Micronutrients in Oncological Intervention
Abstract
:1. Introduction
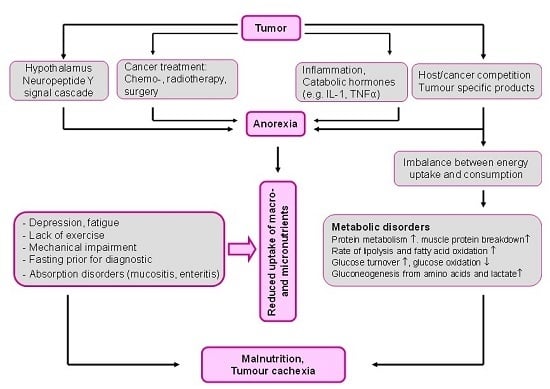
2. Malnutrition: The Overlooked Link to Micronutrient Deficiency
3. Micronutrient Status in Patients with Cancer
4. Micronutrient Supplements for Patients with Cancer
5. Vitamin D
5.1. Vitamin D: Sunlight and Cancer
5.2. Vitamin D: Deficiency and Insufficiency
5.3. Vitamin D Deficiency in Patients with Cancer
5.4. Vitamin D and Cancer Therapy
6. Selenium
6.1. Selenium and the Risk of Cancer
6.2. Use of High-Dose Selenium during Chemo- or Radiotherapy
7. l-Carnitine
7.1. l-Carnitine: Biological Functions
7.2. l-Carnitine Deficiency in Patients with Cancer
- Nutritive l-carnitine deficiency with an inadequate diet (e.g., deficient in iron, vitamin C, and l-methionine).
- Competition with cytostatics (e.g., competition with anthracyclines for the carnitine transporter OCTN2, which is necessary for the transport of l-carnitine into the cells).
- Disruption of l-carnitine biosynthesis by anthracyclines.
- Increase in renal l-carnitine excretion by cisplatin and ifosfamide; formation of non-physiological l-carnitine esters and, hence, increased l-carnitine excretion via the kidneys.
7.3. l-Carnitine Depletion by Cisplatin and Ifosfamide
- l-Carnitine depletion (secondary l-carnitine deficiency in the blood and tissues) with a fall in the plasma l-carnitine levels (<35 µmol/L).
- Impairment of carnitine-palmitoyl transferase 1 activity (an enzyme that catalyzes the carnitine-dependent transport of fatty acids into the mitochondria) and of the cellular carnitine transporter OCTN2.
- Disruption of mitochondrial ATP synthesis, energy deficiency.
- Increased mitochondrial toxicity of cisplatin and ifosfamide.
- Increased risk of fatigue [164] as well as of cisplatin- and ifosfamide-induced adverse neurotoxic and cardiotoxic effects.
7.4. High-Dose l-Carnitine Supplements in Patients with Cancer
8. Vitamin C
8.1. Vitamin C Deficiency
8.2. Preclinical Data: Combination with Chemotherapy and Radiotherapy
8.3. Vitamin C: Effect on Cancer Cells in Vitro
8.4. Vitamin C and Radiotherapy
8.5. Vitamin C in Cancer Therapy
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bray, F.; Jemal, A.; Grey, N.; Ferlay, J.; Forman, D. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncol. 2012, 13, 790–801. [Google Scholar] [CrossRef]
- American Cancer Society. Global Cancer: Facts and Figures, 3rd ed.; American Cancer Society: New York, NY, USA, 2015. [Google Scholar]
- Holzhauer, P.; Gröber, U. Checkliste: Komplementäre Onkologie; Hippokrates Verlag: Stuttgart, Germany, 2010. [Google Scholar]
- Gröber, U.; Mücke, R.; Adamietz, I.A.; Holzhauer, P.; Kisters, K.; Büntzel, J.; Micke, O. Komplementärer Einsatz von Antioxidanzien und Mikronährstoffen in der Onkologie—Update 2013. Der Onkol. 2013, 19, 136–143. [Google Scholar] [CrossRef]
- Gröber, U.; Hübner, J.; Holzhauer, P.; Kleeberg, U.R. Antioxidanzien und andere Mikronährstoffe in der komplementären Onkologie. Der Onkol. 2010, 16, 73–79. [Google Scholar] [CrossRef]
- Micke, O.; Bruns, F.; Glatzel, M.; Schönekaes, K.; Micke, P.; Mücke, R. Predictive factors for the use of complementary and alternative medicine (CAM) in radiation oncology. Eur. J. Integr. Med. 2009, 1, 22–30. [Google Scholar] [CrossRef]
- Zirpoli, G.R.; Brennan, P.M.; Hong, C.C.; McCann, S.E.; Ciupak, G.; Davis, W.; Unger, J.M.; Budd, G.T.; Hershman, D.L.; Moore, H.C.; et al. Supplement use during an intergroup clinical trial for breast cancer (S0221). Breast. Cancer Res. Treat. 2013, 137, 903–913. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G. Use of antioxidants during chemotherapy and radiotherapy should be avoided. CA Cancer J. Clin. 2005, 55, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Block, K.; Koch, A.; Mead, M.; Newman, R.A.; Gyllenhaal, C. Re: Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J. Natl. Cancer Inst. 2009, 101, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Yasueda, A.; Urushima, H.; Ito, T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: A systematic review. Integr. Cancer Ther. 2015, 15, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Block, K.I.; Koch, A.C.; Mead, M.N.; Tothy, P.K.; Newman, R.A.; Gyllenhaal, C. Impact of antioxidant supplementation on chemotherapeutic toxicity: A systematic review of the evidence from randomized controlled trials. Int. J. Cancer 2008, 123, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Nechuta, S.; Lu, W.; Chen, Z.; Zheng, Y.; Gu, K.; Cai, H.; Zheng, W.; Shu, X.O. Vitamin supplement use during breast cancer treatment and survival: A prospective cohort study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Kisters, K.; Adamietz, I.A. Vitamin D in oncology: Update 2015. Med. Monatsschr. Pharm. 2015, 38, 512–516. [Google Scholar] [PubMed]
- Fearon, K.C.; Voss, A.C.; Hustend, D.S. Definition of cancer cachexia: Effect of weight loss, reduced food intake and systemic inflammation on functional status and prognosis. Am. J. Clin. Nutr. 2006, 83, 1345–1350. [Google Scholar] [PubMed]
- Tong, H.; Isenring, E.; Yates, P. The prevalence of nutrition impact symptoms and their relationship to quality of life and clinical outcomes in medical oncology patients. Support Care Cancer 2009, 17, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Laviano, A.; Meguid, M.M. Nutritional issues in cancer management. Nutrition 1996, 12, 358–371. [Google Scholar] [CrossRef]
- Bozzetti, F.; SCRINIO Working Group. Screening the nutritional status in oncology: A preliminary report on 1000 outpatients. Support Care Cancer 2009, 17, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Ströhle, A.; Zänker, K.; Hahn, A. Nutrition in oncology: The case of micronutrients (review). Oncol. Rep. 2010, 24, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Zürcher, G. Mangelernährung bei onkologischen Patienten. In Vitamine, Spurenelemente und Mineralstoffe; Thieme: Stuttgart, Germany, 2002. [Google Scholar]
- Züricher, G. Medikamentöse Strategien zur Gewichtszunahme bei kachektischen Patienten. Akt Ernährungsmed. 2002, 27, 398–407. [Google Scholar]
- Pirlich, M.; Schütz, T.; Norman, K.; Gastell, S.; Lübke, H.J.; Bischoff, S.C.; Bolder, U.; Frieling, T.; Güldenzoph, H.; Hahn, K.; et al. The German hospital malnutrition study. Clin. Nutr. 2006, 25, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Dewys, W.D.; Begg, C.; Lavin, P.T.; Band, PR.; Bennett, J.M.; Bertino, J.R.; Cohen, M.H.; Douglass, H.O., Jr.; Engstrom, P.F.; Ezdinli, E.Z.; et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am. J. Med. 1980, 69, 491–497. [Google Scholar] [CrossRef]
- Barker, L.A.; Gout, B.S.; Crowe, T.C. Hospital malnutrition: Prevalence, identification and impact on patients and the healthcare system. Int. J. Environ. Res. Public Health 2011, 8, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, M.J. Mechanisms of cancer cachexia. Physiol. Rev. 2009, 89, 381–410. [Google Scholar] [CrossRef] [PubMed]
- Seelaender, M.; Laviano, A.; Busquets, S.; Püschel, G.P.; Margaria, T.; Batista, M.L., Jr. Inflammation in Cachexia. Mediators Inflamm. 2015. [Google Scholar] [CrossRef] [PubMed]
- Fedeles, B.I.; Freudenthal, B.D.; Yau, E.; Singh, V.; Chang, S.C.; Li, D.; Delaney, J.C.; Wilson, S.H.; Essigmann, J.M. Intrinsic mutagenic properties of 5-chlorocytosine: A mechanistic connection between chronic inflammation and cancer. Proc. Natl. Acad. Sci. USA 2015, 112, E4571–E4580. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.T.; Tisdale, M.J. The role of glucocorticoids in the induction of zinc-alpha2-glycoprotein expression in adipose tissue in cancer cachexia. Br. J. Cancer 2005, 92, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.; Kravits, K. Symptoms and their impact on nutrition. Semin. Oncol. Nurs. 2000, 16, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Büntzel, J.; Bruns, F.; Glatzel, M.; Garayev, A.; Mücke, R.; Kisters, K.; Schäfer, U.; Schönekaes, K.; Micke, O. Zinc concentrations in serum during head and neck cancer progression. Anticancer Res. 2007, 27, 1941–1943. [Google Scholar] [PubMed]
- Churilla, T.M.; Brereton, H.D.; Klem, M.; Peters, C.A. Vitamin D deficiency is widespread in cancer patients and correlates with advanced stage disease: A community oncology experience. Nutr. Cancer 2012, 64, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Crew, K.D.; Shane, E.; Cremers, S.; McMahon, D.J.; Irani, D.; Hershmann, D.L. High prevalence of vitamin D deficiency despite supplementation in premenopausal women with breast cancer undergoing adjuvant chemotherapy. J. Clin. Oncol. 2009, 27, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, R.A.; Dvorkin, E.; Homel, P.; Culliney, B.; Malamud, S.; Shaiova, L.; Fleishman, S.; Lapin, J.; Klein, E.; Lesage, P.; et al. l-Carnitine supplementation for the treatment of fatigue and depressed mood in cancer patients with carnitine deficiency: A preliminary analysis. Ann. N. Y. Acad. Sci. 2004, 1033, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Kraft, K.; Gärtner, S.; Mayerle, J.; Simon, P.; Weber, E.; Schütte, K.; Stieler, J.; Holzhauer, P.; Gröber, U.; et al. l-Carnitine-supplementation in advanced pancreatic cancer (CARPAN)—A randomized multicentre trial. Nutr. J. 2012, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Mayland, C.R.; Bennett, M.I.; Allan, K. Vitamin C deficiency in cancer patients. Palliat. Med. 2005, 19, 17–20. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, L.L.; Barbosa, J.M.; Ribeiro, A.P.G.; dos Santos, A.C.O.; Pedrosa, F. Nutritional status, dietary intake and serum levels of vitamin C upon diagnosis of cancer in children and adolescents. Nutr. Hosp. 2012, 27, 496–503. [Google Scholar]
- Babaknejad, N.; Sayehmiri, F.; Sayehmiri, K.; Rahimifar, P.; Bahrami, S.; Delpesheh, A.; Hemati, F.; Alizadeh, S. The relationship between selenium levels and breast cancer: A systematic review and meta-analysis. Biol. Trace Elem. Res. 2014, 159, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Wang, F.; Zhao, Y.S.; Pan, H.Z. Evaluation of oxidative stress in colorectal cancer patients. Biomed. Environ. Sci. 2008, 21, 286–289. [Google Scholar] [CrossRef]
- Leung, E.Y.; Crozier, J.E.; Talwar, D.; O’Reilly, D.S.; McKee, R.F.; Horgan, P.G.; McMillan, D.C. Vitamin antioxidants, lipid peroxidation, tumour stage, the systemic inflammatory response and survival in patients with colorectal cancer. Int. J. Cancer 2008, 123, 2460–2464. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Yin, M.C. B vitamins deficiency and decreased anti-oxidative state in patients with liver cancer. Eur. J. Nutr. 2007, 46, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Look, M.P.; Musch, E. Lipid peroxides in the polychemotherapy of cancer patients. Chemotherapy 1994, 40, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, G.; Macciò, A.; Madeddu, C.; Mura, L.; Massa, E.; Gramignano, G.; Lusso, M.R.; Murgia, V.; Camboni, P.; Ferreli, L. Reactive oxygen species, antioxidant mechanisms and serum cytokine levels in cancer patients: Impact of an antioxidant treatment. J. Cell. Mol. Med. 2002, 6, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Macciò, A.; Madeddu, C.; Gramignano, G.; Mulas, C.; Floris, C.; Sanna, E.; Cau, M.C.; Panzone, F.; Mantovani, G. A randomized phase III clinical trial of a combined treatment for cachexia in patients with gynecological cancers: Evaluating the impact on metabolic and inflammatory profiles and quality of life. Gynecol. Oncol. 2012, 124, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Palan, P.R.; Woodall, A.L.; Anderson, P.S.; Mikhail, M.S. Alpha-tocopherol and alpha-tocopheryl quinone levels in cervical intraepithelial neoplasia and cervical cancer. Am. J. Obstet. Gynecol. 2004, 190, 1407–1410. [Google Scholar] [CrossRef]
- Skrzydlewska, E.; Sulkowski, S.; Koda, M.; Zalewski, B.; Kanczuga-Koda, L.; Sulkowska, M. Lipid peroxidation and antioxidant status in colorectal cancer. World J. Gastroenterol. 2005, 11, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.M.; Yin, M.C.; Liu, W.H. Oxidant stress and B vitamins status in patients with non-small cell lung cancer. Nutr. Cancer 2007, 59, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, M. Cutaneous bleeding related to zinc deficiency in two cases of advanced cancer. Cancer 1999, 86, 866–870. [Google Scholar] [CrossRef]
- Ravasco, P.; Monteiro-Grillo, I.; Vidal, P.M.; Camilo, M.E. Dietary counseling improves patient outcomes: A prospective, randomized, controlled trial in colorectal cancer patients undergoing radiotherapy. J. Clin. Oncol. 2005, 23, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Dormand, E.L.; Banwell, P.E.; Goodacre, T.E. Radiotherapy and wound healing. Int. Wound J. 2005, 2, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Muecke, R.; Micke, O.; Schomburg, L.; Glatzel, M.; Reichl, B.; Kisters, K.; Schaefer, U.; Eich, H.T.; Fakhrian, K.; Adamietz, I.A.; et al. German Working Group Trace Elements and Electrolytes in Oncology-AKTE. Multicenter, phase III trial comparing selenium supplementation with observation in gynecologic radiation oncology: Follow-up analysis of the survival data 6 years after cessation of randomization. Integr. Cancer Ther. 2014, 13, 463–467. [Google Scholar] [PubMed]
- Jaakkola, K.; Lähteenmäki, P.; Laakso, J.; Harju, E.; Tykkä, H.; Mahlberg, K. Treatment with antioxidant and other nutrients in combination with chemotherapy and irradiation in patients with small-cell lung cancer. Anticancer Res. 1992, 12, 599–606. [Google Scholar] [PubMed]
- Jatoi, A.; Williams, B.A.; Marks, R.; Nichols, F.C.; Aubry, M.C.; Wampfler, J.; Yang, P. Exploring vitamin and mineral supplementation and purported clinical effects in patients with small cell lung cancer: Results from the Mayo Clinic lung cancer cohort. Nutr. Cancer 2005, 51, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, A.; Williams, B.; Nichols, F.; Aubry, M.C.; Wampfler, J.; Yang, P. Is voluntary vitamin and mineral supplementation associated with better outcome in non-small cell lung cancer patients? Results from the Mayo Clinic lung cancer cohort. Lung Cancer 2005, 49, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Simone, C.B., II; Simone, N.L.; Simone, V.; Simone, C.B. Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase kill and increase survival, Part 2. Altern. Ther. Health Med. 2007, 13, 40–47. [Google Scholar] [PubMed]
- Sieja, K.; Talerczyk, M. Selenium as an element in the treatment of ovarian cancer in women receiving chemotherapy. Gynecol. Oncol. 2004, 93, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Drisko, J.A.; Chapman, J.; Hunter, V.J. The use of antioxidants with firstline chemotherapy in two cases of ovarian cancer. J. Am. Coll. Nutr. 2003, 22, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Lawenda, B.D.; Kelly, K.M.; Ladas, E.J.; Sagar, S.M.; Vickers, A.; Blumberg, J.B. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J. Natl. Cancer Inst. 2008, 100, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Mutschler, E.; Geisslinger, G.; Kroemer, H.K. (Eds.) Mutschler Arzneimittelwirkungen: Pharmakologie—Klinische Pharmakologie—Toxikologie. 10. Auflage; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2013.
- Schmoll, H.J.; Höffken, K.; Possinger, K. Kompendium Internistische Onkologie Standards in Therapie und Diagnostik, Teil I–III; Springer: Heidelberg, Germany, 2005. [Google Scholar]
- Moss, R.W. Should patients undergoing chemotherapy and radiotherapy be prescribed antioxidants? Integr. Cancer. Ther. 2006, 5, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.K.; Bhutani, M.; Guleria, R.; Bal, S.; Mohan, A.; Mohanti, B.K.; Sharma, A.; Pathak, R.; Bhardwaj, N.K.; Prasad, K.N.; et al. Chemotherapy alone vs. chemotherapy plus high dose multiple antioxidants in patients with advanced non small cell lung cancer. J. Am. Coll. Nutr. 2005, 24, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N. Multiple dietary antioxidants enhance the efficacy of standard and experimental cancer therapies and decrease their toxicity. Integr. Cancer. Ther. 2004, 3, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Cole, W.C. Antioxidants in cancer therapy. J. Clin. Oncol. 2006, 24, e8–e9. [Google Scholar] [CrossRef] [PubMed]
- Block, K.; Koch, A.C.; Mend, M.N.; Tothy, P.K.; Newman, R.A.; Gyllenhaal, C. Impact of antioxidant supplementation on chemotherapeutic efficacy: A systematic review of the evidence from randomized controlled trials. Cancer Treat. Rev. 2007, 33, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U. Micronutrients in complementary oncology. Med. Monatsschr. Pharm. 2008, 31, 217–223. [Google Scholar] [PubMed]
- Norman, H.A.; Butrum, R.R.; Feldman, E.; Heber, D.; Nixon, D.; Picciano, M.F.; Rivlin, R.; Simopoulos, A.; Wargovich, M.J.; et al. The role of dietary supplements during cancer therapy. J. Nutr. 2003, 133 (Suppl. 11), S3794–S3799. [Google Scholar]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W. Nutrition and physical activity guidelines for cancer survivors. CA. Cancer J. Clin. 2012, 62, 243–274. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, F.L. The Mortality from Cancer throughout the World; Prudential Press: Newark, NJ, USA, 1916. [Google Scholar]
- Peller, S.; Stephenson, C.S. Skin irritation and Cancer in the US navy. Am. J. Med. Sci. 1937, 194, 326–333. [Google Scholar] [CrossRef]
- Apperly, F.L. The relation of solar radiation to cancer mortality in North America. Cancer Res. 1941, 1, 191–195. [Google Scholar]
- Garland, C.F.; Garland, F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 1980, 9, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Comstock, G.W.; Garland, F.C.; Helsing, K.J.; Shaw, E.K.; Gorham, E.D. Serum 25-hydroxyvitamin D and colon cancer: Eight-year prospective study. Lancet 1989, 2, 1176–1178. [Google Scholar] [CrossRef]
- Lefkowitz, E.S.; Garland, C.F. Sunlight, vitamin D, and ovarian cancer mortality rates in US women. Int. J. Epidemiol. 1994, 23, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Garland, F.C.; Garland, C.F.; Gorham, E.D.; Young, J.F. Geographic variation in breast cancer mortality in the United States: A hypothesis involving exposure to solar radiation. Prev. Med. 1990, 19, 614–622. [Google Scholar] [CrossRef]
- Mizoue, T. Ecological study of solar radiation and cancer mortality in Japan. Health Phys. 2004, 87, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Hanchette, C.L.; Schwartz, G.G. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer 1992, 70, 2861–2869. [Google Scholar] [CrossRef]
- Grant, W.B. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer 2002, 94, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Ecological studies of the UVB-vitamin D-cancer hypothesis. Anticancer Res. 2012, 32, 223–236. [Google Scholar] [PubMed]
- Grant, W.B. Update on evidence that support a role of solar ultraviolet-B irradiance in reducing cancer risk. Anticancer Agents Med. Chem. 2013, 13, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Boscoe, F.P.; Schymura, M.J. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993–2002. BMC Cancer 2006, 6, 264. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.A.; Lesosky, M.; Barnett, H.; Raboud, J.M.; Vieth, R. Vitamin D and reduced risk of breast cancer: A population-based case-control study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Luscombe, C.J.; Fryer, A.A.; French, M.E.; Liu, S.; Saxby, M.F.; Jones, P.W.; Strange, R.C. Exposure to ultraviolet radiation: Association with susceptibility and age at presentation with prostate cancer. Lancet 2001, 358, 641–642. [Google Scholar] [CrossRef]
- Giovannucci, E.; Liu, Y.; Rimm, E.B.; Hollis, B.W.; Stampfer, M.J.; Willett, W.C. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Natl. Cancer Inst. 2006, 98, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Role of solar UVB irradiance and smoking in cancer as inferred from cancer incidence rates by occupation in Nordic countries. Dermato-Endocrinology 2012, 4, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.W.; Wheeler, D.C.; Park, Y.; Cahoon, E.K.; Hollenbeck, A.R.; Freedman, D.M.; Abnet, C.C. Prospective study of ultraviolet radiation exposure and risk of cancer in the United States. Int. J. Cancer 2012, 131, E1015–E1023. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Holick, M.F. Vitamin D. Die Heilkraft des Sonnenvitamins. 3. Auflage, 340 S; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2015. [Google Scholar]
- Wacker, M.; Holick, M.F. Vitamin D—Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.B.; Gorham, E.D.; Kim, J.; Hofflich, H.; Garland, C.F. Meta-analysis of vitamin D sufficiency for improving survival of patients with breast cancer. Anticancer Res. 2014, 34, 1163–1166. [Google Scholar] [PubMed]
- Gröber, U.; Reichrath, J.; Holick, M.F. Live longer with vitamin D? Nutrients 2015, 7, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Linseisen, J.; Slanger, T.; Kropp, S.; Mutschelknauss, E.J.; Flesch-Janys, D.; Chang-Claude, J. Serum 25-hydroxyvitamin D and risk of postmenopausal breast cancer—Results of a large casecontrol study. Carcinogenesis 2008, 29, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Gorham, E.D.; Garland, C.F.; Garland, F.C.; Mohr, S.B.; Lipkin, M.; Newmark, H.L.; Giovannucci, E.; Wei, M.; Holick, M.F. Optimal vitamin D status for colorectal cancer prevention: A quantitative meta analysis. Am. J. Prev. Med. 2007, 32, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Maurer, M.J.; Link, B.K.; Habermann, T.M.; Ansell, S.M.; Micallef, I.N.; Kelly, J.L.; Macon, W.R.; Nowakowski, G.S.; et al. Vitamin D insufficiency and prognosis in non-Hodgkin’s lymphoma. J. Clin. Oncol. 2010, 28, 4191–4198. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Grandi, N.; Raum, E.; Haug, U. Meta-analysis: Longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment. Pharmacol. Ther. 2009, 30, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, P.J.; Ennis, M.; Pritchard, K.I.; Koo, J.; Hood, N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J. Clin. Oncol. 2009, 27, 3757–3763. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.C.; Zhang, Z.L.; Wan, Z.; Wang, L.; Weber, P.; Eggersdorfer, M.; Qin, L.Q.; Zhang, W. Circulating 25-hydroxyvitamin D and risk of lung cancer: A dose-response meta-analysis. Cancer Causes Control 2015, 26, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, S.; Che, X.; Li, X. Vitamin D and lung cancer risk: A comprehensive review and meta-analysis. Cell Physiol. Biochem. 2015, 36, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Maalmi, H.; Ordóñez-Mena, J.M.; Schöttker, B.; Brenner, H. Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients: Systematic review and meta-analysis of prospective cohort studies. Eur. J. Cancer 2014, 50, 1510–1521. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Je, Y. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: A meta-analysis. Br. J. Cancer 2014, 110, 2772–2784. [Google Scholar] [CrossRef] [PubMed]
- Schöttker, B.; Jorde, R.; Peasey, A.; Thorand, B.; Jansen, E.H.; Groot, L.D.; Streppel, M.; Gardiner, J.; Ordóñez-Mena, J.M.; Perna, L.; et al. Vitamin D and mortality: Meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ 2014, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Printz, C. High vitamin D levels increase survival rates in patients with metastatic colorectal cancer. Cancer 2015, 121, 2105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Song, L. Association between vitamin D receptor gene polymorphisms and breast cancer risk: A meta-analysis of 39 studies. PLoS ONE 2014, 9, e96125. [Google Scholar] [CrossRef] [PubMed]
- Lappe, J.M.; Travers-Gustafson, D.; Davies, K.M.; Recker, R.R.; Heaney, R.P. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am. J. Clin. Nutr. 2007, 85, 1586–1591. [Google Scholar] [PubMed]
- Bade, B.; Zdebik, A.; Wagenpfeil, S.; Gräber, S.; Geisel, J.; Vogt, T.; Reichrath, J. Low serum 25-hydroxyvitamin D concentrations are associated with increased risk for melanoma and unfavourable prognosis. PLoS ONE 2014, 9, e112863. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Galluzzo, S.; Vincenzi, B.; Zoccoli, A.; Ferraro, E.; Lippi, C.; Altomare, V.; Tonini, G.; Bertoldo, F. Longitudinal evaluation of vitamin D plasma levels during anthracycline—And docetaxel-based adjuvant chemotherapy in early-stage breast cancer patients. Ann. Oncol. 2010, 21, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Fink, M. Vitamin D deficiency is a cofactor of chemotherapy-induced mucocutaneous toxicity and dysgeusia. J. Clin. Oncol. 2011, 29, e81–e82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xie, W.; Krasowski, M.D. PXR: A xenobiotic receptor of diverse function implicated in pharmacogenetics. Pharmacogenomics 2008, 9, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Kisters, K. Influence of drugs on vitamin D and calcium metabolism. Dermato-Endocrinology 2012, 4, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Khan, Q.J.; Reddy, P.S.; Kimler, B.F.; Sharma, P.; Baxa, S.E.; O’Dea, A.P.; Klemp, J.R.; Fabian, C.J. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res. Treat. 2010, 119, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, A.L.; Taylor, M.E.; Gao, F.; Armamento-Villareal, R.; Jamalabadi-Majidi, S.; Napoli, N.; Ellis, M.J. Vitamin D and aromatase inhibitor-induced musculoskeletal symptoms (AIMSS): A phase II, double-blind, placebo-controlled, randomized trial. Breast Cancer Res. Treat. 2011, 129, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Carmel, A.S.; Shieh, A.; Bang, H.; Bockman, R.S. The 25(OH)D level needed to maintain a favorable bisphosphonate response is ≥33 ng/mL. Osteoporos. Int. 2012, 23, 2479–2487. [Google Scholar] [CrossRef] [PubMed]
- Berruti, A.; Cook, R.; Saad, F.; Buttigliero, C.; Lipton, A.; Tampellini, M.; Lee, K.A.; Coleman, R.E.; Smith, M.R.; Berruti, A.; et al. Prognostic role of serum parathyroid hormone levels in advanced prostate cancer patients undergoing zoledronic acid administration. Oncologist 2012, 17, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Hokugo, A.; Christensen, R.; Chung, E.M.; Sung, E.C.; Felsenfeld, A.L.; Sayre, J.W.; Garrett, N.; Adams, J.S.; Nishimura, I. Increased prevalence of bisphosphonate-related osteonecrosis of the jaw with vitamin D deficiency in rats. J. Bone Miner. Res. 2010, 25, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Favus, M.J. Bisphosphonates for osteoporosis. N. Engl. J. Med. 2010, 363, 2027–2035. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, A.; Saia, G.; Bettini, G.; Tronchet, A.; Totola, A.; Bedogni, G.; Tregnago, P.; Valenti, M.T.; Bertoldo, F.; Ferronato, G.; et al. Osteomalacia: The missing link in the pathogenesis of bisphosphonate-related osteonecrosis of the jaws? Oncologist 2012, 17, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Epperla, N.; Pathak, R. Hypocalcemia secondary to zoledronate therapy in a patient with low vitamin D level. WMJ 2015, 114, 163–166. [Google Scholar] [PubMed]
- Bergman, P.; Sperneder, S.; Höijer, J. Low vitamin D levels are associated with higher opioid dose in palliative cancer patients—Results from an observational study in Sweden. PLoS ONE 2015, 10, e0128223. [Google Scholar] [CrossRef] [PubMed]
- Bittenbring, J.T.; Neumann, F.; Altmann, B.; Achenbach, M.; Reichrath, J.; Ziepert, M.; Geisel, J.; Regitz, E.; Held, G.; Pfreundschuh, M. Vitamin D deficiency impairs rituximab-mediated cellular cytotoxicity and outcome of patients with diffuse large B-cell lymphoma treated with but not without rituximab. J. Clin. Oncol. 2014, 32, 3242–3248. [Google Scholar] [CrossRef] [PubMed]
- Baggerly, C.A.; Cuomo, R.E.; French, C.B.; Garland, C.F.; Gorham, E.D.; Grant, W.B.; Heaney, R.P.; Holick, M.F.; Hollis, B.W.; McDonnell, S.L.; et al. Sunlight and vitamin D: Necessary for public health. J. Am. Coll. Nutr. 2015, 34, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Grant, W. Roles of solar UVB and vitamin D in reducing cancer risk and increasing survival. Anticancer Res. 2016, 36. in press. [Google Scholar]
- Fearon, K.C. Cancer cachexia: Developing multimodal therapy for a multidimensional problem. Eur. J. Cancer 2008, 44, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Polly, P.; Tan, T.C. The role of vitamin D in skeletal and cardiac muscle function. Front. Physiol. 2014, 5, 145. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.H.; Owen, J.; Ahearn, T.; Fedirko, V.; Flanders, W.D.; Jones, D.P.; Bostick, R.M. Effects of supplemental vitamin D and calcium on biomarkers of inflammation in colorectal adenoma patients: A randomized, controlled clinical trial. Cancer Prev Res. (Phila.) 2011, 4, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, J.; Zaritsky, J.J.; Sea, J.L.; Chun, R.F.; Lisse, T.S.; Zavala, K.; Nayak, A.; Wesseling-Perry, K.; Westerman, M.; Hollis, B.W.; et al. Suppression of iron-regulatory hepcidin by vitamin D. J. Am. Soc. Nephrol. 2014, 25, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh-Moghaddam, A.; Gholamrezaei, A.; Hemati, S. Vitamin D deficiency is associated with the severity of radiation-induced proctitis in cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.B. The environment and disease: Association or causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Potischman, N.; Weed, D.L. Causal criteria in nutritional epidemiology. Am. J. Clin. Nutr. 1999, 69, 1309S–1314S. [Google Scholar] [PubMed]
- Grant, W.B. How strong is the evidence that solar ultraviolet B and vitamin D reduce the risk of cancer? An examination using hill's criteria for causality. Dermato-Endocrinology 2009, 1, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Long follow-up time and different sensitivities of cancer types may have obscured the effect of 25-hydroxyvitamin D on cancer incidence and mortality rates. Am. J. Clin. Nutr. 2015, 102, 230. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, F.P.; Raman, A.V.; Reeves, M.A.; Berry, M.J. Regulation and function of selenoproteins in human disease. Biochem. J. 2009, 422, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Wallenberg, M.; Misra, S.; Wasik, A.M.; Marzano, C.; Björnstedt, M.; Gandin, V.; Fernandes, A.P. Selenium induces a multi-targeted cell death process in addition to ROS formation. J. Cell Mol. Med. 2014, 18, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Wallenberg, M.; Misra, S.; Björnstedt, M. Selenium cytotoxicity in cancer. Basic Clin. Pharmacol. Toxicol. 2014, 114, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C.; Combs, G.F., Jr.; Turnbull, B.W. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 1996, 276, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N.; White, D.A.; Schneider, C.J. Cancer mortality correlation studies-III: Statistical associations with dietary selenium intakes. Bioinorg. Chem. 1977, 7, 23–31. [Google Scholar] [CrossRef]
- Willett, W.C.; Polk, B.F.; Morris, J.S.; Stampfer, M.J.; Pressel, S.; Rosner, B.; Taylor, J.O.; Schneider, K.; Hames, C.G. Prediagnostic serum selenium and risk of cancer. Lancet 1983, 2, 130–134. [Google Scholar] [CrossRef]
- Whanger, P.D. Selenium and its relationship to cancer: An update dagger. Br. J. Nutr. 2004, 91, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Saez, J.B.; Senra-Varela, A.; Pousa-Estevez, L. Selenium in breast cancer. Oncology 2003, 64, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Shamberger, R.J.; Willis, C.E. Selenium distribution and human cancer mortality. CRC Crit. Rev. Clin. Lab. Sci. 1971, 2, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.J.; Fedirko, V.; Jenab, M.; Schomburg, L.; Méplan, C.; Freisling, H.; Bueno-de-Mesquita, H.B.; Hybsier, S.; Becker, N.P.; Czuban, M.; et al. Selenium status is associated with colorectal cancer risk in the European prospective investigation of cancer and nutrition cohort. Int. J. Cancer 2015, 136, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Pourmand, G.; Salem, S.; Moradi, K. Serum selenium level and prostate cancer: A case-control study. Nutr. Cancer 2008, 60, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.E.; Duffield-Lillico, A.J.; Slate, E.; Natarajan, N.; Turnbull, B.; Jacobs, E. The nutritional prevention of cancer: 400 mcg per day selenium treatment. Nutr. Cancer 2008, 60, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Facompre, N.; El-Bayoumy, K. Potential stages for prostate cancer prevention with selenium: Implications for cancer survivors. Cancer Res. 2009, 69, 2699–2703. [Google Scholar] [CrossRef] [PubMed]
- Duffield-Lillico, A.J.; Dalkin, B.L.; Reid, M.E. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: An analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003, 91, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenoproteins and human health: Insights from epidemiological data. Biochim. Biophys. Acta 2009, 1790, 1533–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennert, G.; Zwahlen, M.; Brinkman, M.; Vinceti, M.; Zeegers, M.P.; Horneber, M. Selenium for preventing cancer (review). Cochrane Database Syst. Rev. 2011, 11. [Google Scholar] [CrossRef]
- Vinceti, M.; Dennert, G.; Crespi, C.M.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; del Giovane, C. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2014, 3. [Google Scholar] [CrossRef]
- Lippmann, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The selenium and vitamin E cancer prevention trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Bleys, J.; Navas-Acien, A.; Guallar, E. Serum selenium levels and all cause, cancer, and cardiovascular mortality among US adults. Arch. Intern. Med. 2008, 168, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Chiang, E.C.; Shen, S.; Kengeri, S.S.; Xu, H.; Combs, G.F.; Morris, J.S.; Bostwick, D.G.; Waters, D.J. Defining the optimal selenium dose for prostate cancer risk reduction: Insights from the U-Shaped relationship between selenium status, DNA damage, and apoptosis. Dose Response 2009, 8, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Kristal, A.R.; Darke, A.K.; Morris, J.S.; Tangen, C.M.; Goodman, P.J.; Thompson, I.M.; Meyskens, F.L., Jr.; Goodman, G.E.; Minasian, L.M.; Parnes, H.L.; et al. Baseline selenium status and effects of selenium and vitamin E supplementation on prostate cancer risk. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Geybels, M.S.; van den Brandt, P.A.; Schouten, L.J.; van Schooten, F.J.; van Breda, S.G.; Rayman, M.P.; Green, F.R.; Verhage, B.A. Selenoprotein gene variants, toenail selenium levels, and risk for advanced prostate cancer. J. Natl. Cancer. Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.J.; Chen, Y.; Zhang, Y.Q.; Zhou, M.Z.; Song, X.M.; Zhang, B.Z.; Luo, L.; Xu, P.M.; Zhao, Y.N.; Zhao, Y.B.; et al. The protective role of selenium on the toxicity of cisplatinum-contained chemotherapy regimen in cancer patients. Biol. Trace Elem. Res. 1997, 56, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Asfour, I.A.; Shazly, S.E.; Fayek, M.H. Effect of high-dose sodium selenite therapy on polymorphonuclear leukocyte apoptosis in non-Hodgkin’s lymphoma patients. Biol. Trace Elem. Res. 2006, 110, 19–32. [Google Scholar] [CrossRef]
- Asfour, I.A.; Fayek, M.H.; Raouf, S. The impact of high-dose sodium selenite therapy on Bcl-2 expression in adult non-Hodgkin’s lymphoma patients: Correlation with response and survival. Biol. Trace Elem. Res. 2007, 120, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Büntzel, J.; Riesenbeck, D.; Glatzel, M.; Berndt-Skorka, R.; Riedel, T.; Mücke, R.; Kisters, K.; Schönekaes, K.G.; Schäfer, U.; Bruns, F.; et al. Limited effects of selenium substitution in the prevention of radiation-associated toxicities. Results of a randomized study in head and neck cancer patients. Anticancer Res. 2010, 30, 1829–1832. [Google Scholar] [PubMed]
- Mücke, R.; Schomburg, L.; Glatzel, M. Multicenter, phase III trial comparing selenium supplementation with observation in gynecologic radiation oncology. Int. J. Radiat. Oncol. Biol. Phys. 2010, 70, 825–835. [Google Scholar]
- Muecke, R.; Micke, O.; Schomburg, L.; Buentzel, J.; Glatzel, M.; Baaske, D.; Berndt-Skorka, R.; Prott, F.J.; Reichl, B.; Kisters, K.; et al. Impact of treatment planning target volumen (PTV) size on radiation induced diarrhoea following selenium supplementation in gynecologic radiation oncology—A subgroup analysis of a multicenter, phase III trial. Radiat. Oncol. 2013, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Brodin, O.; Eksborg, S.; Wallenberg, M.; Asker-Hagelberg, C.; Larsen, E.H.; Mohlkert, D.; Lenneby-Helleday, C.; Jacobsson, H.; Linder, S.; Misra, S.; et al. Pharmacokinetics and toxicity of sodium selenite in the treatment of patients with carcinoma in a phase I clinical trial: The SECAR Study. Nutrients 2015, 7, 4978–4994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlic, H.; Lohninger, A. Supplementation of l-carnitine in athletes: Does it make sense? Nutrition 2004, 20, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Vitali, G.; Caraceni, A.; Ravaglia, S.; Capri, G.; Cundari, S.; Zanna, C.; Gianni, L. Symptomatic and neurophysiological responses of paclitaxel- or cisplatin-induced neuropathy to oral acetyl-l-carnitine. Eur. J. Cancer 2005, 41, 1746–1750. [Google Scholar] [CrossRef] [PubMed]
- De Grandis, D. Acetyl-l-carnitine for the treatment of chemotherapy-induced peripheral neuropathy: A short review. CNS Drugs 2007, 21 (Suppl. 1), 39–43. [Google Scholar] [CrossRef] [PubMed]
- Haschke, M.; Vitins, T.; Lüde, S. Urinary excretion of carnitine as a marker of proximal tubular damage associated with platin-based antineoplastic drugs. Nephrol. Dial. Transplant. 2010, 25, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Hockenberry, M.J.; Hooke, M.C.; Gregurich, M.; McCarthy, K. Carnitine plasma levels and fatigue in children/adolescents receiving cisplatin, ifosfamide, or doxorubicin. J. Pediatr. Hematol. Oncol. 2009, 31, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Graziano, F.; Bisonni, R.; Catalano, V. Potential role of levocarnitine supplementation for the treatment of chemotherapy-induced fatigue in non-anaemic cancer patients. Br. J. Cancer 2002, 86, 1854–1857. [Google Scholar] [CrossRef] [PubMed]
- Sayed-Ahmed, M.M. Role of carnitine in cancer chemotherapy-induced multiple organ toxicity. Saudi Pharm. J. 2010, 18, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Al-Majed, A.A.; Sayed-Ahmed, M.M.; Al-Yahya, A.A. Propionyl-l-carnitine prevents the progression of cisplatin-induced cardiomyopathy in a carnitine-depleted rat model. Pharmacol. Res. 2006, 53, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Iwase, S.; Kawaguchi, T.; Yotsumoto, D. Efficacy and safety of an amino acid jelly containing coenzyme Q10 and l-carnitine in controlling fatigue in breast cancer patients receiving chemotherapy: A multi-institutional, randomized, exploratory trial (JORTC-CAM01). Support. Care Cancer 2016, 24, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, U.; Nickel, A.; Daniel, H. Increased carnitine-dependent fatty acid uptake into mitochondria of human colon cancer cells induces apoptosis. J. Nutr. 2005, 135, 1510–1514. [Google Scholar] [PubMed]
- Heuberger, W.; Berardi, S.; Jacky, E.; Pey, P. Increased urinary excretion of carnitine in patients treated with cisplatin. Eur. J. Clin. Pharmacol. 1998, 54, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Marthaler, N.P.; Visarius, T.; Küpfer, A.; Lauterburg, B.H. Increased urinary losses of carnitine during ifosfamide chemotherapy. Cancer Chemother. Pharmacol. 1999, 44, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Gelehrter, S.K.; Vase, T.; Venkatramani, R.; Landier, W.; Wilson, K.D.; Herrera, C.; Reichman, L.; Menteer, J.D.; et al. Carnitine and cardiac dysfunction in childhood cancer survivors treated with anthracyclines. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Alshabanah, O.A.; Hafez, M.M.; Al-Harbi, M.M.; Hassan, Z.K.; Al Rejaie, S.S.; Asiri, Y.A.; Sayed-Ahmed, M.M. Doxorubicin toxicity can be ameliorated during antioxidant l-carnitine supplementation. Oxid. Med. Cell. Longev. 2010, 3, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Dubourg, L.; Michoudet, C.; Cochat, P.; Baverel, G. Human kidney tubules detoxify chloroacetaldehyde, a presumed nephrotoxic metabolite of ifosfamide. J. Am. Soc. Nephrol. 2001, 12, 1615–1623. [Google Scholar] [PubMed]
- Andrieu-Abadie, N.; Jaffrezou, J.P.; Hatem, S. l-Carnitine prevents doxorubicin-induced apoptosis of cardiac myocytes: Role of inhibition of ceramide generation. FASEB J. 1999, 13, 1501–1510. [Google Scholar] [PubMed]
- Chao, H.H.; Liu, J.C.; Hong, H.J. l-Carnitine reduces doxorubicin-induced apoptosis through a prostacyclin-mediated pathway in neonatal rat cardiomyocytes. Int. J. Cardiol. 2011, 146, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Delaney, C.E.; Hopkins, S.P.; Addison, C.L. Supplementation with l-carnitine does not reduce the efficacy of epirubicin treatment in breast cancer cells. Cancer Lett. 2007, 252, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.W.; Flatters, S.J.; Xiao, W.H. Prevention of paclitaxel-evoked painful peripheral neuropathy by acetyl-l-carnitine: Effects on axonal mitochondria, sensory nerve fiber terminal arbors, and cutaneous Langerhans cells. Exp. Neurol. 2008, 210, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.R.; Hong, Y.M.; Boriack, R.L.; Bennett, M.J. Effect of l-carnitine supplementation on cardiac carnitine palmitoyltransferase activities and plasma carnitine concentrations in adriamycin-treated rats. Pediatr. Res. 2003, 53, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Campone, M.; Berton-Rigaud, D.; Joly-Lobbedez, F.; Baurain, J.F.; Rolland, F.; Stenzl, A.; Fabbro, M.; van Dijk, M.; Pinkert, J.; et al. A double-blind, randomized phase II study to evaluate the safety and efficacy of acetyl-l-carnitine in the prevention of sagopilone-induced peripheral neuropathy. Oncologist 2013, 18, 1190–1191. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Galli, M.A.; Tancini, G.; Barni, S. Prevention by l-carnitine of interleukin-2 related cardiac toxicity during cancer immunotherapy. Tumori 1993, 79, 202–204. [Google Scholar] [PubMed]
- Waldner, R.; Laschan, C.; Lohninger, A.; Gessner, M.; Tüchler, H.; Huemer, M.; Spiegel, W.; Karlic, H. Effects of doxorubicin-containing chemotherapy and a combination with l-carnitine on oxidative metabolism in patients with non-Hodgkin lymphoma. J. Cancer Res. Clin. Oncol. 2006, 132, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Maestri, A.; de Pasquale Ceratti, A.; Cundari, S.; Zanna, C.; Cortesi, E.; Crinò, L. A pilot study on the effect of acetyl-l-carnitine in paclitaxel- and cisplatin-induced peripheral neuropathy. Tumori 2005, 91, 135–138. [Google Scholar] [PubMed]
- Hershman, D.L.; Unger, J.M.; Crew, K.D.; Minasian, L.M.; Awad, D.; Moinpour, C.M.; Hansen, L.; Lew, D.L.; Greenlee, H.; et al. Randomized double-blind placebo-controlled trial of acetyl-l-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J. Clin. Oncol. 2013, 31, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Baorui, L.; Ping, L.; Wu, C.; Zheng, R.; Zhang, X.; Zhuang, Z.; Deng, Y.; Zheng, L.; Xu, Q.; et al. A prospective study to evaluate the efficacy and safety of oral acetyl-l-carnitine (ALC) in treatment of chemotherapy-induced peripheral neuropathy (CPIN). In Proceedings of the 48th Annual Meeting of the American-Society-of-Clinical-Oncology (ASCO), Chicago, IL, USA, 1–5 June 2012; abstract 9017.
- Cruciani, R.A.; Zhang, J.J.; Manola, J. l-Carnitine supplementation for the management of fatigue in patients with cancer: An eastern cooperative oncology group phase III, randomized, double-blind, placebo-controlled trial. J. Clin. Oncol. 2012, 30, 3864–3869. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, R.A.; Dvorkin, E.; Homel, P.; Malamud, S.; Culliney, B.; Lapin, J.; Portenoy, R.K.; Esteban-Cruciani, N. Safety, tolerability and symptom outcomes associated with l-carnitine supplementation in patients with cancer, fatigue, and carnitine deficiency: A phase I/II study. J. Pain Symptom. Manag. 2006, 32, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.; Richardson, A.; Ream, E.; Smith, A.G.; Kerr, D.J.; Kearney, N. Cancer-related fatigue: Inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Cancer Fatigue Forum. Ann. Oncol. 2000, 11, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, C.; Dessì, M.; Panzone, F.; Serpe, R.; Antoni, G.; Cau, M.C.; Montaldo, L.; Mela, Q.; Mura, M.; Astara, G.; et al. Randomized phase III clinical trial of a combined treatment with carnitine + celecoxib ± megestrol acetate for patients with cancer-related anorexia/cachexia syndrome. Clin. Nutr. 2012, 31, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, R.A.; Dvorkin, E.; Homel, P.; Culliney, B.; Malamud, S.; Lapin, J. l-Carnitine supplementation in patients with advanced cancer and carnitine deficiency: A double-blind, placebo-controlled study. J. Pain Symptom. Manag. 2009, 37, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Gramignano, G.; Lusso, M.R.; Madeddu, C.; Massa, E.; Serpe, R.; Deiana, L. Efficacy of l-carnitine administration on fatigue, nutritional status, oxidative stress, and related quality of life in 12 advanced cancer patients undergoing anticancer therapy. Nutrition 2006, 22, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Laviano, A.; Rianda, S.; Molfino, A.; Fanelli, F.R. Omega-3 fatty acids in cancer. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Azrad, M.; Turgeon, C.; Demark-Wahnefried, W. Current evidence linking polyunsaturated fatty acids with cancer risk and progression. Front Oncol. 2013, 3, 224. [Google Scholar] [CrossRef] [PubMed]
- Arshad, A.; Isherwood, J.; Mann, C.; Cooke, J.; Pollard, C.; Runau, F.; Morgan, B.; Steward, W.; Metcalfe, M.; Dennison, A. Intravenous ω-3 fatty acids plus gemcitabine: Potential to improve response and quality of life in advanced pancreatic cancer. JPEN J. Parenter. Enteral. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Hübner, J.; Holzhauer, P. Vitamin C in der komplementären Onkologie. Der Onkol. 2010, 16, 303–313. [Google Scholar] [CrossRef]
- Günes-Bayir, A.; Kiziltan, H.S. Palliative vitamin C application in patients with radiotherapy-resistant bone metastases: A retrospective study. Nutr. Cancer 2015, 67, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, L.J.; Robitaille, L.; Zakarian, R.; Melnychuk, D.; Kavan, P.; Agulnik, J.; Cohen, V.; Small, D.; Miller, W.H., Jr. High-dose intravenous vitamin C combined with cytotoxic chemotherapy in patients with advanced cancer: A phase I-II clinical trial. PLoS ONE 2015, 10, e0120228. [Google Scholar] [CrossRef] [PubMed]
- Cieslak, J.A.; Cullen, J.J. Treatment of pancreatic cancer with pharmacological ascorbate. Curr. Pharm. Biotechnol. 2015, 16, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Kurbacher, C.M.; Wagner, U.; Kolster, B.; Andreotti, P.E.; Krebs, D.; Bruckner, H.W. Ascorbic acid (vitamin C) improves the antineoplastic activity of doxorubicin, cisplatin, and paclitaxel in human breast carcinoma cells in vitro. Cancer Lett. 1996, 103, 183–189. [Google Scholar] [CrossRef]
- Guerriero, E.; Sorice, A.; Capone, F.; Napolitano, V.; Colonna, G.; Storti, G.; Castello, G.; Costantini, S. Vitamin C effect on mitoxantrone-induced cytotoxicity in human breast cancer cell lines. PLoS ONE 2014, 9, e115287. [Google Scholar] [CrossRef] [PubMed]
- Yiang, G.T.; Chou, P.L.; Hung, Y.T.; Chen, J.N.; Chang, W.J.; Yu, Y.L.; Wei, C.W. Vitamin C enhances anticancer activity in methotrexate-treated Hep3B hepatocellular carcinoma cells. Oncol. Rep. 2014, 32, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Yang, C.M.; Su, C.M.; Liao, J.W.; Hu, M.L. Inhibitory effect of vitamin C in combination with vitamin K3 on tumor growth and metastasis of Lewis lung carcinoma xenografted in C57BVL/6 mice. Nutr. Cancer 2011, 63, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Shimpo, K.; Nagatsu, T.; Yamada, K.; Sato, T.; Niimi, H.; Shamoto, M.; Takeuchi, T.; Umezawa, H.; Fujita, K. Ascorbic acid and adriamycin toxicity. Am. J. Clin. Nutr. 1991, 54 (Suppl. 6), 1298S–1301S. [Google Scholar] [PubMed]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Pooput, C.; Kirk, K.L.; Krishna, M.C.; Khosh, D.B.; Drisko, J.; Levine, M. Pharmacologic doses of ascorbate act as prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 11105–11109. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Lee, J.H.; Krishna, M.C.; Shacter, E.; Choyke, P.L.; Pooput, C.; Kirk, K.L.; Buettner, G.R.; et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 8749–8754. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U. Vitamin C in complementary oncology—Update 2009. Med. Monatsschr. Pharm. 2009, 32, 263–267. [Google Scholar] [PubMed]
- Ohno, S.; Ohno, Y.; Suzuki, N.; Soma, G.; Inoue, M. High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer. Anticancer Res. 2009, 29, 809–815. [Google Scholar] [PubMed]
- Herst, P.M.; Broadley, K.W.; Harper, J.L.; McConnell, M.J. Pharmacological concentrations of ascorbate radiosensitize glioblastoma multiforme primary cells by increasing oxidative DNA damage and inhibiting G2/M arrest. Free Radic. Biol. Med. 2012, 52, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Hosokawa, Y.; Hazawa, M.; Kashiwakura, I.; Okumura, K.; Kaku, T.; Nakayama, E. Ascorbic acid enhances radiation-induced apoptosis in an HL60 human leukemia cell line. J. Radiat. Res. 2011, 52, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.L.; McConnell, M.J.; Herst, P.M. Radiosensitisation by pharmacological ascorbate in glioblastoma multiforme cells, human glial cells, and HUVECs depends on their antioxidant and DNA repair capabilities and is not cancer specific. Free Radic. Biol. Med. 2014, 74, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Vollbracht, C.; Schneider, B.; Leendert, V.; Weiss, G.; Auerbach, L.; Beuth, J. Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: Results of a retrospective, multicentre, epidemiological cohort study in Germany. Vivo 2011, 25, 983–990. [Google Scholar]
- Drisko, J.A.; Chapman, J.; Hunter, V.J. The use of antioxidant therapies during chemotherapy. Gynecol Oncol. 2003, 88, 434–439. [Google Scholar] [CrossRef]
- Welsh, J.L.; Wagner, B.A.; van‘t Erve, T.J.; Zehr, P.S.; Berg, D.J.; Halfdanarson, T.R.; Yee, N.S.; Bodeker, K.L.; Du, J.; Roberts, L.J., 2nd; et al. Pharmacological ascorbate with gemcitabine for the control of metastastic and node-positive pancreatic cancer (PACMAN): Results from a phase I clinical trial. Cancer Chemother. Pharmacol. 2013, 71, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.A.; Mitchell, E.; Bazzan, A.J.; Littman, S.; Zabrecky, G.; Yeo, C.J.; Pillai, M.V.; Newberg, A.B.; Deshmukh, S.; Levine, M. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS ONE 2012, 7, e29794. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.S.; Kim, J.K.; Shim, J.Y. High-dose vitamin C promotes regression of multiple pulmonary metastases originating from hepatocellular carcinoma. Yonsei Med. J. 2015, 56, 1449–1452. [Google Scholar] [CrossRef] [PubMed]
- PDQ Cancer Complementary and Alternative Medicine Editorial Board. High-Dose Vitamin C (PDQ): Health Professional Version. PDQ Cancer Information Summaries; National Cancer Institute: Bethesda, MD, USA, 2002–2015. [Google Scholar]



| Type of Tumor | Proportions of Patients (%) |
|---|---|
| Pancreatic carcinoma | 83 |
| Gastric carcinoma | 83 |
| Esophageal carcinoma | 79 |
| Carcinomas of head and neck | 72 |
| Colorectal carcinoma | 55–60 |
| Pulmonary carcinoma | 50–66 |
| Prostate carcinoma | 56 |
| Mammary carcinoma | 10–35 |
| Cytostatic Agent | Micro-nutrient | Mechanism | Possible Consequences |
|---|---|---|---|
| Cisplatin | l-carnitine | Increased renal excretion of l-carnitine | Cisplatin-induced carnitine insufficiency, increased risk of complications (e.g., fatigue) |
| Cisplatin | Magnesium, potassium | Increased renal excretion of magnesium and potassium | Hypomagnesaemia, hypokalaemia, disorders of lipid metabolism, glucose intolerance, increased nephrotoxicity |
| Cyclo-phosphamide | Vitamin D | Increased breakdown of calcidiol and calcitriol to inactive metabolites by 24-hydroxylase | Vitamin D deficiency (calcidiol <20 ng/mL), risk of metabolic bone disorders and impaired immunocompetence |
| Fluorouracil | Vitamin B1 | Inhibition of phosphorylation of thiamine to active coenzyme thiamine diphosphate | Risk of cardiac failure, lactic acidosis, neurotoxicity |
| Ifosfamide | l-carnitine | Increased renal excretion of l-carnitine | Ifosfamide-induced carnitine insufficiency, increased risk of complications (e.g., fatigue) |
| Methotrexate | Folic acid | Folic acid antagonism | Folate deficiency, homocysteinaemia, mucositis |
| Paclitaxel | Vitamin D | Increased breakdown of calcidiol and calcitriol to inactive metabolites by 24-hydroxylase | Vitamin D deficiency (calcidiol <20 ng/mL), risk of metabolic bone disorders and impaired immunocompetence |
| Pemetrexed | Folic acid | Folic acid antagonism | Mucositis, diarrhea, thrombocytopenia, neutropenia, homocysteinaemia |
| Author | Design | Outcomes |
|---|---|---|
| Hu et al., 1997 [153] | Patients with various solid tumors and chemotherapy containing cisplatin (n = 41) Randomized crossover study; administration of selenium (as seleno-kappacarrageenan) 4 mg/day for four days prior to and four days after chemotherapy in the first or second cycle | With selenium supplements: clearly higher leucocyte counts 14 days after chemotherapy (3.35 ± 2.01 × 109/L vs. 2.31 ± 1.38 × 109/L; p < 0.05) Less need for granulocyte colony stimulating factor (110.1 IU vs. 723.6 IU, p < 0.05) Less need for blood transfusion (0 mL vs. 62 ± 38 mL, p < 0.05) |
| Sieja et al., 2004 [54] | Patients with ovarian cancer on chemotherapy (cisplatin, cyclophosphamide; n = 31): • Selenium 200 µg/day • Control patients not given any selenium preparations | Significant increases in serum selenium levels, and glutathione peroxidase activity in red blood cells (after 2 and 3 months), and in the leucocyte count (3 months); significant reduction in alopecia, flatulence, abdominal pain, weakness, loss of appetite |
| Asfour et al., 2006/2007 [154,155] | Patients recently diagnosed with non-Hodgkin’s lymphoma (n = 50); Randomized, open-label study: • Chemotherapy plus sodium selenite 200 µg/kg/day; • Chemotherapy according to CHOP regimen | Significant fall in tumor marker Bcl-2 in the group taking supplements after 30 days (end value: 8.6 ± 6.9 ng/mL vs. 36.9 ± 7.9 ng/mL; p < 0.05 for test substance vs. placebo); complete response rate 60% vs. 40%; median overall survival in patients with complete remission 21.9 ± 1.4 months vs. 19.7 ± 2.0 months; p = 0.01 |
| Büntzel et al., 2010 [156] | Patients with advanced head/neck cancer and radiotherapy (n = 39) Randomized, open-label study: • Group A: with sodium selenite (500 µg on radiotherapy days, 300 µg on the other days; n = 22); • Group B: no selenium replacement (n = 17) | Dysphagia (difficulty swallowing): 22.7% vs. 35.3%; alteration in taste: 22.7% vs. 47.1%; dry mouth: 22.7% vs. 23.5%; stomatitis: 36.4% vs. 23.5%; only the decrease in difficulties swallowing in the last week of radiotherapy was statistically significant |
| Mücke et al., 2010 [157] | Patients with cancer of the cervix or uterus (n = 81) in the radiotherapy phase following surgical removal of the tumor and with a serum selenium concentration below 84 µg/L; randomized, open-label study: • Group A: with sodium selenite (500 µg on radiotherapy days, 300 µg on the other days; n = 39) • Group B: no selenium replacement (n = 42) | Significantly increased serum selenium concentration in group A at the end of the study; radiogenic diarrhea (grade ≥ 2) at the end of the study 20.5% vs. 44.5% (p = 0.04); no difference with respect to blood tests, functional status or quality of life |
| Author | Design | Outcomes |
|---|---|---|
| Iwase et al., 2016 [168] | Women with breast cancer (n = 57) and cancer-related fatigue undergoing chemotherapy; intervention: semi-solid, orally administrable dietary supplement containing coenzyme Q10 and l-carnitine; once daily or regular care for 21 days; multi-institutional, randomized, exploratory trial. | Changes in the global fatigue score, GFS, and current feeling of fatigue were significantly different between the intervention and control groups; HADS, EORTC QLQ-C30, and EORTC QLQ-BR23 scores were not significantly different between the two groups |
| Hershman et al., 2013 [184] | Women with breast cancer (n = 409) undergoing adjuvant taxane-based chemotherapy; intervention: Acetyl-l-carnitine 3 g/day for 24 weeks, control: placebo; randomized, two arms, parallel, blinded, placebo control, 24 weeks follow-up | Chemotherapy induced peripheral neuropathy was significantly increased after 24 weeks Functional status increased CrF unchanged |
| Campone et al., 2013 [180] | Patients with ovarian cancer or castration-resistant prostate cancer and no evidence of neuropathy (n = 150), intervention: Sagopilone, SAG (16 mg/m(2)) intravenously over 3 h every 3 weeks) with Acetyl-l-carnitine (1 g every 3 days) or placebo; Prospective, placebo-controlled, double-blind, randomized trial | No significant difference in overall peripheral neuropathy (PN) incidence was observed between treatment arms, but the incidence of grade ≥3 PN was significantly lower in the acetyl-l-carnitine arm in patients with ovarian cancer compared with a placebo. |
| Kraft et al., 2012 [33] | Patients with advanced pancreatic cancer (n = 72), intervention: 4 g l-carnitine/day for 12 weeks; randomized, two arms, parallel, blinded, placebo control, 12 weeks follow up. | BMI increased, nutritional status increased, quality of life increased, cancer related fatigue unchanged. |
| Cruciani et al., 2012 [186] | Patients with invasive malignancies and moderate to severe fatigue (n = 326); intervention: l-carnitine 1 g, twice daily for 4 weeks or placebo; Randomized, two arms, parallel, blinded, placebo control, four weeks follow-up. | Cancer-related fatigue unchanged, pain unchanged, depression unchanged |
| Cruciani et al., 2006 [187] | Patients (n = 27) with various advanced malignancies (stage unclear) and low plasma carnitine levels, no concurrent chemo-/radiotherapy; intervention: l-carnitine, starting dose: 250 mg/day, increments of 500 mg to a maximum target dose of 3 g/day; quasi-experimental (phase I/II), uncontrolled, pre-post test, one week follow-up. | Cancer-related fatigue decreased, depression decreased, and quality of life increased. |
| Bianchi et al., 2005 [161] | Patients (n = 25) with various cancers (stages unclear) during paclitaxel or cisplatinum chemotherapy and chemotherapy-induced polyneuropathy (CIPN) grade II/III; intervention: Acetyl-l-Carnitine 1 g, twice daily for eight weeks; Quasi-experimental, uncontrolled, pre-post test, eight weeks follow-up. | Sensory and motor neuropathy improved (NCI-CTC scale) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gröber, U.; Holzhauer, P.; Kisters, K.; Holick, M.F.; Adamietz, I.A. Micronutrients in Oncological Intervention. Nutrients 2016, 8, 163. https://doi.org/10.3390/nu8030163
Gröber U, Holzhauer P, Kisters K, Holick MF, Adamietz IA. Micronutrients in Oncological Intervention. Nutrients. 2016; 8(3):163. https://doi.org/10.3390/nu8030163
Chicago/Turabian StyleGröber, Uwe, Peter Holzhauer, Klaus Kisters, Michael F. Holick, and Irenäus A. Adamietz. 2016. "Micronutrients in Oncological Intervention" Nutrients 8, no. 3: 163. https://doi.org/10.3390/nu8030163