolyzed, especially by ACE[29]. In the presence of ACE inhibition and after chronic administration of AT1 receptor blockers, Ang-(1-7) levels are raised several times, suggesting that this heptapeptide contributes to the actions of RAS blockers[283031]. Many studies have indicated the physiopathological importance of Ang-(1-7) in human diseases[32–36]. In general, Ang-(1-7) has opposing actions to Ang II, which gives the system a dual influence over various tissues[2021]. For instance, Ang-(1-7) have vasodilator and anti-proliferative effects on blood vessels[37–39]. This effect allows RAS to influence vascular growth, either by stimulating through Ang II or inhibiting through Ang-(1-7)[2137–39]. Ang II is clearly mitogenic in vascular smooth muscle cell (VSMC) culture and in intact arteries, whereas Ang-(1-7) inhibits VSMC growth and reduces neointimal formation[373840]. To date, Ang II and Ang-(1-7) exhibit opposite effects on the regulation of cell growth as demonstrated by Gallagher and Tallant (2004) in lung cancer cells[41]. Receptor Mas appears to be involved in the antiproliferative effect of Ang-(1-7) in VSMC[42] and in stent-induced neointima proliferation[43]. Furthermore, it has been demonstrated that Ang-(1-7) inhibits vascular growth through prostaglandin-mediated intracellular events inducing cAMP production and reduction of Ang II-stimulated ERK1/2 activities[44].
RAS can be envisioned as a dual function system, in which the vasoconstrictor/proliferative or vasodilator/antiproliferative actions are primarily driven by the ACE/ACE2 balance[2145]. According to that, an increased ACE/ACE2 activity ratio will lead to increased Ang II generation and increased catabolism of Ang-(1-7) favoring vasoconstriction while an opposite ratio will decrease Ang II and increase Ang-(1-7) levels facilitating vasodilation[2145]. The fact that Ang-(1-7)/Mas directly antagonizes many actions of Ang II, provides an additional layer of counter regulation in the system[46].
THE RAS’S ROLE IN LIVER FIBROSIS
A growing body of evidence indicates that the RAS takes part in the pathogenesis of liver fibrosis[47–61]. In this regard, plasma renin activity and aldosterone levels were both elevated in patients with liver cirrhosis, especially those with hepatorenal syndrome[47–50]. Indeed, Ang II and aldosterone actions lead to renal vasoconstriction, blood flow redistribution and increases in sodium and water tubular reabsorption[47–49]. All of these effects tend to normalize the organ perfusion pressure and plasma effective volume. The elevation in circulating levels of PRA and aldosterone is due to an excessive production of these substances and not from a diminished hepatic catabolism[4748]. This higher production could be attributed either to a physiological response to systemic vasodilation that occurs in cirrhotic patients[4861] or to an intrahepatic RAS activation[53–60].
Tissue fibrosis is a common response in numerous chronic diseases, regardless of etiology, resulting in the production of, for example, liver cirrhosis, glomerulosclerosis, interstitial lung fibrosis and cardiac hypertrophy. Thus, resembling what happens in renal[6263] and cardiac[16] fibrosis, several studies suggest that Ang II could mediate and exacerbate liver fibrosis through HSC activation and by stimulating TGFβ-1 via AT1 receptors[1–6141553–55]. Bataller et al[53] have shown the presence of AT1 receptors in human activated HSC cultures. Experimental studies with AT1a receptor knockout mice showed an attenuated liver inflammation and fibrosis following bile duct ligation[56]. Immunohistochemistry analysis revealed decreased infiltration by inflammatory cells, reduced lipid peroxidation products and decreased phosphorylation of c-Jun and p42/44 MAPK in AT1a knockout mice compared to AT1 wild type animals[56]. On the other hand, the genetic deletion of Ang II AT2 receptors worsened the fibrosis induced by CCl4 by stimulating oxidative stress, which lead to HSC activation[57]. While AT1 receptors play an important role in the development of fibrosis, the AT2 signal has anti-fibrogenic and/or cytoprotective effects on oxidative stress-induced liver fibrosis[5657]. Taken together, these experimental studies suggest that RAS-associated liver fibrogenesis may be determined by the balance between AT1 and AT2 signals.
Therefore, by activating AT1 receptors, Ang II induces contraction and proliferation of HSCs, which is considered the principal effector of hepatic acinar fibrosis[53–55]. A similar effect has been observed in mesangial and VSMC. AT1 receptors are found in most of the mesenchymal cells and mediate the majority of the Ang II biological effects, including the increase in intracellular calcium, cellular contraction and proliferation[5859]. The magnitude of the HSC contractile response to Ang II is comparable to the effect elicited by endothelin-1, which is considered the most powerful contractile agent to this cell line. Ang II contractile effects were attenuated in the presence of powerful vasodilators, such as nitric oxide and prostaglandins[53], and completely blocked by pre-incubation with the AT1 receptor blocker, losartan[55]. Additionally, Ang II mediates key biological actions involved in hepatic tissue repair, including myofibroblast proliferation, infiltration of inflammatory cells, and collagen synthesis[60]. Activated HSCs secrete Ang II, which induces fibrogenic actions through the activation of NADPH oxidase[1–4].
Although the mechanisms of hepatic fibrosis are not fully understood, such as in other tissues, experimental evidence indicates that TGFβ-1 has a key role in this process[1415]. In the heart and in the kidneys, many vasoactive peptides have shown themselves capable of enhancing TGFβ-1 expression, including Ang II[166062]. Jonsson et al[54] have investigated functional polymorphisms of TGFβ-1 and angiotensinogen genes and the influence of these genotypes in liver fibrosis of patients with chronic C hepatitis. These authors found a significant relation between TGFβ-1 and angiotensinogen genotypes and the development of liver fibrosis[54]. Patients that did not exhibit a profibrotic genotype normally did not develop fibrosis. Ang II also increases TGFβ-1 and the genetic expression of collagen 1 via AT1 receptors in the liver[5558]. TGFβ-1 also induces HSC activation, which, in turn, increases TGFβ-1 expression[1415]. Thus, there is a formation of autocrine and paracrine loops that assure the continuous production of this fibrogenic cytokine[161415].
RAS inhibition reduces collagen IV expression and interstitial expansion in different tissues. The response to treatments with AT1 receptor blockers and ACE inhibitors clearly illustrate the importance of the RAS in renal and cardiac fibrosis[16176465]. Kidney tubulointerstitial fibrosis induced by cyclosporine was ameliorated by RAS inhibition[66]. Similarly, RAS pharmacological blockade also reduced collagen IV expression and interstitial expansion in rats with renal obstruction[67]. Treatment with ACE inhibitors and/or AT1 receptor blockers has also shown beneficial effects in liver diseases[5152545558]. Some studies have shown reductions in TGFβ-1 and procollagen α1 mRNA levels in the liver of rats treated with captopril after common bile duct ligation (BDL), supporting the hypothesis of an Ang II action on HSCs[5859]. In addition to antifibrotic effects, captopril has improved hemodynamic alterations, renal function and cholestasis[5152545558]. Paizis et al[59] demonstrated that the RAS blockade by irbesartan, an AT1 receptor antagonist, in BDL rats, reduced the expression of TGFβ-1 and of collagen 1 in the liver. These findings are consistent with the concept that the Ang II-TGFβ-1 axis may work in the liver as a pathway towards organic fibrosis, as previously demonstrated in other experimental models, like the use of carbon tetrachloride[68] in mansonic schistosomiasis[69] and, more recently, in transgenic mice with high expression of TGFβ-1[70]. In fact, the local production of Ang II, as well as circulating RAS activation, may be a significant part of the tissue overall response to injuries.
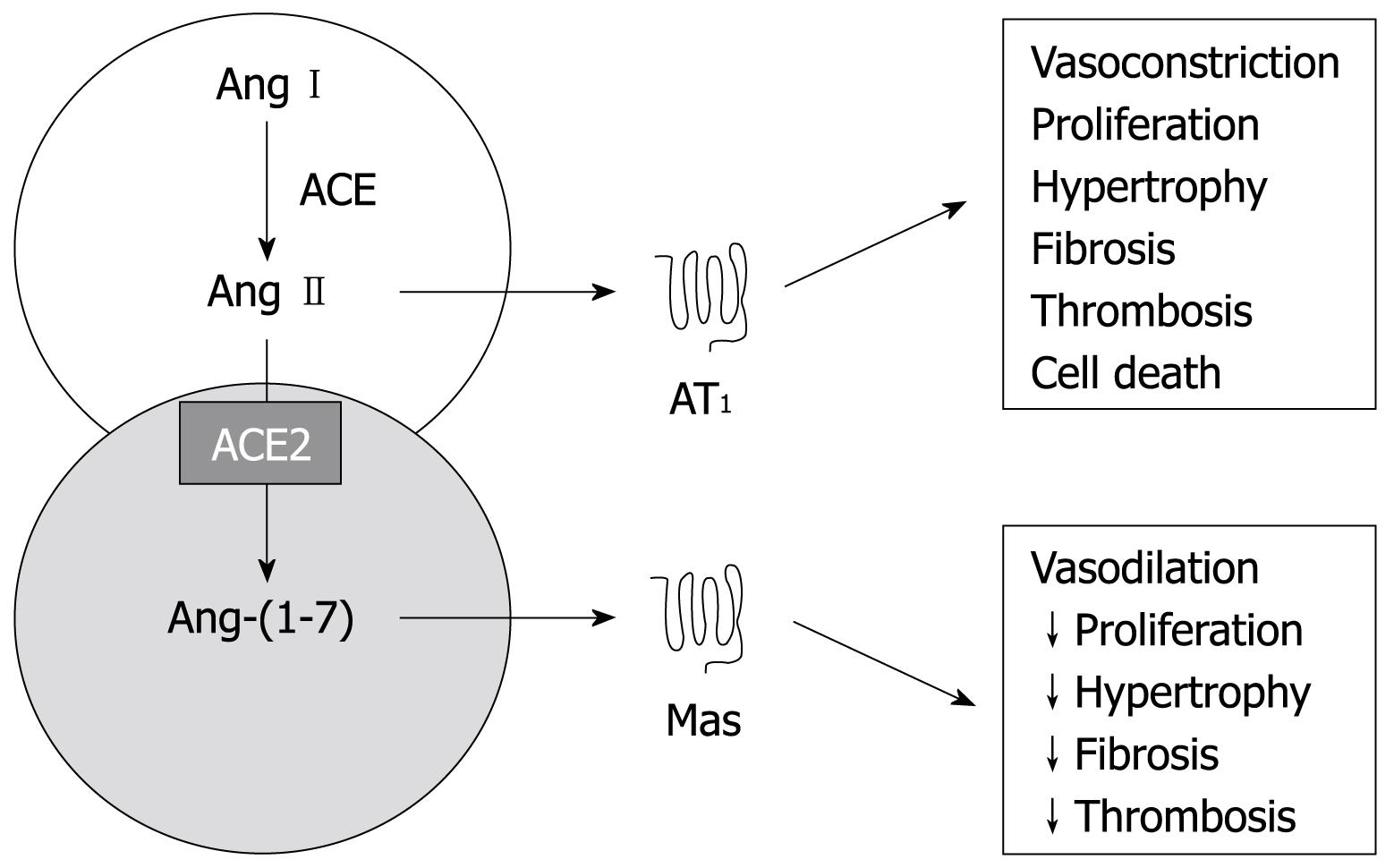
However, there are few studies exploring the role of Ang-(1-7) in liver fibrosis[4571–75]. Our group recently showed that the progression of liver dysfunction in BDL rats is characterized by marked changes in Ang-(1-7) levels and that the overall activation of the circulating RAS was associated in time with the progression of hepatic fibrosis[72]. Furthermore, the pharmacological blockade of the Ang-(1-7) receptor Mas accelerated liver fibrosis by increasing the liver content of collagen and TGFβ-1[72]. In line with these findings, Paizis et al[71] (2005) observed an upregulation of ACE2 and its widespread expression throughout the liver in BDL animals and in human cirrhosis. A few years later, the same group showed that as BDL rats developed advanced fibrosis, increased expression of components of the classic RAS such as ACE, AT1 receptor and Ang II was accompanied by increased hepatic and plasma ACE2 activity, increased Mas expression in the liver and a major rise in plasma levels of Ang-(1-7)[73]. More recently, Lubel et al[75] (2009) corroborated these previous studies regarding the protective role of Ang-(1-7) against liver fibrosis. The authors reported that, in BDL rats, Ang-(1-7) not only improved the histological fibrosis stage and reduced hydroxyproline content but also decreased gene expression of collagen 1A1, α-SMA, VEGF, CTGF, ACE and receptor Mas[75]. In addition, cultured hepatic cells expressed AT1 and Mas receptors, and when treated with Ang-(1-7) or the Mas receptor agonist, AVE 0991, produced less α-SMA and hydroxyproline, an effect reversed by the Mas receptor antagonist, A779. Ang-(1-7) is upregulated in human liver disease and has antifibrotic actions in a rat model of cirrhosis[75]. Indeed, the current studies[4571–75] raise the possibility that upregulation of hepatic ACE2 and Mas, and the generation of Ang-(1-7) represent a counter regulatory response to RAS-mediated liver injury (Figure 3).
Figure 3 A schematic diagram of both RAS arms.
The counter-regulatory arm of the RAS, ACE2-Ang-(1-7)-Mas axis, produces effects that oppose those of the ACE-Ang II-AT1 receptor axis. Ang: Angiotensin; Mas: G-protein coupled receptor of Ang-(1-7); AT1: Type 1 receptor of Ang II.
There is substantial evidence to suggest that Ang-(1-7) is involved in the beneficial actions of AT1 receptor blockers, ACE and vasopeptidase inhibitors[76]. Supporting this theory Maia et al[77] have shown that the antagonist of Ang-(1-7) receptor Mas, A-779[78], has attenuated the hypotensive response to bradykinin in animals treated with ACE inhibitors, suggesting the involvement of Ang-(1-7) in the cardiovascular effects of ACE inhibitors. The studies with BDL rats are also evidence that RAS blocking agents may attenuate liver fibrosis not only by antagonizing Ang II, but also by elevating Ang-(1-7) levels[4572–75]. Thus, the administration of Ang-(1-7) or its oral agonist, the compound AVE0991[7579], could be useful for understanding the mechanisms of fibrosis and should be further investigated for the treatment of liver diseases associated with fibrosis.
CONCLUSION
The better understanding of the underlying mechanisms involved in liver fibrosis makes effective antifibrotic therapy an imminent reality. However, treating this disease remains a challenge and, up to this moment, no antifibrotic agent has been approved for routine human use. It is important to mention that Ang-(1-7) is quickly hydrolyzed, especially by ACE and, in presence of ACE inhibition and after chronic administration of AT1 receptor blockers, its levels increase several times[303135], suggesting that this heptapeptide may contribute to RAS blockade[2021]. Furthermore, Kostenis et al[46] have recently demonstrated that the Ang-(1-7) Mas receptor can hetero-oligomerize with the AT1 receptor and by so doing inhibits the actions of Ang II. So, it is believed that the Mas receptor acts in vivo as an antagonist to the AT1 receptor[46]. Hence, it has raised the hypothesis that the RAS acts through two pathways: the first one, responsible for the main actions of the system, composed of the ACE-Ang II-AT1 receptor system and the second one, the counter-regulatory pathway, formed by the ACE2-Ang-(1-7)-receptor Mas system[202145]. Finally, the use of ACE inhibitors, AT1 receptor antagonists and, perhaps, Ang-(1-7) receptor Mas agonists[78] could become important tools in this study, and maybe to the therapeutic approach of liver fibrosis.
Peer reviewers: Ramon Bataller, MD, Liver Unit, Hospital Clinic, Villarroel 170, Barcelona 08036, Spain; Wendy M Mars, PhD, Department of Pathology, University of Pittsburgh, S-411B South Biomedical Science Tower Pittsburgh, PA 15261, United States