Delirium Severely Worsens Outcome in Patients with COVID-19—A Retrospective Cohort Study from Temporary Critical Care Hospitals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
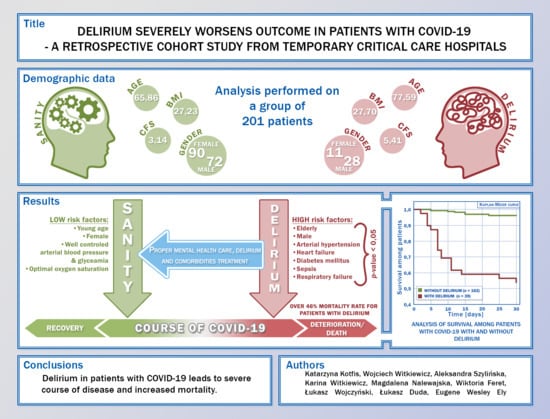
2.2. Study Population
2.3. Data Collection
2.4. Statistical Analysis
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Opening Remarks at the Media Briefing on COVID-19. World Health Organization, 11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11march2020 (accessed on 1 July 2021).
- Kotfis, K.; Roberson, S.W.; Wilson, J.E.; Dabrowski, W.; Pun, B.T.; Ely, E.W. COVID-19: ICU Delirium Management during SARS-CoV-2 Pandemic. Crit. Care 2020, 24, 176. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Rogers, J.P.; Chesney, E.; Oliver, D.; A Pollak, T.; McGuire, P.; Fusar-Poli, P.; Zandi, M.; Lewis, G.; David, A.S. Psychiatric and Neuropsychiatric Presentations Associated with Severe Coronavirus Infections: A Systematic Review and Meta-Analysis with Comparison to the COVID-19 Pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Helms, J.; Kremer, S.; Merdji, H.; Schenck, M.; Severac, F.; Clere-Jehl, R.; Studer, A.; Radosavljevic, M.; Kummerlen, C.; Monnier, A.; et al. Delirium and Encephalopathy in Severe COVID-19: A Cohort Analysis of ICU Patients. Crit. Care 2020, 24, 491. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H.; Lindroth, H.; Perkins, A.J.; Jamil, Y.; Wang, S.; Roberts, S.; Farber, M.; Rahman, O.; Gao, S.; Marcantonio, E.R.; et al. Delirium Incidence, Duration, and Severity in Critically Ill Patients With Coronavirus Disease 2019. Crit. Care Explor. 2020, 2, e0290. [Google Scholar] [CrossRef]
- Pun, B.T.; Badenes, R.; La Calle, G.H.; Orun, O.M.; Chen, W.; Raman, R.; Simpson, B.-G.K.; Wilson-Linville, S.; Olmedillo, B.H.; de la Cueva, A.V.; et al. Prevalence and Risk Factors for Delirium in Critically Ill Patients with COVID-19 (COVID-D): A Multicentre Cohort Study. Lancet Respir. Med. 2021, 9, 239–250. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Devlin, J.W.; Skrobik, Y.; Gélinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marra, A.; Kotfis, K.; Hosie, A.; MacLullich, A.M.J.; Pandharipande, P.P.; Ely, E.W.; Pun, B.T. Delirium Monitoring: Yes or No? That Is the Question. Am. J. Crit. Care 2019, 28, 127–135. [Google Scholar] [CrossRef]
- Devlin, J.W.; O’Neal, H.R.; Thomas, C.; Barnes Daly, M.A.; Stollings, J.L.; Janz, D.R.; Ely, E.W.; Lin, J.C. Strategies to Optimize ICU Liberation (A to F) Bundle Performance in Critically Ill Adults With Coronavirus Disease 2019. Crit. Care Explor. 2020, 2, e0139. [Google Scholar] [CrossRef] [PubMed]
- Kotfis, K.; Roberson, S.W.; Wilson, J.E.; Pun, B.T.; Wesley Ely, E.; Jeżowska, I.; Jezierska, M.; Dabrowski, W. COVID-19: What Do We Need to Know about ICU Delirium during the SARS-CoV-2 Pandemic? Anaesthesiol. Intensive Ther. 2020, 52, 132–138. [Google Scholar] [CrossRef]
- Rood, P.; de Waal, G.H.; Vermeulen, H.; Schoonhoven, L.; Pickkers, P.; Boogaard, M.V.D. Effect of Organisational Factors on the Variation in Incidence of Delirium in Intensive Care Unit Patients: A Systematic Review and Meta-Regression Analysis. Aust. Crit. Care 2018, 31, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Slooter, A.J.C.; Otte, W.M.; Devlin, J.W.; Arora, R.C.; Bleck, T.P.; Claassen, J.; Duprey, M.S.; Ely, E.W.; Kaplan, P.W.; Latronico, N.; et al. Updated Nomenclature of Delirium and Acute Encephalopathy: Statement of Ten Societies. Intensive Care Med. 2020, 46, 1020–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandharipande, P.P.; Girard, T.D.; Jackson, J.C.; Morandi, A.; Thompson, J.L.; Pun, B.T.; Brummel, N.E.; Hughes, C.G.; Vasilevskis, E.E.; Shintani, A.K.; et al. Long-Term Cognitive Impairment after Critical Illness. N. Engl. J. Med. 2013, 369, 1306–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girard, T.D.; Thompson, J.L.; Pandharipande, P.P.; Brummel, N.E.; Jackson, J.C.; Patel, M.B.; Hughes, C.G.; Chandrasekhar, R.; Pun, B.T.; Boehm, L.M.; et al. Clinical Phenotypes of Delirium during Critical Illness and Severity of Subsequent Long-Term Cognitive Impairment: A Prospective Cohort Study. Lancet Respir. Med. 2018, 6, 213–222. [Google Scholar] [CrossRef]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef] [PubMed]
- Duggan, M.C.; Van, J.; Ely, E.W. Delirium Assessment in Critically Ill Older Adults: Considerations During the COVID-19 Pandemic. Crit. Care Clin. 2021, 37, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Pranata, R.; Huang, I.; Lim, M.A.; Yonas, E.; Vania, R.; Kuswardhani, R.A.T. Delirium and Mortality in Coronavirus Disease 2019 (COVID-19)—A Systematic Review and Meta-Analysis. Arch. Gerontol. Geriatr. 2021, 95, 104388. [Google Scholar] [CrossRef]
- Velásquez-Tirado, J.D.; Trzepacz, P.T.; Franco, J.G. Etiologies of Delirium in Consecutive COVID-19 Inpatients and the Relationship Between Severity of Delirium and COVID-19 in a Prospective Study With Follow-Up. J. Neuropsychiatry Clin. Neurosci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Rebora, P.; Rozzini, R.; Bianchetti, A.; Blangiardo, P.; Marchegiani, A.; Piazzoli, A.; Mazzeo, F.; Cesaroni, G.; Chizzoli, A.; Guerini, F.; et al. Delirium in Patients with SARS-CoV-2 Infection: A Multicenter Study. J. Am. Geriatr. Soc. 2021, 69, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.; Helfand, B.K.I.; Gou, R.Y.; Gartaganis, S.L.; Webb, M.; Moccia, J.M.; Bruursema, S.N.; Dokic, B.; McCulloch, B.; Ring, H.; et al. Delirium in Older Patients with COVID-19 Presenting to the Emergency Department. JAMA Netw. Open 2020, 3, 2029540. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.; Azari Jafari, A.; Mirmoeeni, S.; Sadeghian, S.; Heidari, M.E.; Sadeghian, S.; Assarzadegan, F.; Puormand, S.M.; Ebadi, H.; Fathi, D.; et al. Central Nervous System Manifestations in COVID-19 Patients: A Systematic Review and Meta-Analysis. Brain Behav. 2021, 11, e02025. [Google Scholar] [CrossRef]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male Sex Identified by Global COVID-19 Meta-Analysis as a Risk Factor for Death and ITU Admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef]
- Zazzara, M.B.; Penfold, R.S.; Roberts, A.L.; Lee, K.A.; Dooley, H.; Sudre, C.H.; Welch, C.; Bowyer, R.C.E.; Visconti, A.; Mangino, M.; et al. Probable Delirium Is a Presenting Symptom of COVID-19 in Frail, Older Adults: A Cohort Study of 322 Hospitalised and 535 Community-Based Older Adults. Age Ageing 2021, 50, 40–48. [Google Scholar] [CrossRef]
- Andrés-Esteban, E.M.; Quintana-Diaz, M.; Ramírez-Cervantes, K.L.; Benayas-Peña, I.; Silva-Obregón, A.; Magallón-Botaya, R.; Santolalla-Arnedo, I.; Juárez-Vela, R.; Gea-Caballero, V. Outcomes of Hospitalized Patients with COVID-19 According to Level of Frailty. PeerJ 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Krewulak, K.D.; Stelfox, H.T.; Leigh, J.P.; Wesley Ely, E.; Fiest, K.M. Incidence and Prevalence of Delirium Subtypes in an Adult ICU: A Systematic Review and Meta-Analysis. Crit. Care Med. 2018, 46, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Raabe, A.; Wissing, H.; Zwissler, B. Brain Cell and S-100B Increase after Acute Lung Injury. Anesthesiology. 2005, 102, 713–714. [Google Scholar] [CrossRef]
- Hopkins, R.O.; Weaver, L.K.; Pope, D.; Orme, J.F.; Bigler, E.D.; Larson-Lohr, V. Neuropsychological Sequelae and Impaired Health Status in Survivors of Severe Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 1999, 160, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Pérez, M.; Roca, O.; Poulakou, G.; Souto, J.; Laborda, C.; Balcells, J.; Serra, J.; Masclans, J.R.; Anglès, R.; et al. High-Flow Nasal Therapy in Adults with Severe Acute Respiratory Infection. A Cohort Study in Patients with 2009 Influenza A/H1N1v. J. Crit. Care 2012, 27, 434–439. [Google Scholar] [CrossRef]
- Frat, J.-P.; Thille, A.W.; Mercat, A.; Girault, C.; Ragot, S.; Perbet, S.; Prat, G.; Boulain, T.; Morawiec, E.; Cottereau, A.; et al. High-Flow Oxygen through Nasal Cannula in Acute Hypoxemic Respiratory Failure. N. Engl. J. Med. 2015, 372, 2185–2196. [Google Scholar] [CrossRef] [Green Version]
- McNeil, J.B.; Hughes, C.G.; Girard, T.; Ware, L.B.; Ely, E.W.; Chandrasekhar, R.; Han, J.H. Plasma Biomarkers of Inflammation, Coagulation, and Brain Injury as Predictors of Delirium Duration in Older Hospitalized Patients. PLoS ONE 2019, 14, e0226412. [Google Scholar] [CrossRef]
- Knopp, P.; Miles, A.; Webb, T.E.; Mcloughlin, B.C.; Mannan, I.; Raja, N.; Wan, B.; Davis, D. Presenting Features of COVID-19 in Older People: Relationships with Frailty, Inflammation and Mortality. Eur. Geriatr. Med. 2020, 11, 1089–1094. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Wang, R.; Feng, Z.; Tan, Y.; Wang, H.; Wang, C.; Liu, L.; Liu, Y.; Liu, Y.; et al. Human Kidney Is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Perico, L.; Benigni, A.; Remuzzi, G. Should COVID-19 Concern Nephrologists? Why and to What Extent? The Emerging Impasse of Angiotensin Blockade. Nephron 2020, 144, 213–221. [Google Scholar] [CrossRef]
- Li, Z.; Wu, M.; Yao, J.; Guo, J.; Liao, X.; Song, S.; Li, J.; Duan, G.; Zhou, Y.; Wu, X.; et al. Caution on Kidney Dysfunctions of COVID-19 Patients. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Toklu, H.; Ganti, L.; Crimi, E.; Cintron, C.; Hagan, J.; Serrano, E. Cerebrospinal Fluid Findings and Hypernatremia in COVID-19 Patients with Altered Mental Status. Int. J. Emerg. Med. 2020, 13, 63. [Google Scholar] [CrossRef]
- Maguire, D.; Woods, M.; Richards, C.; Dolan, R.; Veitch, J.W.; Sim, W.M.J.; Kemmett, O.E.H.; Milton, D.C.; Randall, S.L.W.; Bui, L.D.; et al. Prognostic Factors in Patients Admitted to an Urban Teaching Hospital with COVID-19 Infection. J. Transl. Med. 2020, 18. [Google Scholar] [CrossRef]
- Díaz-Pérez, C.; Ramos, C.; López-Cruz, A.; Muñoz Olmedo, J.; Lázaro González, J.; De Vega-Ríos, E.; González-Ávila, C.; Hervás, C.; Trillo, S.; Vivancos, J. Acutely Altered Mental Status as the Main Clinical Presentation of Multiple Strokes in Critically Ill Patients with COVID-19. Neurol. Sci. 2020, 41, 2681–2684. [Google Scholar] [CrossRef]
- Carrascosa, M.F.; Batán, A.M.; Novo, M.F.A. Delirium and Pulmonary Embolism in the Elderly. Mayo Clin. Proc. 2009, 84, 91–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannink, M. Old, short of breath and confused; delirium as a manifestation of pulmonary embolism in geriatric patients. Ned Tijdschr Geneeskd 1998, 142, 1045. [Google Scholar] [PubMed]
- Soysal, P.; Isik, A.T. Hypoactive Delirium Caused by Pulmonary Embolus in an Elderly Adult. J. Am. Geriatr. Soc. 2014, 62, 586–587. [Google Scholar] [CrossRef]
- Imazio, M.; Klingel, K.; Kindermann, I.; Brucato, A.; De Rosa, F.G.; Adler, Y.; De Ferrari, G.M. COVID-19 Pandemic and Troponin: Indirect Myocardial Injury, Myocardial Inflammation or Myocarditis? Heart 2020, 106, 1127–1131. [Google Scholar] [CrossRef]
- Sandoval, Y.; Januzzi, J.L.; Jaffe, A.S. Cardiac Troponin for Assessment of Myocardial Injury in COVID-19: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 1244–1258. [Google Scholar] [CrossRef]
- Eegberts, A.; Mattace-Rraso, F.U.S. Increased Neutrophil-Lymphocyte Ratio in Delirium: A Pilot Study. Clin. Interv. Aging 2017, 12, 1115–1121. [Google Scholar] [CrossRef] [Green Version]
- Kotfis, K.; Bott-Olejnik, M.; Szylińska, A.; Rotter, I. Could Neutrophil-to-Lymphocyte Ratio (NLR) Serve as a Potential Marker for Delirium Prediction in Patients with Acute Ischemic Stroke? A Prospective Observational Study. J. Clin. Med. 2019, 8, 1075. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Yue, J.; Lei, P.; Lin, T.; Peng, X.; Xie, D.; Gao, L.; Shu, X.; Wu, C. Neutrophil-Lymphocyte Ratio as a Predictor of Delirium in Older Internal Medicine Patients: A Prospective Cohort Study. BMC Geriatr. 2021, 21, 334. [Google Scholar] [CrossRef]
- He, R.; Wang, F.; Shen, H.; Zeng, Y.; Zhang, L. Association between Increased Neutrophil-to-Lymphocyte Ratio and Postoperative Delirium in Elderly Patients with Total Hip Arthroplasty for Hip Fracture. BMC Psychiatry 2020, 20, 496. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; He, L.; Zhang, G.; Yu, J.; Chen, Y.; Yin, H.; Goyal, H.; Zhang, G.M.; Xiao, Y.; Gu, C.; et al. Normal Reference Intervals of Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, Lymphocyte-to-Monocyte Ratio, and Systemic Immune Inflammation Index in Healthy Adults: A Large Multi-Center Study from Western China. Clin. Lab. 2019, 65, 255–265. [Google Scholar] [CrossRef]
- Moosazadeh, M.; Maleki, I.; Alizadeh-Navaei, R.; Kheradmand, M.; Hedayatizadeh-Omran, A.; Shamshirian, A.; Barzegar, A. Normal Values of Neutrophil-to-Lymphocyte Ratio, Lymphocyte-to-Monocyte Ratio and Platelet-to-Lymphocyte Ratio among Iranian Population: Results of Tabari Cohort. Casp. J. Intern. Med. 2019, 10, 320–325. [Google Scholar] [CrossRef]
- Christiansen, M.H.; Barup, K.; Samson, M.H. Neutrophil-Lymphocyte-Ratio Distributions in a Danish Population from General Practice. Scand. J. Clin. Lab. Investig. 2019, 79, 75–79. [Google Scholar] [CrossRef]
- Völk, S.; Koedel, U.; Pfister, H.W.; Schwankhart, R.; Op Den Winkel, M.; Mühlbauer, K.; Klein, M. Impaired Consciousness in the Emergency Department. Eur. Neurol. 2019, 80, 179–186. [Google Scholar] [CrossRef]
- Tsuruta, R.; Oda, Y. A Clinical Perspective of Sepsis-Associated Delirium. J. Intensiv. Care 2016, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Crippa, I.A.; Subirà, C.; Vincent, J.L.; Fernandez, R.F.; Hernandez, S.C.; Cavicchi, F.Z.; Creteur, J.; Taccone, F.S. Impaired Cerebral Autoregulation Is Associated with Brain Dysfunction in Patients with Sepsis. Crit. Care 2018, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcez, F.B.; Aliberti, M.J.R.; Poco, P.C.E.; Hiratsuka, M.; Takahashi, S.D.F.; Coelho, V.A.; Salotto, D.B.; Moreira, M.L.V.; Jacob-Filho, W.; Avelino-Silva, T.J. Delirium and Adverse Outcomes in Hospitalized Patients with COVID-19. J. Am. Geriatr. Soc. 2020, 68, 2440–2446. [Google Scholar] [CrossRef]

| Variables | Total | No Delirium (n = 162) | Delirium (n = 39) | p-Value |
|---|---|---|---|---|
| Demographic data | ||||
| Age [years], mean ± SD; Me | 68.14 ± 13.82; 69.0 | 65.86 ± 13.65; 68.0 | 77.59 ± 10.13; 81.0 | <0.001 |
| Gender [male], n (%) | 100 (49.75) | 72 (44.44) | 28 (71.79) | 0.002 |
| BMI [kg/m2], mean ± SD; Me | 27.32 ± 5.21; 27.5 | 27.23 ± 5.33; 27.4 | 27.70 ± 4.69; 28.3 | 0.416 |
| Smoking, n (%) | 29 (14.43) | 25 (15.82) | 4 (10.26) | 0.531 |
| EF [%] (mean ± SD; Me) | 47.22 ± 16.11; 55.0 | 51.00 ± 13.78; 55.0 | 28.33 ± 15.28; 25.0 | 0.051 |
| CFS (1–9), (mean ± SD; Me) | 3.57 ± 1.92; 3.0 | 3.14 ± 1.58; 3.0 | 5.41 ± 2.15; 6.0 | <0.001 |
| Comorbidities | ||||
| Arterial Hypertension, n (%) | 131 (65.17) | 99 (61.11) | 32 (82.05) | 0.023 |
| Chronic Coronary Syndrome, n (%) | 42 (20.89) | 31 (19.14) | 11 (28.21) | 0.211 |
| Myocardial Infarction, n (%) | 22 (10.95) | 18 (11.11) | 4 (10.26) | 0.878 |
| Chronic Heart Failure, n (%) | 36 (17.91) | 24 (14.81) | 12 (30.77) | 0.019 |
| Atrial Fibrillation, n (%) | 37 (18.41) | 27 (16.67) | 10 (25.64) | 0.194 |
| Previous ischemic stroke, n (%) | 15 (7.46) | 10 (6.17) | 5 (12.82) | 0.281 |
| Previous hemorrhagic stroke, n (%) | 1 (0.49) | 0 (0.00) | 1 (2.63) | 0.428 |
| Transient Ischemic Attack, n (%) | 2 (0.99) | 1 (0.62) | 1 (2.63) | 0.841 |
| CKD (GFR < 60) [mL/min/1.73 m2], n (%) | 35 (17.41) | 24 (14.81) | 11 (28.21) | 0.048 |
| Post-renal transplant, n (%) | 4 (1.99) | 4 (2.47) | 0 (0.00) | 0.724 |
| Dialysis, n (%) | 4 (1.99) | 4 (2.47) | 0 (0.00) | 0.724 |
| Impaired insulin tolerance, n (%) | 5 (2.49) | 4 (2.47) | 1 (2.63) | 0.590 |
| Diabetes [oral medications/diet], n (%) | 39 (19.40) | 32 (19.75) | 7 (17.95) | 0.976 |
| Diabetes [insulin], n (%) | 23 (11.44) | 13 (8.02) | 10 (25.64) | 0.002 |
| Gout/hyperuricemia, n (%) | 11 (5.47) | 6 (3.70) | 5 (12.82) | 0.064 |
| ICA stenosis, n (%) | 10 (4.97) | 6 (3.70) | 4 (10.26) | 0.201 |
| Chronic peripheral ischemia, n (%) | 11 (5.47) | 7 (4.32) | 4 (10.26) | 0.284 |
| Venous thrombosis, n (%) | 4 (1.99) | 2 (1.35) | 2 (5.13) | 0.408 |
| Pulmonary Embolism, n (%) | 2 (0.99) | 2 (1.35) | 0 (0.00) | 0.885 |
| COPD, n (%) | 14 (6.97) | 9 (5.56) | 5 (12.82) | 0.211 |
| Asthma, n (%) | 16 (7.96) | 12 (7.41) | 4 (10.26) | 0.794 |
| Active neoplasm, n (%) | 17 (8.46) | 15 (.26) | 2 (5.13) | 0.609 |
| Medications | No Delirium (n = 162) | Delirium (n = 39) | p-Value |
|---|---|---|---|
| ASA, n (%) | 36 (22.22) | 13 (34.21) | 0.181 |
| ADP inhibitors, n (%) | 11 (6.83) | 0 (0.00) | 0.207 |
| OAC/NOAC, n (%) | 19 (11.80) | 5 (13.16) | 0.963 |
| B-blockers, n (%) | 78 (48.45) | 22 (57.89) | 0.386 |
| ACE-I/Sartans, n (%) | 71 (44.10) | 14 (36.84) | 0.523 |
| CCBs, n (%) | 34 (21.12) | 9 (23.68) | 0.899 |
| Statins/fibrates, n (%) | 42 (26.09) | 10 (26.32) | 0.860 |
| Nitrates, n (%) | 3 (1.86) | 1 (2.63) | 0.735 |
| Diuretics, n (%) | 53 (32.92) | 20 (52.63) | 0.037 |
| MCRAsm, n (%) | 13 (8.07) | 5 (13.16) | 0.504 |
| Bronchodilators, n (%) | 19 (11.80) | 3 (7.89) | 0.687 |
| Oral antidiabetic drugs, n (%) | 33 (20.50) | 8 (21.05) | 0.883 |
| Insulin, n (%) | 13 (8.07) | 10 (26.32) | 0.002 |
| Thyroid hormones/thyrostatics, n (%) | 24 (14.91) | 3 (7.89) | 0.383 |
| NSAIDs, n (%) | 9 (5.59) | 0 (0.00) | 0.290 |
| Immunosuppression, n (%) | 11 (6.83) | 0 (0.00) | 0.207 |
| Opioids, n (%) | 2 (1.24) | 4 (10.53) | 0.013 |
| Symptoms on Admission | No Delirium (n = 162) | Delirium (n = 39) | p-Value |
|---|---|---|---|
| Low-grade fever/Fever, n (%) | 101 (62.35) | 25 (64.10) | 0.838 |
| Dyspnea, n (%) | 88 (54.32) | 24 (61.54) | 0.525 |
| Cough, n (%) | 79 (48.77) | 13 (33.33) | 0.119 |
| Chest pain, n (%) | 27 (16.67) | 6 (15.38) | 0.963 |
| Weakness, n (%) | 110 (67.90) | 32 (82.05) | 0.122 |
| Nausea, n (%) | 22 (13.58) | 2 (5.13) | 0.235 |
| Vomiting, n (%) | 19 (11.73) | 1 (2.56) | 0.156 |
| Diarrhea, n (%) | 26 (16.05) | 2 (5.13) | 0.131 |
| Musculo-articular pains, n (%) | 23 (14.20) | 3 (7.69) | 0.412 |
| Lack of taste, n (%) | 17 (10.49) | 3 (7.69) | 0.821 |
| Lack of smell, n (%) | 15 (9.26) | 1 (2.56) | 0.290 |
| Headache, n (%) | 13 (8.02) | 1 (2.56) | 0.394 |
| Laboratory Data on Admission | No Delirium (n = 162) | Delirium (n = 39) | p-Value |
|---|---|---|---|
| Mean ± SD; Me | Mean ± SD; Me | ||
| HbA1c (%) | 6.73 ± 1.40; 6.2 | 6.68 ± 1.82; 5.90 | 0.495 |
| TC [mg/dL] | 147.91 ± 51.88; 137.0 | 153.0 ± 55.26; 135.0 | 0.716 |
| LDL [mg/dL] | 84.82 ± 39.31; 85.5 | 83.90 ± 38.87; 70.5 | 0.900 |
| HDL [mg/dL] | 36.38 ± 12.43; 33.5 | 39.50 ± 21.31; 33.5 | 0.922 |
| TG [mg/dL] | 159.09 ± 116.90; 124.5 | 144.30 ± 71.32; 133.0 | 0.989 |
| WBC [109/L] | 6.94 ± 3.92; 6.0 | 9.85 ± 5.56; 9.1 | 0.001 |
| Neutrophils [109/L] | 5.10 ± 3.27; 4.2 | 8.15 ± 5.37; 7.6 | <0.001 |
| Lymphocytes [109/L] | 1.17 ± 0.78; 1.0 | 1.03 ± 0.41; 1.1 | 0.984 |
| NLR | 5.75 ± 4.91; 4.5 | 9.25 ± 6.81; 6.9 | 0.001 |
| NLR ≥ 6.51, n (%) | 38 (27.94%) | 22 (61.11%) | <0.001 |
| PLT [109/L] | 241.85 ± 118.89; 214.0 | 255.42 ± 118.40; 239.0 | 0.420 |
| PWR | 39.76 ± 18.48; 36.6 | 30.93 ± 16.58; 29.2 | 0.004 |
| PLR | 263.70 ± 183.03; 227.22 | 280.95 ± 170.56; 220.6 | 0.314 |
| HGB [mmol/L] | 7.82 ± 1.50; 8.0 | 8.09 ± 1.49; 8.0 | 0.548 |
| HCT [L/L] | 0.36 ± 0.07; 0.4 | 0.38 ± 0.05; 0.4 | 0.199 |
| Creatinine [mg/dL] | 1.17 ± 1.07; 0.9 | 1.83 ± 1.94; 1.2 | 0.001 |
| GFR [mL/min/1.73 m2] | 76.73 ± 32.07; 75.2 | 60.91 ± 35.90; 53.9 | 0.007 |
| Urea [mg/dL] | 49.35 ± 30.69; 40.0 | 85.22 ± 75.43; 54.1 | 0.040 |
| CRP [mg/dL] | 69.59 ± 57.68; 55.0 | 98.31 ± 74.30; 81.1 | 0.018 |
| IL-6 [pg/mL] | 57.30 ± 76.69; 37.4 | 266.43 ± 1005.49; 57.9 | 0.023 |
| PCT [ng/mL] | 0.56 ± 2.89; 0.1 | 4.80 ± 18.30; 0.2 | <0.001 |
| AST [U/L] | 46.54 ± 54.40; 32.5 | 64.32 ± 64.72; 47.0 | 0.163 |
| Alanine transaminase (ALT) [U/L] | 45.90 ± 79.82; 24.0 | 37.39 ± 29.91; 28.0 | 0.892 |
| GGTP [U/L] | 71.60 ± 77.21; 38.0 | 81.65 ± 166.66; 40.0 | 0.766 |
| APRI | 0.34 ± 1.15; 0.17 | 0.33 ± 0.48; 0.17 | 0.326 |
| INR | 1.30 ± 1.03; 1.1 | 1.78 ± 2.25; 1.2 | 0.016 |
| APTT [s] | 33.59 ± 9.06; 30.8 | 32.47 ± 7.42; 31.6 | 0.980 |
| LDH [U/L] | 322.42 ± 109.70; 308.0 | 427.13 ± 183.54; 346.0 | 0.012 |
| Fibrinogen [g/L] | 4.69 ± 1.65; 4.7 | 4.63 ± 2.28; 3.8 | 0.590 |
| D-Dimer [ng/mL] | 1753.61 ± 2229.58; 833.0 | 2953.18 ± 2779.1; 1518.0 | 0.004 |
| CKMB [U/L] | 20.37 ± 10.98; 18.0 | 25.88 ± 17.27; 21.3 | 0.099 |
| TnT [ug/L] | 0.07 ± 0.30; 0.01 | 0.05 ± 0.06; 0.03 | 0.005 |
| Kalium [mmol/L] | 4.08 ± 0.56; 4.1 | 4.23 ± 0.70; 4.21 | 0.305 |
| Natrium [mmol/L] | 136.10 ± 4.88; 137.0 | 137.72 ± 5.53; 138.0 | 0.157 |
| Chloride [mmol/L] | 98.46 ± 6.04; 99.0 | 99.70 ± 4.6; 100.0 | 0.482 |
| Respiratory Parameters on Admission | No Delirium (n = 162) | Delirium (n = 39) | p-Value | |
|---|---|---|---|---|
| SpO2 (mean ± SD; Me) | 94.85 ± 3.17; 95.0 | 94.26 ± 4.46; 95.0 | 0.668 | |
| Nasal cannula, n (%) | 51 (31.68%) | 15 (38.46%) | 0.419 | |
| Non-rebreather mask, n (%) | 20 (12.42%) | 12 (30.77%) | 0.010 | |
| HFNOT | Yes, n (%) | 6 (3.7 %) | 9 (23.08 %) | <0.001 |
| Day started (mean ± SD; Me) | 5.00 ± 3.95; 3.5 | 3.13 ± 3.00; 2.5 | 0.272 | |
| Flow [L/min] (mean ± SD; Me) | 6.02 ± 3.83; 5.0 | 10.04 ± 8.68; 7.0 | 0.013 | |
| pH (mean ± SD; Me) | 7.47 ± 0.06; 7.5 | 7.48 ± 0.06; 7.5 | 0.588 | |
| pO2 (mmHg), (mean ± SD; Me) | 76.05 ± 24.06; 71.0 | 70.72 ± 29.72; 62.0 | 0.043 | |
| pCO2 (mmHg), (mean ± SD; Me) | 34.66 ± 6.72; 34.0 | 32.04 ± 5.56; 31.0 | 0.105 | |
| FiO2 (mean ± SD; Me) | 0.45 ± 0.29; 0.3 | 0.63 ± 0.28; 0.8 | 0.006 | |
| HCO3- (mean ± SD; Me) | 25.25 ± 4.87; 25.1 | 25.11 ± 5.28; 25.2 | 0.907 | |
| BE (mean ± SD; Me) | 1.89 ± 4.62; 1.9 | 1.26 ± 5.76; 0.4 | 0.466 | |
| pO2/FiO2, (mean ± SD; Me) | 252.89 ± 161.44; 26.36 | 158.45 ± 134.38; 82.7 | 0.005 | |
| ARDS, n (%) | without | 25 (39.68%) | 4 (16.00%) | 0.029 |
| mild | 11 (17.46%) | 2 (8.00%) | ||
| moderate | 11 (17.46%) | 5 (20.00%) | ||
| severe | 16 (25.40%) | 14 (56.00%) | ||
| COVID-19-Specific Treatment | No Delirium (n = 162) | Delirium (n = 39) | p-Value |
|---|---|---|---|
| LMWH, n (%) | 152 (93.83%) | 39 (100.00%) | 0.111 |
| Prophylactic dose [40 mg once a day], n (%) | 68 (41.98%) | 11(28.21%) | 0.114 |
| Intermediate dose [1 mg/kg once a day], n (%) | 60 (37.04%) | 13 (33.33%) | 0.805 |
| Therapeutic dose [1 mg/kg twice a day], n (%) | 39 (24.07%) | 19 (48.72%) | 0.004 |
| Antibiotic therapy, n (%) | 145 (89.51%) | 37 (94.87%) | 0.469 |
| Ceftriaxone, n (%) | 127 (78.40%) | 35(89.74%) | 0.167 |
| Azithromycin, n (%) | 108 (66.67%) | 34 (87.18%) | 0.019 |
| Levofloxacin, n (%) | 14 (8.64%) | 3 (7.69%) | 0.897 |
| Other antibiotic, n (%) | 26 (16.05%) | 12 (30.77%) | 0.035 |
| Steroid therapy, n (%) | 113 (69.75%) | 32 (82.05%) | 0.181 |
| Dexamethasone, n (%) | 108 (66.67%) | 30 (76.92%) | 0.295 |
| Prednisone, n (%) | 5 (3.09%) | 0 (0.00%) | 0.590 |
| Hydrocortisone, n (%) | 1 (0.62%) | 6 (15.38%) | <0.001 |
| Other steroid, n (%) | 1 (0.62%) | 1 (2.56%) | 0.841 |
| Max. dexamethasone dose (or equivalent) (mean ± SD; Me) | 6.19 ± 3.04; 4.0 | 13.41 ± 34.20; 8.0 | 0.037 |
| Time of steroid therapy [days] (mean ± SD; Me) | 8.16 ± 4.91; 7.0 | 8.00 ± 6.70; 7.0 | 0.377 |
| Vitamin D3, n (%) | 41 (25.31%) | 11 (28.21%) | 0.867 |
| Remdesivir, n (%) | 31 (19.14%) | 5 (12.82%) | 0.489 |
| Complications | No Delirium (n = 162) | Delirium (n = 39) | p-Value | |
|---|---|---|---|---|
| Cardiological complications (n/%) | 10 (6.17%) | 14 (35.90%) | <0.001 | |
| Heart Failure, n (%) | 5 (3.09%) | 5 (12.82%) | 0.036 | |
| Myocardial Infarction, n (%) | 1 (0.62%) | 0 (0.00%) | 0.438 | |
| Atrial Fibrillation, n (%) | 7 (4.32%) | 7 (17.95%) | 0.008 | |
| Atrial Flutter, n (%) | 0 (0.00%) | 1 (2.56%) | 0.438 | |
| Other arrhythmias (including ventricular, supraventricular arrhythmias and atrioventricular conduction disorders), n (%) | 2 (1.23%) | 0 (0.00%) | 0.841 | |
| Pulmonary complications, n (%) | 105 (64.81%) | 35 (89.74%) | 0.004 | |
| Respiratory failure (pO2 < 60 mmHg and/or pCO2 > 45 mmHg), n (%) | 23 (14.20%) | 23 (58.97%) | <0.001 | |
| Radiological signs of pneumonia, n (%) | 136 (84.47%) | 37 (94.87%) | 0.148 | |
| Clinical manifestation of pneumonia, n (%) | 117 (72.22%) | 35 (89.74%) | 0.038 | |
| Fibrosis, n (%) | 2 (1.24%) | 7 (17.95%) | <0.001 | |
| Pneumothorax, n (%) | 2 (1.24%) | 1 (2.56%) | 0.901 | |
| Hydrothorax, n (%) | 15 (9.32%) | 7 (17.95%) | 0.207 | |
| Renal complications, n (%) | 22 (13.58%) | 13 (33.33%) | 0.004 | |
| AKI or decompensation of CKD (creatinine level ratio (last measurement/admission)), n (%) | 21 (12.96%) | 12 (30.77%) | 0.014 | |
| Urinary Tract Infection, n (%) | 12 (7.41%) | 7 (17.95%) | 0.086 | |
| Neurological complications, n (%) | 0 (0.00%) | 30 (76.92%) | <0.001 | |
| Transient Ischemic Attack, n (%) | 0 (0.00%) | 1 (2.56%) | 0.438 | |
| Ischemic stroke, n (%) | 0 (0.00%) | 1 (2.56%) | 0.438 | |
| Seizures, n (%) | 1 (0.62%) | 0 (0.00%) | 0.438 | |
| Venous Thromboembolism, n (%) | 3 (1.85%) | 3 (7.69%) | 0.161 | |
| Deep Vein Thrombosis, n (%) | 1 (0.62%) | 0 (0.00%) | 0.438 | |
| Pulmonary Embolism, n (%) | 3 (1.85%) | 3 (7.69%) | 0.161 | |
| Other complications, n (%) | 12 (7.41%) | 8 (20.51%) | 0.031 | |
| Pressure ulcers, n (%) | 2 (1.23%) | 3 (7.69%) | 0.079 | |
| Gastrointestinal hemorrhage, n (%) | 3 (1.85%) | 2 (5.13%) | 0.544 | |
| Mucosal bleeding, n (%) | 3 (1.85%) | 1 (2.56%) | 0.724 | |
| HIT, n (%) | 0 (0.00%) | 1 (2.56%) | 0.438 | |
| Sepsis, n (%) | 8 (4.94%) | 7 (17.95%) | 0.014 | |
| CDI, n (%) | 4 (2.47%) | 0 (0.00%) | 0.724 | |
| FOLLOW-UP | ||||
| Time of stay in the ward (including the day of admission and discharge) (mean ± SD; Me) | 10.48 ± 5.22; 10.0 | 11.46 ± 9.10; 9.0 | 0.588 | |
| Timing of death (until day 30) | 13.17 ± 5.71; 13.5 | 9.56 ± 7.24; 7.0 | 0.096 | |
| Death, n (%) | 6 (3.70%) | 18 (46.15%) | <0.001 | |
| Discharge, n (%) | Discharged home | 136 (83.95%) | 14 (35.90%) | <0.001 |
| Transferred to another unit | 12 (7.41%) | 1 (2.56%) | ||
| Transferred to ICU | 8 (4.94%) | 6 (15.38%) | ||
| Complications | Delirium(Unadjusted) | Delirium *(Adjusted by Age and Gender) | ||
|---|---|---|---|---|
| OR | p-Value | OR | p-Value | |
| Cardiological complications | 8.512 (3.409–21.256) | <0.001 | 7.720 (2.668–22.335) | <0.001 |
| Heart Failure | 4.618 (1.266–16.840) | 0.020 | 4.449 (1.090–18.160) | 0.038 |
| Atrial Fibrillation | 4.844 (1.589–14.766) | 0.006 | 3.112 (0.886–10.930) | 0.077 |
| Pulmonary complications | 4.750 (1.607–14.037) | 0.005 | 8.788 (2.604–29.661) | <0.001 |
| Respiratory failure (pO2 < 60 mmHg and/or pCO2 > 45 mmHg) | 8.687 (3.999–18.871) | <0.001 | 6.285 (2.657–14.865) | <0.001 |
| Radiological signs of pneumonia | 3.401 (0.770–15.021) | 0.106 | 3.812 (0.792–18.356) | 0.095 |
| Clinical manifestation of pneumonia | 3.365 (1.131–10.011) | 0.029 | 3.921 (1.187–12.957) | 0.025 |
| Pulmonary Fibrosis | 17.391 (3.453–87.589) | <0.001 | 8.124 (1.458–45.271) | 0.017 |
| Pneumothorax | 2.092 (0.185–23.680) | 0.551 | 2.763 (0.225–33.987) | 0.427 |
| Hydrothorax | 2.129 (0.803–5.647) | 0.129 | 1.366 (0.451–4.132) | 0.581 |
| Renal complications | 3.182 (1.425–7.105) | 0.005 | 2.411 (0.945–6.149) | 0.065 |
| AKI/Decompensation of CKD (creatinine level ratio (last measurement/admission) | 2.984 (1.314–6.776) | 0.009 | 1.665 (0.649–4.268) | 0.289 |
| Urinary Tract Infection | 2.734 (0.999–7.487) | 0.050 | 2.602 (0.767–8.824) | 0.125 |
| Pulmonary Embolism | 4.417 (0.856–22.785) | 0.076 | 3.231 (0.458–22.774) | 0.239 |
| Other complications | 3.226 (1.217–8.549) | 0.019 | 2.321 (0.739–7.291) | 0.149 |
| Pressure ulcers | 6.667 (1.074–41.368) | 0.042 | 3.525 (0.469–26.519) | 0.221 |
| Gastrointestinal hemorrhage | 2.865 (0.462–17.764) | 0.258 | 1.766 (0.257–12.137) | 0.563 |
| Mucosal bleeding | 1.395 (0.141–13.783) | 0.776 | 1.855 (0.162–21.194) | 0.619 |
| Sepsis | 4.211 (1.425–12.443) | 0.009 | 3.991 (1.151–13.841) | 0.029 |
| FOLLOW-UP | ||||
| Time of stay in ward (including the day of admission and discharge) | 1.024 (0.971–1.081) | 0.374 | 1.009 (0.951–1.070) | 0.766 |
| Timing of death (until day 30) | 0.931 (0.819–1.060) | 0.279 | 0.913 (0.772–1.081) | 0.291 |
| Death | 22.286 (7.955–62.434) | <0.001 | 17.212 (5.108–58.003) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotfis, K.; Witkiewicz, W.; Szylińska, A.; Witkiewicz, K.; Nalewajska, M.; Feret, W.; Wojczyński, Ł.; Duda, Ł.; Ely, E.W. Delirium Severely Worsens Outcome in Patients with COVID-19—A Retrospective Cohort Study from Temporary Critical Care Hospitals. J. Clin. Med. 2021, 10, 2974. https://doi.org/10.3390/jcm10132974
Kotfis K, Witkiewicz W, Szylińska A, Witkiewicz K, Nalewajska M, Feret W, Wojczyński Ł, Duda Ł, Ely EW. Delirium Severely Worsens Outcome in Patients with COVID-19—A Retrospective Cohort Study from Temporary Critical Care Hospitals. Journal of Clinical Medicine. 2021; 10(13):2974. https://doi.org/10.3390/jcm10132974
Chicago/Turabian StyleKotfis, Katarzyna, Wojciech Witkiewicz, Aleksandra Szylińska, Karina Witkiewicz, Magdalena Nalewajska, Wiktoria Feret, Łukasz Wojczyński, Łukasz Duda, and Eugene Wesley Ely. 2021. "Delirium Severely Worsens Outcome in Patients with COVID-19—A Retrospective Cohort Study from Temporary Critical Care Hospitals" Journal of Clinical Medicine 10, no. 13: 2974. https://doi.org/10.3390/jcm10132974