Stimulator of Interferon Genes-Associated Vasculopathy With Onset in Infancy: A Systematic Review of Case Reports
- Department of Respiratory, National Children's Medical Center, China National Clinical Research Center for Respiratory Diseases, Beijing Children's Hospital, Capital Medical University, Beijing, China
Objective: To summarize and analyze the manifestations of stimulator of interferon genes (STING)-associated vasculopathy with onset in infancy (SAVI).
Methods: A systematic literature review was performed including cases from January 1, 2014, to February 1, 2020, using PubMed, OVID, CNKI, and WanFang. This included all the literature containing comparatively complete clinical data. Statistical analysis was performed using SPSS 20.0 to analyze the difference in age of onset, severity of skin lesions, and respiratory symptoms between SAVI patients with p.N154S and p.V155M mutations.
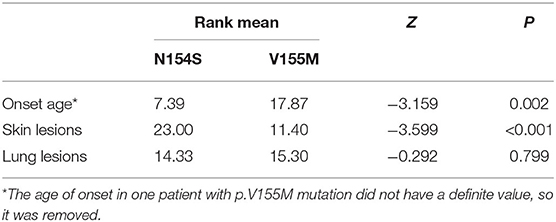
Results: A total of 25 papers were included reporting on 51 individuals, of whom 17 had familiar inheritance of their mutation. Patients included 27 males and 24 females, and 8 fatal cases were observed. A total of 10 mutation sites have been reported in the STING gene, with p.V155M being the most prevalent. We identified SAVI as an early-onset disease with a median age of onset of 3 months after birth. Skin lesions were the most common symptoms of SAVI, found in 94.1% (48/51) of patients, while 76% (19/25) who had undergone a skin biopsy showed vasculopathy. Involvement of the lungs was identified in 68.6% (35/51) of patients, while only 22.2% (4/18) who had undergone a lung biopsy showed vasculopathy. Of 20 patients, 19 had increased immunoglobulin, mainly IgG. Furthermore, 45.1% (23/51) of patients had a positive low titer or were transiently positive for antinuclear antibodies. Of the 18 patients treated with JAK inhibitors, 6 relapsed and 2 died of acute respiratory failure caused by viral infection. Patients with p.N154S mutation had an earlier disease onset (p = 0.002) and more severe skin lesions (p < 0.001) than those patients with p.V155M mutation.
Conclusion: SAVI is an early-onset disease accompanied by skin and lung lesions whose clinical presentation varies among patients with different genotypes. Therapeutic effects of JAK inhibitors are unsatisfactory.
Introduction
Stimulator of interferon genes (STING)-associated vasculopathy with onset in infancy (SAVI), first reported in 2014 (1), is an interferonopathy caused by gain-of-function mutations in STING1 gene. It usually involves skin and pulmonary lesions, accompanied by systematic inflammatory symptoms such as a recurrent fever (1). However, the initial manifestations and therapeutic effectiveness differ across reported cases. Here, we conducted a systematic review of all reported SAVI cases, summarizing the characteristics of disease presentation and provide clinical support for early diagnosis and prognosis.
Materials and Methods
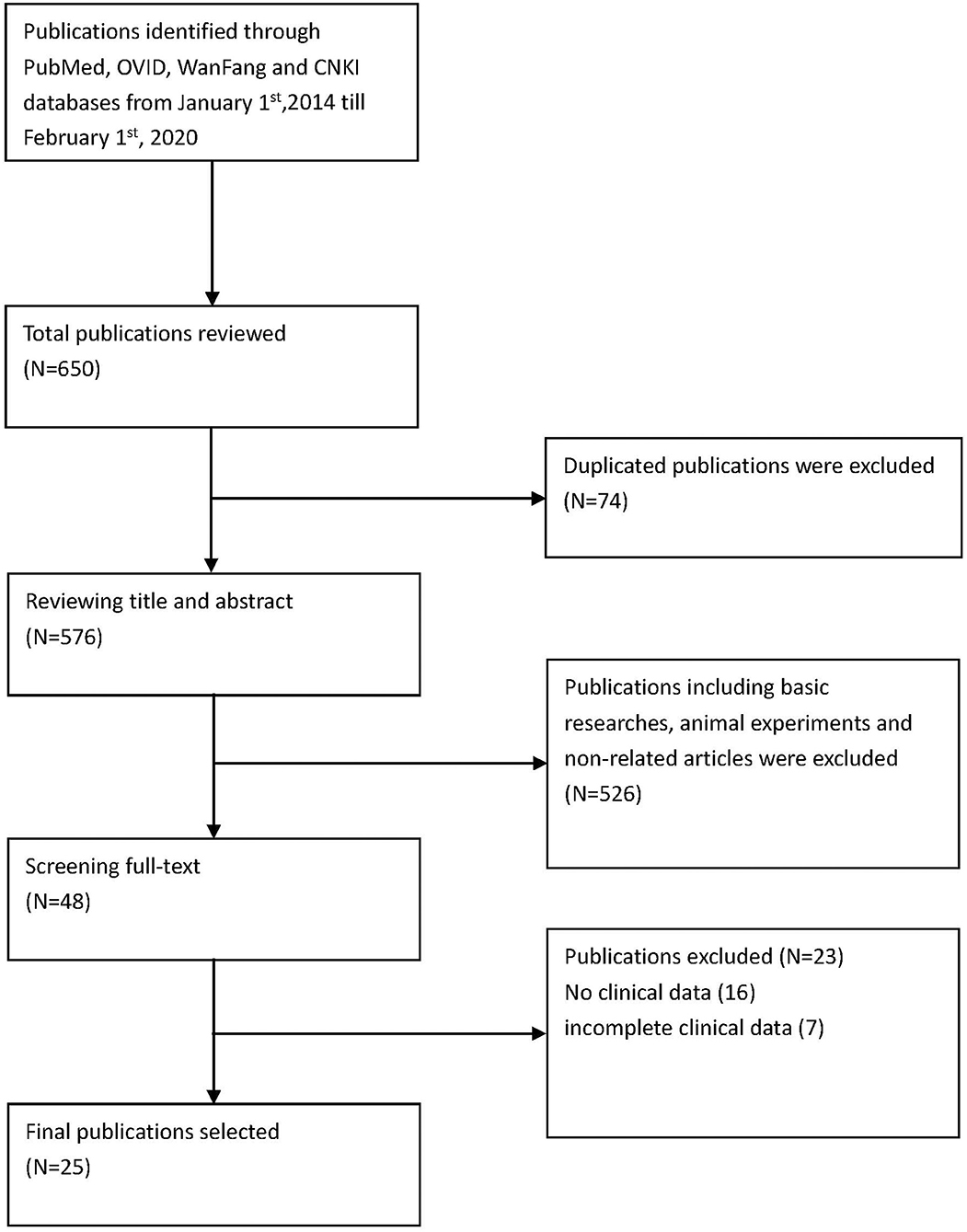
Methods are summarized in Figure 1.
Search Strategy
Via PubMed, OVID, CNKI, and WanFang, the English terms searched were “sting-associated vasculopathy with onset in infancy” and “stimulator of interferon genes associated vasculopathy with onset in infancy,” “STING,” “TMEM173,” and “mutation.” We searched for literature published from January 1, 2014, to February 1, 2020 (Figure 1). The specific search queries utilized are listed below.
PubMed
Formula 1: [(STING-associated vasculopathy with onset in infancy) OR stimulator of interferon genes associated vasculopathy with onset in infancy] AND 2014/01/01:2020/02/01[dp]
Formula 2: {[(stimulator of interferon genes) OR TMEM173] AND mutation} AND 2014/01/01:2020/02/01[dp]
OVID
Formula 1: (STING-associated vasculopathy with onset in infancy) OR (stimulator of interferon genes associated vasculopathy with onset in infancy)
Formula 2: [(stimulator of interferon genes OR TMEM173) AND mutation]
We also searched CNKI and WanFang Database for literature published in Chinese using a similar search strategy.
Inclusion criteria: (1) including case reports, (2) complete clinical data, (3) articles written in English and Chinese.
Results
Summary of Patients in the Included Literature
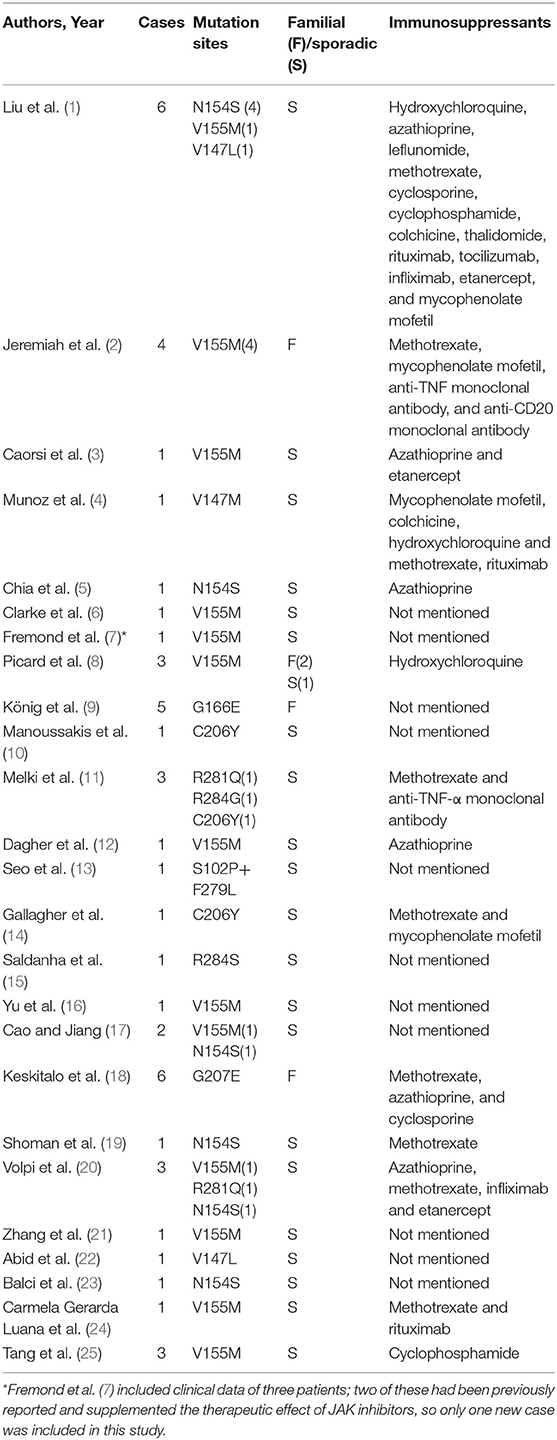
We included 25 articles (1–25) that met the search criteria, 23 in English and 2 in Chinese. These articles comprised 43 non-fatal and 8 fatal cases, with a sex ratio of 1.25:1 (27 males to 24 females). Moreover, there were 17 familial cases with autosomal dominant inheritance. A total of 10 mutation sites have been reported, with p.V155M being the most prevalent (Table 1).
Manifestations of SAVI Patients
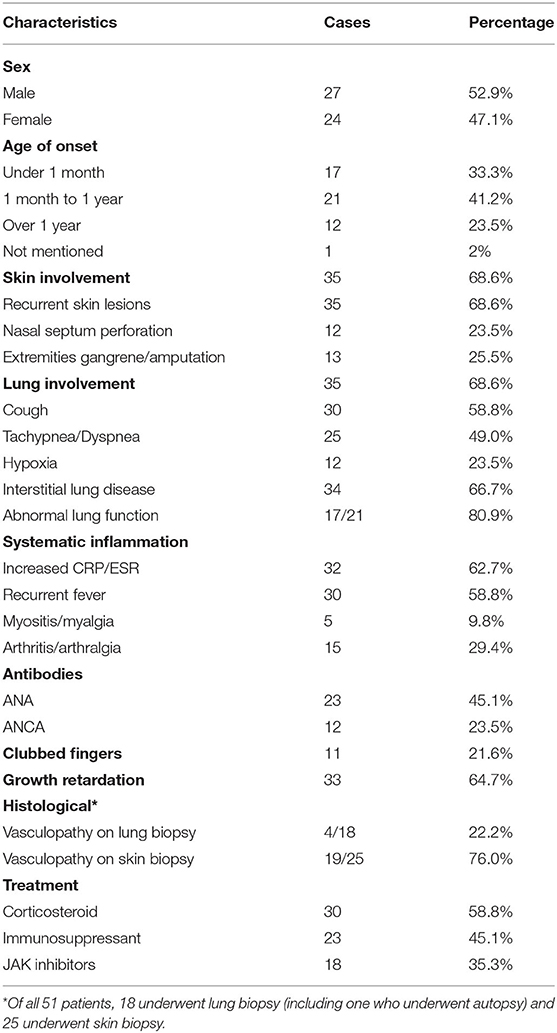
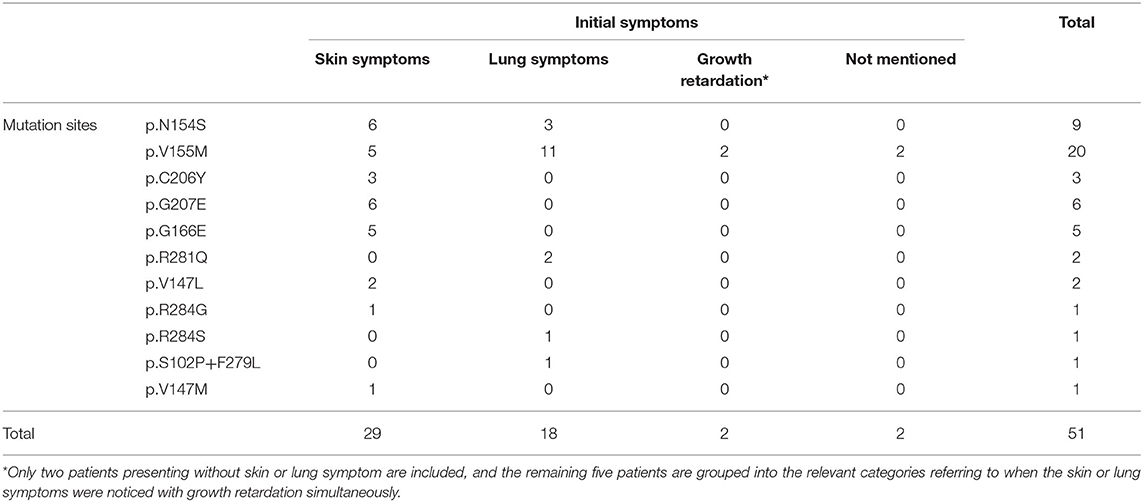
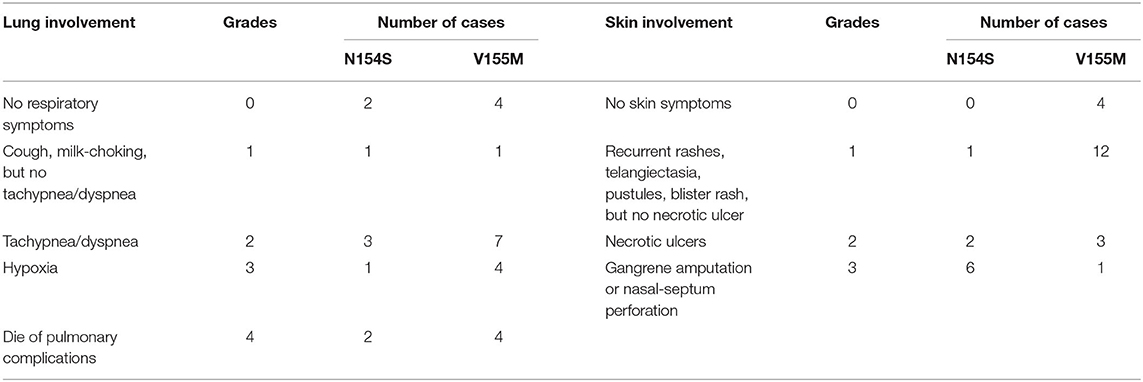
Our systematic review of the literature found that the age of onset of SAVI ranged from neonatal to adulthood, while the median age of presentation was 3 months after birth (Table 2). The initial symptoms of 35.3% (18/51) of patients were respiratory symptoms, such as tachypnea, dyspnea, cough, and milk-choking, while 56.86% (29/51) initially suffered from skin lesions, accompanied by growth retardation (Table 3). As the most typical skin lesions, chilblain lesions were usually cold-related, including telangiectatic, pustular, erythema, blistering rashes, and ulcers, predominantly on the cheek, auricle, and extremities. Aggravation of the skin lesions resulted in tissue loss, such as nasal septum perforation (12 patients), nail loss (11 patients), and gangrene of the extremities or amputation (13 patients). A total of 35 patients had lung involvements. Among them, 30 cases suffered from respiratory symptoms and 34 cases had signs of interstitial lung disease (ILD) on chest image. Five patients without respiratory symptoms had ILD signs on chest image, while one patient without ILD image change had respiratory symptoms. Other accompanying symptoms included a recurrent fever (30 patients), growth retardation (33 patients), myositis/myalgia (5 patients), arthritis/arthralgia (15 patients), renal impairment (3 patients), and brain impairment (3 patients).
Analysis of Patient Test Data
The inflammatory markers, C-reactive protein, and erythrocyte sedimentation rate were elevated in 62.7% (32/51) of patients. Of the 20 patients who underwent immune function testing, 19 had hyperimmunoglobulinemia, mainly IgG (18/19 patients) (Table 2). The lymphocyte profile was abnormal in 12/18 of patients, with 8 showing decreased CD4+ T lymphocytes and 3 showing elevated CD19+ B lymphocytes. Twenty-three patients presented with a low titer or transiently positive antinuclear antibodies (with the maximum titer of 1:640), and 12 had positive or transient positive anti-neutrophil cytoplasmic antibodies. A total of 10 patients were positive for rheumatoid factor (RF); because 60% (6/10) of patients suffered with arthralgia, we suspected that RF presence and arthralgia symptoms were interconnected. Other related autoantibodies in the patient data included anti-double-stranded DNA antibody (two patients), antiphospholipid antibodies (six patients), anti-cyclic citrullinated peptide antibody (two patients), and lupus anticoagulant (one patient). Over half of the patients (34/51) had signs of ILD on chest high-resolution computed tomography (HRCT), presenting as ground glass opacity, cysts, reticulations, interlobular septal thickening, or pleural thickening. Additional symptoms may also appear in SAVI patients, including consolidations, bronchiectasis, emphysema, lymphadenopathy, and pulmonary hypertension. Only one patient with the p.S102P+F279L mutation presented with indicated obliterans bronchitis. Meanwhile, the familial p.G166E and p.G207E cases had neither respiratory symptoms nor pulmonary lesions on HRCT. Skin biopsies were performed on 25 patients, revealing that 76.0% (19/25) of them had vasculopathy, including vasculitis (13 patients) and perivascular inflammation (6 patients). Another two patients presented with nodular granulomatous dermatitis. All the eight patients who did skin biopsies, with p.N154S mutation, presented vasculopathy, whereas only 4 of 18 patients showed vasculopathy upon pulmonary biopsy in patients with p.V155M mutation.
Treatment and Prognosis
Previous studies indicated that corticosteroid and multiple immunosuppressive therapies were not effective (1, 21). Among the 51 patients, 30 were treated with corticosteroids and 23 were treated with immunosuppressants, including hydroxychloroquine, mycophenolate mofetil, azathioprine, leflunomide, methotrexate, cyclosporine, cyclophosphamide, colchicine, thalidomide, rituximab, tocilizumab, infliximab, and etanercept. Only 10 patients showed a partial or transient improvement with these therapies; however, in 3 patients, treatment with steroids combined with immunosuppressants (azathioprine, cyclosporine, and methotrexate) stabilized their condition. Since IFN-β stimulates downstream inflammation mainly through the JAK-STAT pathway, JAK inhibitors (tofacitinib, ruxolitinib, and baricitinib) were prescribed to 18 patients. Although 11 patients showed improvement in both skin and respiratory symptoms and 7 patients benefited from partially symptom relief, 6 patients relapsed and 2 patients died of acute respiratory failure caused by viral infection.
Disease Manifestations Differ Based on Genotype
When summarizing the manifestations of SAVI, the different clinical phenotypes were noticeable. Therefore, the features of patients with p.N154S and p.V155M mutations were compared, including the age of onset and respiratory and skin symptoms. A classification of each patient was made to evaluate the severity of their symptoms (Table 4). A two-samples rank sum tests was used in SPSS20.0 to determine any statistical significance between the genotypes. The results were as follows: patients with p.N154S mutation had an earlier disease onset (p = 0.002) and more severe skin lesions (p < 0.001) than those patients with p.V155M mutation, whereas there was no difference in respiratory symptom presentation (Table 5).
Discussion
Several mutations have been identified in the STING1 gene, which encodes a transmembrane protein localizing to the endoplasmic reticulum that is composed of 379 amino acids. STING is a significant signal transduction molecule in innate immunity with important antiviral roles and has functions in cancer (26). It stimulates type I IFN-mediated pro-inflammatory cytokines through the interferon regulatory factor 3 or nuclear Factor kappa B pathways after identifying exogenous ds-DNA or RNA in the cytoplasm (26–33). Various cell types express STING, including endothelial cells, skin cells, hematopoietic cells, T cells, macrophages, dendritic cells, type 2 bronchial epithelial cells, and alveolar cells (1). Therefore, multiple tissues, such as the skin vasculature and pulmonary system, are likely affected by the various mutations, thus resulting in several phenotypes and disease manifestations. The literature describes SAVI as an early-onset disease, with chilblain lesions, ILD, and recurrent fever as its features (1). Our systematic review of the literature showed that the median age of onset in our study was 3 months after birth, with 64.7% (33/51) of patients suffering from both skin and respiratory lesions. However, the spectrum of disease manifestations continues to grow as more genotypes related to SAVI are discovered, including arthritis, myositis, kidney damage, brain damage, photosensitivity, hair loss, and thyroid damage (2, 3, 8, 9, 11, 18, 20, 22, 25). In support of this, 3 cases without skin lesion (21, 25) and 16 cases without pulmonary impairment (1, 2, 11, 18) have been reported, suggesting the clinical phenotypes of SAVI are more diverse than was previously thought.
As hotspot mutations, clinical manifestations of p.N154S and p.V155M were compared, including age of onset, skin, and respiratory symptoms. Compared to patients with p.V155M mutation, p.N154S mutations had an earlier onset and more severe skin lesions. Similar heterogeneity on phenotypes was observed in mouse models. Motwani et al. (34) confirmed that only the V154M mice developed lung fibrosis and the V154M mutant was more active than the N153S mutant inducing four-fold greater levels of IFN-β reporter gene in transfected 293T cells. Meanwhile, lung impairment was not involved in all members of the p.G166E and p.G207E family cases (9, 18). Besides, p.G207E families' patients presented with livedo reticularis and suppurative necrosis of the skin rather than typical chilblain lesions. These families also presented with peculiar features, including light sensitivity, hair loss, abnormal thyroid function, and recurrent sinusitis (9, 18). Above all, we conclude that the clinical phenotypes vary in relation to the different genotypes of SAVI.
Previous research has indicated that SAVI is refractory to corticosteroid and multiple immunosuppressants, while JAK inhibitors may have a curative effect via decreasing the expression of IFN and downstream pro-inflammatory factors. However, when summarizing the 18 patients who had been treated with JAK inhibitors, we found the treatment to be unsatisfactory. Although most JAK inhibitor-treated patients had achieved total or partial transient relief of symptoms, there were six patients who relapsed and two patients who died due to pulmonary complications. The poor therapeutic effect may be explained by the fact found in mouse models that the STING-associated disease depended on T cells but not type I interferon or IRF3 (Interferon Regulating Factor 3) (34–37). Besides, the risk of viral infection might increase in patients treated with JAK inhibitors (5, 7), as infection has been shown to aggravate pulmonary fibrosis in rats (38). Moreover, Saldanha et al. (15) reported on a patient who did not suffer any serious pulmonary infection for 2 years while on immunoglobulin therapy and sulfamethoxazole to prevent infection. Therefore, it can be speculated that preventing infection may delay the aggravation of pulmonary fibrosis and reduce the occurrence of JAK inhibitor side effects. In addition, nitro fatty acids and nitrofuran are direct inhibitors of the STING pathway and could be used to treat STING-related autoimmune diseases in the future (39, 40).
Conclusion
The clinical phenotypes of SAVI are diverse and related to the genotypes. Compared to the patients with p.V155M mutation, the onset of p.N154S mutation was earlier, with skin lesions of greater severity. The efficacy of JAK inhibitors leaves something to be desired based on previous reports, and our data presented here. Using antibiotics plus immunotherapy to prevent infection may improve patient prognosis.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
YD acquired and analyzed the data and wrote the manuscript draft. XL contributed to design the search criteria and summarized the conclusion. ZZ, JH, and QY made critical revisions to the manuscript. All authors reviewed the manuscript and completed a final approval.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, Montealegre Sanchez GA, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. (2014) 371:507–518. doi: 10.1056/NEJMoa1312625
2. Jeremiah N, Neven B, Gentili M, Callebaut I, Maschalidi S, Stolzenberg MC, et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest. (2014) 124:5516–20. doi: 10.1172/JCI79100
3. Caorsi R, Rice G, Cardinale F, Volpi S, Buoncompagni A, Crow Y, et al. AB1014 enlarging the clinical spectrum of sting-associated vasculopathy with onset in infancy (SAVI). Ann Rheum Dis. (2015) 74(Suppl 2):1237–3. doi: 10.1136/annrheumdis-2015-eular.6115
4. Munoz J, Rodiere M, Jeremiah N, Rieux-Laucat F, Oojageer A, Rice GI, et al. Stimulator of interferon genes-associated vasculopathy with onset in infancy: a mimic of childhood granulomatosis with polyangiitis. JAMA Dermatol. (2015) 151:872–77. doi: 10.1001/jamadermatol.2015.0251
5. Chia J, Eroglu FK, Ozen S, Orhan D, Montealegre-Sanchez G, de Jesus AA, et al. Failure to thrive, interstitial lung disease, and progressive digital necrosis with onset in infancy. J Am Acad Dermatol. (2016) 74:186–9. doi: 10.1016/j.jaad.2015.10.007
6. Clarke SL, Pellowe EJ, de Jesus AA, Goldbach-Mansky R, Hilliard TN, Ramanan AV. Interstitial lung disease caused by STING-associated vasculopathy with onset in infancy. Am J Respir Crit Care Med. (2016) 194:639–42. doi: 10.1164/rccm.201510-2102LE
7. Fremond ML, Rodero MP, Jeremiah N, Belot A, Jeziorski E, Duffy D, et al. Efficacy of the Janus kinase 1/2 inhibitor ruxolitinib in the treatment of vasculopathy associated with TMEM173-activating mutations in 3 children. J Allergy Clin Immunol. (2016) 138:1752–5. doi: 10.1016/j.jaci.2016.07.015
8. Picard C, Thouvenin G, Kannengiesser C, Dubus J-C, Jeremiah N, Rieux-Laucat F, et al. Severe pulmonary fibrosis as the first manifestation of interferonopathy (TMEM173 Mutation). Chest. (2016) 150:e65–71. doi: 10.1016/j.chest.2016.02.682
9. König N, Fiehn C, Wolf C, Schuster M, Cura Costa E, Tüngler V, et al. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann Rheum Dis. (2017) 76:468–72. doi: 10.1136/annrheumdis-2016-209841
10. Manoussakis MN, Mavragani CP, Nezos A, Zampeli E, Germenis A, Moutsopoulos HM. Type I interferonopathy in a young adult. Rheumatology. (2017) 56:2241–3. doi: 10.1093/rheumatology/kex316
11. Melki I, Rose Y, Uggenti C, Van Eyck L, Fremond ML, Kitabayashi N, et al. Disease-associated mutations identify a novel region in human STING necessary for the control of type I interferon signaling. J Allergy Clin Immunol. (2017) 140:543–52 e545. doi: 10.1016/j.jaci.2016.10.031
12. Dagher R, Ghiye R, Nicolas G, Feghali H, Fadous KM, Seabra L, et al. Sting-associated vasculopathy with onset in infancy (SAVI): a differential diagnosis of inflammatory interstitial lung disease. Ann Rheum Dis. (2017) 76(Suppl 2):406. doi: 10.1136/annrheumdis-2017-eular.4489
13. Seo J, Kang J-A, Suh DI, Park E-B, Lee C-R, Choi SA, et al. Tofacitinib relieves symptoms of stimulator of interferon genes (STING)-associated vasculopathy with onset in infancy caused by 2 de novo variants in TMEM173. J Allergy Clin Immunol. (2017) 139:1396–9. doi: 10.1016/j.jaci.2016.10.030
14. Gallagher K, Brogan P, Burrows N, Gass J, Bale P, Armon K. STING: associated vasculopathy with onset in infancy (SAVI). Rheumatology. (2018) 57:key273.006. doi: 10.1093/rheumatology/key273.006
15. Saldanha RG, Balka KR, Davidson S, Wainstein BK, Wong M, Macintosh R, et al. A Mutation outside the dimerization domain causing atypical STING-associated vasculopathy with onset in infancy. Front Immunol. (2018) 9:1535. doi: 10.3389/fimmu.2018.01535
16. Yu ZX, Zhong LQ, Song HM, Wang CY, Wang W, Li J, et al. Stimulator of interferon genes-associated vasculopathy with onset in infancy: first case report in China. Zhonghua Er Ke Za Zhi. (2018) 56:179–85. doi: 10.3760/cma.j.issn.0578-1310.2018.03.005
17. Cao Y, Jiang LP. The challenge of diagnosing SAVI: case studies. Pediatr Allergy Immunol Pulmonol. (2019) 32:167–72. doi: 10.1089/ped.2019.1054
18. Keskitalo S, Haapaniemi E, Einarsdottir E, Rajamaki K, Heikkila H, Ilander M, et al. Novel TMEM173 mutation and the role of disease modifying alleles. Front Immunol. (2019) 10:2770. doi: 10.3389/fimmu.2019.02770
19. Shoman W, El Chazli Y, ElSawy I, Arostegui JI. First Egyptian patient with STING-associated vasculopathy with onset in infancy. Scand J Rheumatol. (2019) 48:338–9. doi: 10.1080/03009742.2018.1550212
20. Volpi S, Insalaco A, Caorsi R, Santori E, Messia V, Sacco O, et al. Efficacy and adverse events during janus kinase inhibitor treatment of SAVI syndrome. J Clin Immunol. (2019) 39:476–85. doi: 10.1007/s10875-019-00645-0
21. Zhang Y, Yan XL, Meng C, Song GH, Wang LL. Stimulator of interferon genes-associated vasculopathy with onset in infancy: one case report and literature review. Chin J Evid Based Pediatr. (2019) 14:196–200. doi: 10.3969/j.issn.1673-5501.2019.03.007
22. Abid Q, Best Rocha A, Larsen CP, Schulert G, Marsh R, Yasin S, et al. APOL1-Associated collapsing focal segmental glomerulosclerosis in a patient with stimulator of interferon genes (STING)-associated vasculopathy with onset in infancy (SAVI). Am J Kidney Dis. (2020) 75:287–90. doi: 10.1053/j.ajkd.2019.07.010
23. Balci S, Ekinci RMK, de Jesus AA, Goldbach-Mansky R, Yilmaz M. Baricitinib experience on STING-associated vasculopathy with onset in infancy: a representative case from Turkey. Clin Immunol. (2020) 212:108273. doi: 10.1016/j.clim.2019.108273
24. Carmela Gerarda Luana R, Virginia M, Gianmarco M, Ivan C, Silvia F, Manuela P, et al. A patient with stimulator of interferon genes-associated vasculopathy with onset in infancy without skin vasculopathy. Rheumatology. (2020) 59:905–7. doi: 10.1093/rheumatology/kez444
25. Tang X, Xu H, Zhou C, Peng Y, Liu H, Liu J, et al. STING-Associated vasculopathy with onset in infancy in three children with new clinical aspect and unsatisfactory therapeutic responses to tofacitinib. J Clin Immunol. (2020) 40:114–22. doi: 10.1007/s10875-019-00690-9
26. Barber GN. STING: infection, inflammation and cancer. Nat Rev Immunol. (2015) 15:760–70. doi: 10.1038/nri3921
27. Jiaxi W, Lijun S, Xiang C, Fenghe D, Heping S, Chuo C, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. (2013) 339:826–30. doi: 10.1126/science.1229963
28. Osamu T, Shizuo A. Innate immunity to virus infection. Immunol Rev. (2009) 227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x
29. Palm Noah W, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. (2009) 227:221–33. doi: 10.1111/j.1600-065X.2008.00731.x
30. Hiroki I, N BG. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. (2008) 455:674–8. doi: 10.1038/nature07317
31. Seng-Ryong W, Leticia C, F GT. The STING pathway and the T cell-inflamed tumor microenvironment. Trends Immunol. (2015) 36:250–6. doi: 10.1016/j.it.2015.02.003
32. Hiroki I, Zhe M, N BG. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. (2009) 461:788–92. doi: 10.1038/nature08476
33. Bruce AB. TLRs and innate immunity. Blood. (2009) 113:1399–407. doi: 10.1182/blood-2008-07-019307
34. Motwani M, Pawaria S, Bernier J, Moses S, Henry K, Fang T, et al. Hierarchy of clinical manifestations in SAVI N153S and V154M mouse models. Proc Natl Acad Sci USA. (2019) 116:7941–50. doi: 10.1073/pnas.1818281116
35. Delphine B, Peggy K, Florent A, Delphine L, Virginia D, Sophie J, et al. Severe combined immunodeficiency in stimulator of interferon genes (STING) V154M/wild-type mice. J Allergy Clin Immunol. (2019) 143:712–25.e5. doi: 10.1016/j.jaci.2018.04.034
36. Hella L, Stinson WA, Derek JP, Wei Q, Gowri K, Cathrine AM, et al. STING-associated lung disease in mice relies on T cells but not type I interferon. J Allergy Clin Immunol. (2019) 144:254–66.e8. doi: 10.1016/j.jaci.2019.01.044
37. James DW, Ricardo AI-C, Brock GB, Teresa LA, Amber MS, Cathrine AM, et al. STING-associated vasculopathy develops independently of IRF3 in mice. J Exp Med. (2017) 214:3279–92. doi: 10.1084/jem.20171351
38. Bennion Brock G, Ingle H, Ai Teresa L, Miner Cathrine A, Platt Derek J, Smith Amber M, et al. A human gain-of-function STING mutation causes immunodeficiency and gammaherpesvirus-induced pulmonary fibrosis in mice. J Virol. (2019) 93:e01806–18. doi: 10.1128/JVI.01806-18
39. Louise HA, J BG, Michael R, Kojiro M, R SS, Emari O, et al. Nitro-fatty acids are formed in response to virus infection and are potent inhibitors of STING palmitoylation and signaling. Proc Natl Acad Sci USA. (2018) 115:E7768–75. doi: 10.1073/pnas.1806239115
Keywords: STING-associated vasculopathy with onset in infancy, interstitial lung disease, interferon genes, systematic review, children
Citation: Dai YF, Liu XY, Zhao ZP, He JX and Yin QQ (2020) Stimulator of Interferon Genes-Associated Vasculopathy With Onset in Infancy: A Systematic Review of Case Reports. Front. Pediatr. 8:577918. doi: 10.3389/fped.2020.577918
Received: 30 June 2020; Accepted: 18 November 2020;
Published: 17 December 2020.
Edited by:
Marzia Duse, Sapienza University of Rome, ItalyReviewed by:
Adriana Almeida De Jesus, National Institutes of Health (NIH), United StatesDonato Rigante, Catholic University of the Sacred Heart, Italy
Neslihan Edeer Karaca, Ege University, Turkey
Copyright © 2020 Dai, Liu, Zhao, He and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: XiuYun Liu, liuxiu_yun@126.com
 YunFan Dai
YunFan Dai XiuYun Liu
XiuYun Liu ZhiPeng Zhao
ZhiPeng Zhao