- 1Department of Liver Surgery and Liver Transplantation Centre, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Liver Surgery, The First Clinical Medical College of Lanzhou University, Lanzhou, China
Background: Robotic distal pancreatectomy (RDP) and laparoscopic distal pancreatectomy (LDP) are the two principal minimally invasive surgical approaches for patients with pancreatic body and tail adenocarcinoma. The use of RDP and LDP for pancreatic ductal adenocarcinoma (PDAC) remains controversial, and which one can provide a better R0 rate is not clear.
Methods: A comprehensive search for studies that compared robotic versus laparoscopic distal pancreatectomy for PDAC published until July 31, 2021, was conducted. Data on perioperative outcomes and oncologic outcomes (R0-resection and lymph node dissection) were subjected to meta-analysis. PubMed, Cochrane Central Register, Web of Science, and EMBASE were searched based on a defined search strategy to identify eligible studies before July 2021.
Results: Six retrospective studies comprising 572 patients (152 and 420 patients underwent RDP and LDP) were included. The present meta-analysis showed that there were no significant differences in operative time, tumor size, and lymph node dissection between RDP and LDP group. Nevertheless, compared with the LDP group, RDP results seem to demonstrate a possibility in higher R0 resection rate (p<0.0001).
Conclusions: This systematic review and meta-analysis suggest that RDP is a technically and oncologically safe and feasible approach for selected PDAC patients. Large randomized and controlled prospective studies are needed to confirm this data.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/#recordDetails, identifier [CRD42021269353].
Introduction
The incidence of pancreatic cancer has risen and is likely to become the second most frequent cause of cancer-related death by 2030 (1). Pancreatic duct adenocarcinoma (PDAC) is the most common type of pancreatic cancer and is usually located in the head of the pancreas (2). Distal pancreatectomy is the fundamental surgery for the treatment of body-tail tumors of the pancreas. Since Cuscheri et al. reported the first laparoscopic distal pancreatectomy (LDP) in 1996 (3), and Melvin et al. performed the first robotic distal pancreatectomy (RDP) in 2003 (4). RDP and LDP applied to the surgical treatment of pancreatic tumors have increased over the last decade. Thanks to 3D high-definition visualization, tremor filtration, and instrument dexterity of robotic surgery systems, robotic surgery has emerged as a viable alternative approach to conventional laparoscopic surgery (5). Some literatures have confirmed the safety and feasibility of RDP and emphasized its advantages in less bleeding, lower conversion rate, shorter hospital stay, and higher spleen preservation rate (6–9). These studies involved patients with pancreatic ductal adenocarcinoma, pancreatic neuroendocrine tumor, intraductal papillary mucinous neoplasm, solid pseudopapillary tumor, or some benign tumors; therefore, RDP and LDP, which one is the better approach for PDAC, is unclear. To the best of our knowledge, no system review and meta-analysis has been performed to analyze the perioperative short-term oncological outcomes of minimally invasive distal pancreatectomy (RDP and LDP) for PDAC. This systematic review and meta-analysis aimed to compare the perioperative and oncologic outcomes of RDP versus LDP for PDAC.
Methods
Data Sources and Search Strategy
This study has registered at PROSPERO, and registration number is CRD42021269353 and reported on the basis of the PRISMA guidelines (10). Studies that investigated RDP versus LDP for PDCA were systematically searched in PubMed, Web of Science, EMBASE, Cochrane Central Register, and ClinicalTrials.gov databases before July 31, 2021, by two independent investigators (QF, CJ). The search terms used were “robotic surgery,” “laparoscopic surgery,” “distal pancreatectomy,” “left pancreatectomy,” “pancreatic cancer,” “pancreatic ductal adenocarcinoma,” and “adenocarcinoma,” either individually or in combination. The “related articles” function was used to broaden the search, and all citations were considered for relevance. Manual search of the references of publication was adopted to prevent missing relevant researches.
Inclusion and Exclusion Criteria
Two investigators (QF, CJ) reviewed currently available literature and screened all titles and abstracts independently and identified eligible studies according to the following criteria.
Inclusion criteria were as follows: (1) Participants: patients with pancreatic body and tail adenocarcinoma, and PDAC was defined by histologically; (2) Types of interventions: RDP and LDP; (3) Study type: randomized controlled trials (RCTs), propensity score matching studies, retrospective studies, cohort studies, and case-control studies comparing RDP to LDP with PDAC patients; (4) At least one outcome was reported in the literature, including operation time, intraoperative bleeding, tumor size, R0 rate, conversion rate, lymph node harvested, and spleen preservation rate; (5) Language restrictions: English.
Exclusion criteria were the following: (1) Conference abstracts, editorials, letters, case reports; (2) No comparative analysis between RDP and LDP.
Data Extraction and Quality Assessment
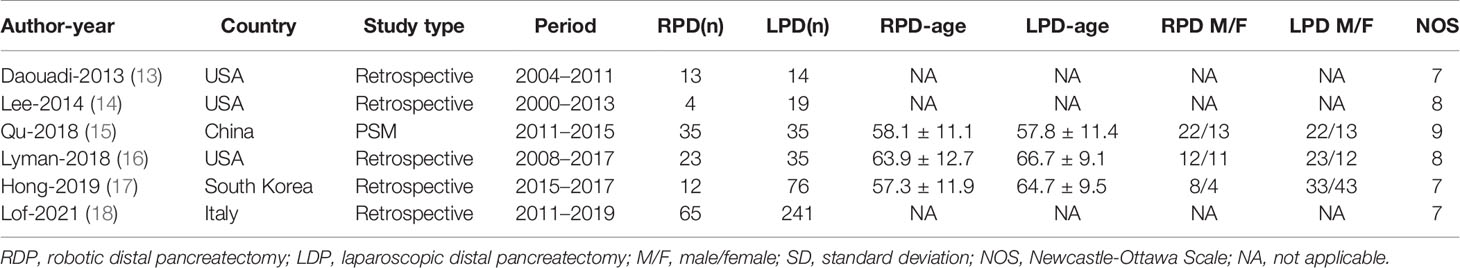
The original data from all candidate articles were independently assessed and extracted by two reviewers (QF, CJ) by using a unified datasheet, and any ambiguity was resolved by a third researcher (XF). The major data extraction includes the following: name of first author, publication year, study design, country, number of patients, mean age, gender, operative times, tumor size, bleeding, hospitalization, overall complication, overall complications, mortality, blood transfusion, R0 rate. The quality of the eligible studies was assessed by Newcastle-Ottawa Scale (NOS) by two different assessors (11). Every included study was independently evaluated by two authors (QF, XF), and NOS score ≥6 is considered as being of high quality.
Statistical Analysis
The Review Manager 5.3 software was used for statistical analyses. The 95% confidence interval (CI) and mean difference (MD) were used for continuous data, while categorical variables using odds ratio (OR). The method originally described by Hozo et al. to convert medians with ranges into means with standard deviations was used (12). Potential publication bias was visually assessed by Begg’s funnel plot and Egger’s test. Statistical heterogeneity was quantified using I2 value. A fixed-effects model (FEM) was adopted when heterogeneity was low or moderate (I2 <50%), and when heterogeneity was high (I2 ≥50%), a random-effects model (REM) was used.
Results
Characteristics of the Included Studies
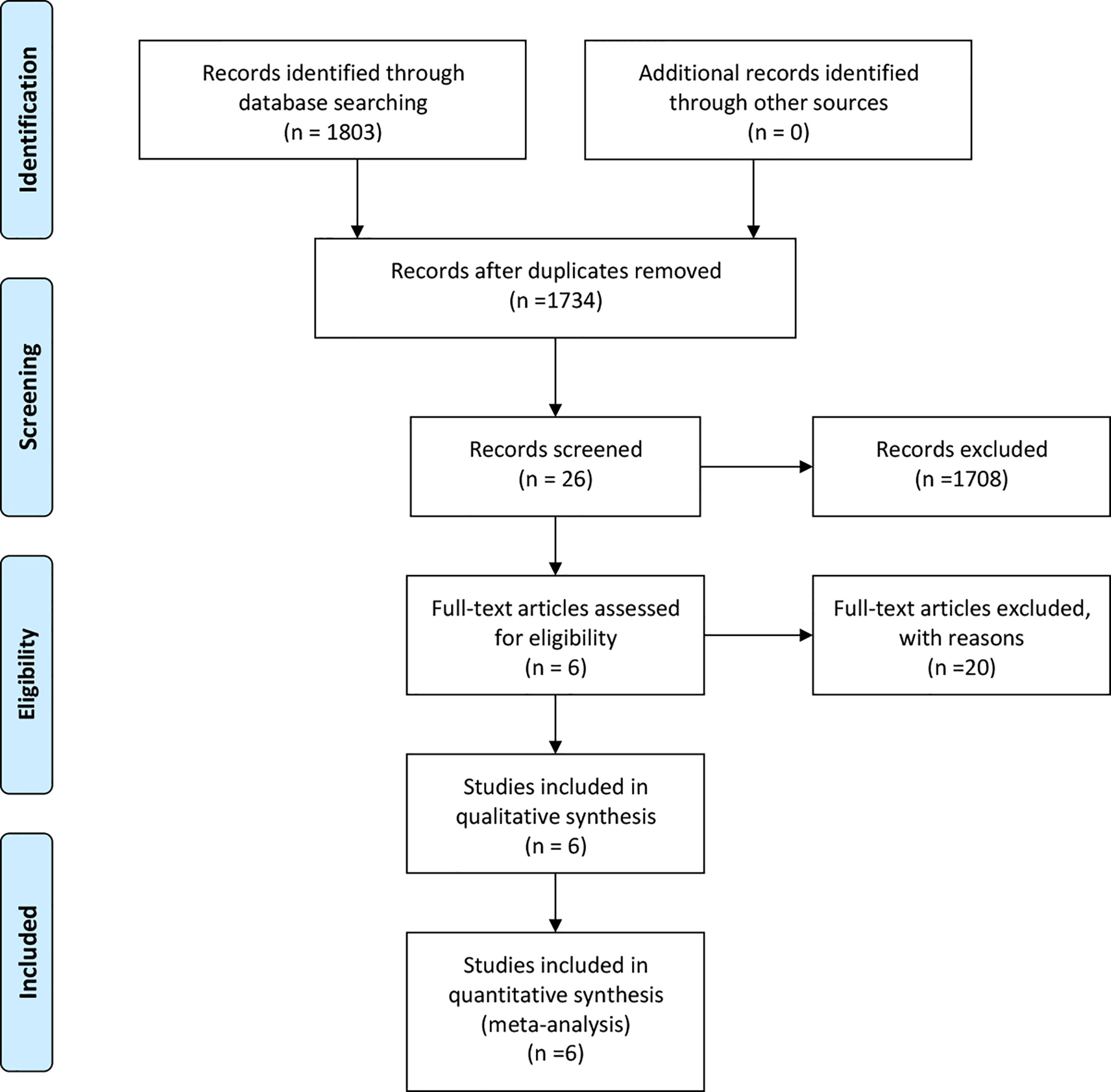
Finally, a total of 1,734 relevant English publications from the various electronic databases were yielded. According to the inclusion criteria, six retrospective studies (13–18) comparing RDP and LDP in a total of 572 patients (152 and 420 underwent RDP and LDP, respectively) were included for further analysis. A flow diagram of our analysis protocol is shown in Figure 1. The general information and summary of NOS scores of all the included studies are given in Table 1.
Perioperative Outcomes
Operative Time
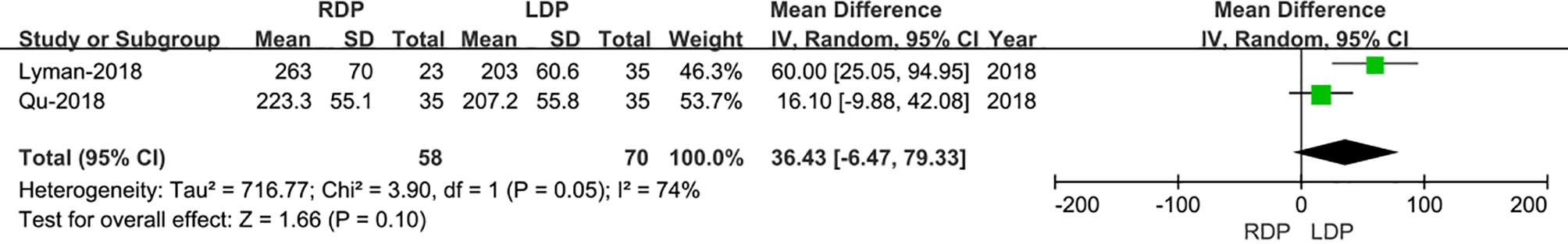
Only two studies (15, 16) that encompassed 128 patients (58 and 70 underwent RDP and LDP, respectively) reported operative times. The meta-analysis showed no difference in operative time in the two groups (WMD: 36.43 min; 95% CI −6.47 to 79.33; p=0.10). Heterogeneity was high (I2 = 74%) and analyzed in REM (Figure 2).
Postoperative Outcomes
Tumor Size
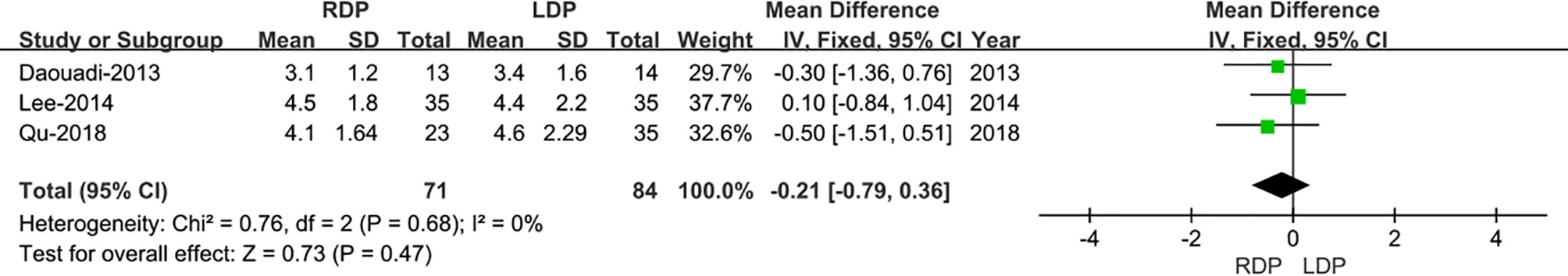
Three studies (13, 15, 16) that encompassed 155 patients (71 and 84 underwent RDP and LDP, respectively) recorded the tumor size, and pooled data didn’t show any differences in tumor size in the two approaches (WMD: −0.21; 95% CI −0.79 to 0.36; I2 = 0%, p =0.47) (Figure 3).
Short−Term Oncological Outcomes
R0 Resection Rate
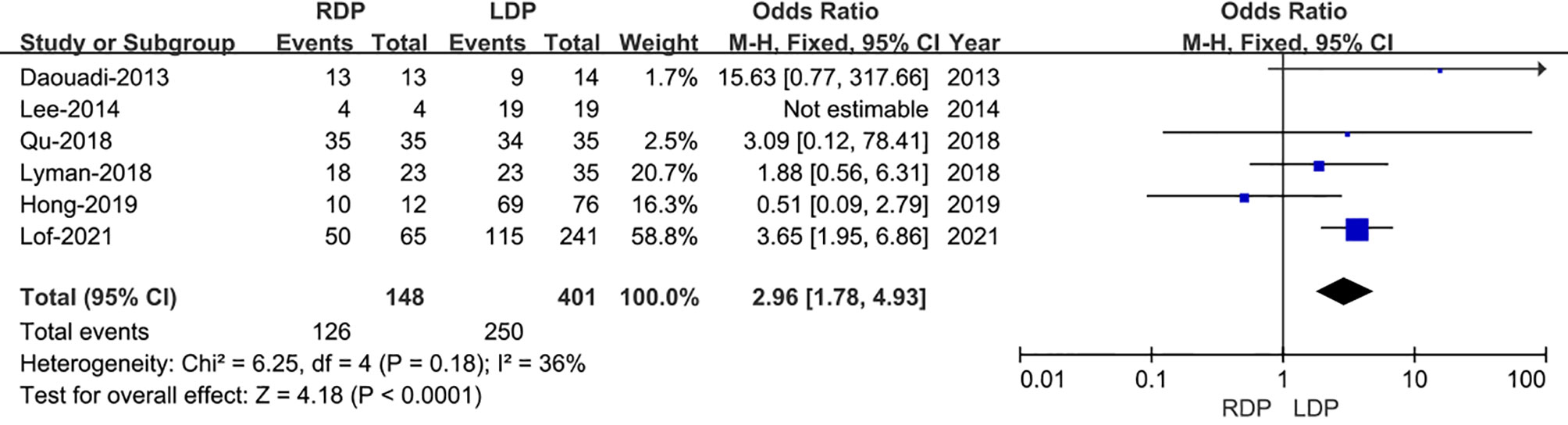
Regarding R0 resection rate, data were provided in all the six studies including 572 patients (13–18). And a meta-analysis of these data suggested that RDP was associated with a higher R0 resection rate (OR: 2.96; 95% CI 1.78–4.93; I2 = 36%, p<0.0001) as shown in the FEM (Figure 4).
Lymph Node Dissection
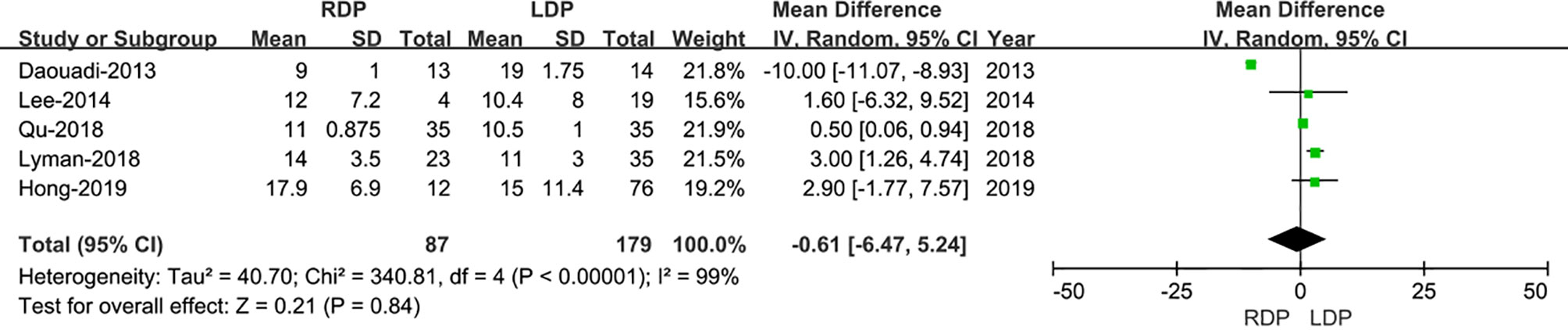
Five studies (13–17) assessed the number of lymph node dissection. These eight studies had great heterogeneity (I2 = 99%), and therefore, the REM was used. The results revealed no difference in lymph node dissection (WMD: −0.61; 95% CI −6.47 to 5.24; p= 0.84) (Figure 5).
Long−Term Oncological Outcomes
Long-Term Survival
Only two studies (15, 17) reported OS and DFS of RDP and LDP in the treatment of PDAC. Qu et al. (15) compared the survival data after PSM of 70 patients with PDAC (35 underwent RDP and 35 underwent LDP) from China and suggested that RDP and LDP can achieve a median overall survival of 27 and 25 months, respectively (p = 0.15), and the median disease-free survival (11 months vs. 11 months, respectively, p = 0.25). The largest overall survival outcomes data of RDP and LDP in the treatment of PDAC comes from Korea. Hong et al. reported 88 patients with PDAC underwent RDP or LDP (12 underwent RDP and 76 underwent LDP) and revealed a non-significant difference in median OS (not reached vs. 32.1 months, p = 0.359) and disease-free survival (11.9 vs. 14.6 months, p = 0.381) (17).
Publication Bias
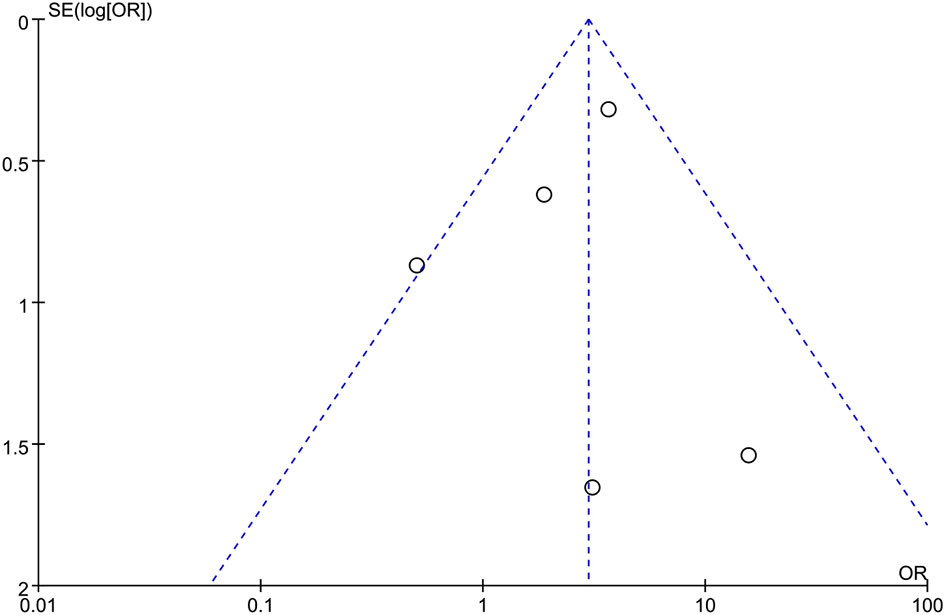
Funnel plot of R0 resection rate was drawn to investigate the potential publication bias. All of the studies lie inside the 95% Cis, and the funnel plot of R0 rate indicated no obvious publication bias (Figure 6).
Discussion
As a standard surgical method for the treatment of benign and malignant diseases of the body and tail of the pancreas, the development of distal pancreatectomy has experienced laparotomy, laparoscopy, and robot surgery. Previous investigations indicate that LDP has a shorter length of stay, less blood loss, less pain, earlier oral intake, and faster recovery in comparison with open distal pancreatectomy (19–21). As a result of the robotic system providing better visualization and reducing natural tremors, many researchers believe that robotic surgery system can conquer the technical limitations of LDP and thus potentially provide better oncological outcomes. Despite that there have been published several other meta-analyses assessing surgical and oncological outcomes between two minimally invasive techniques of distal pancreatectomy, there are no studies focusing on PDAC (22–25). To compare the real difference between RDP and LDP in the treatment of PDAC, we analyzed the data from the literature that cases were pathologically diagnosed as PDAC. To the best of our knowledge, this is the first meta-analysis that compares RDP and LDP for patients with PDAC. The present study included 572 PDAC patients (152 and 420 patients underwent RDP and LDP, respectively). In short, this meta-analysis did not detect any statistically significant differences in operative time, tumor size, and lymph node dissection. However, the R0 resection rate was significantly higher in the RDP group than in the LDP group (85.13 vs. 62.34%; p<0.0001).
Surgical margins and lymph node dissections are two important malignancy prognosis factors in distal pancreatectomy. In terms of oncologic outcome, pooled data of this meta-analysis revealed that RDP has a higher rate of R0 rection than LDP. We think that this may be explained by patients with PDAC in early stage who were selected to perform RDP. From the perspective of tumor radical effect, the results of this study show that the two surgical methods have the same effect in the number of lymph node dissections, suggesting that RDP and LDP have the same tumor radical effect, which is consistent with the results of most existing clinical studies.
When it comes to long-term survival, according to our search, there are still no RCTs comparing the long-term survival between RDP to LDP in patients with PDAC. The largest overall survival outcome data of RDP and LDP in the treatment of PDAC comes from Korea. Hong et al. reported 88 patients with PDAC underwent RDP or LDP (12 underwent RDP and 76 underwent LDP) and revealed a non-significant difference in median OS (not reached vs. 32.1 months, p = 0.359) and disease-free survival (11.9 vs. 14.6 months, p = 0.381) (17). But Qu et al. (15) compared the survival data after PSM of 70 patients with PDAC (35 underwent RDP and 35 underwent LDP) from China and suggested that RDP and LDP can achieve a median overall survival of 27 and 25 months, respectively (p = 0.15), and the median disease-free survival (11 months vs. 11 months respectively, p = 0.25). In some ways, the pooled data demonstrated that RDP is not ontologically inferior to LDP and can even achieve superior oncologic outcomes compared to LDP.
The conversion rate, overall and major complications rate, pancreatic fistula, spleen preservation rate, and costs during RDP and LDP for PDAC were not analyzed due to data being unavailable in these studies. But Kamarajah’s study that included 3,112 patients only focuses on safety of RDP and shows no significant differences in overall and major complications, overall and high-grade pancreatic fistula, and compared to LDP, RDP was associated with lower conversion rate (23). And Guerrini’s meta-analysis shows RDP was associated with higher spleen preservation rate than LDP (24).
To evaluate the safety and efficiency of RDP for PDAC, this meta-analysis included six studies and showed that RDP was comparable to LDP. However, this review has some limitations that should be considered. First, most of the included studies were retrospective research, and there were no RCTs, which may have contributed to selection bias. Furthermore, of the six included studies, the TNM stage, tumor size, and differentiation degree of patients with PDAC have not been reported in some studies. What’s more, few studies reported long-term survival outcomes such as overall survival and 3-year survival time of RDP. Therefore, further studies, in particular large-scale prospective studies and RCTs, are expected to assess the effectiveness and safety of RDP for patients with PDAC.
In conclusion, this system review and meta-analysis suggests that RDP is a technically and oncologically safe and feasible approach for PDAC patients and seems to provide a better R0 rate. Large randomized and controlled prospective studies are needed to confirm the superiority of RDP.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
QF, CJ, and WL: Study concept and design. QF and CJ: Literature review. QF, XF, and HJ: Statistical analysis. QF, ML, and YD: Draft of the manuscript and preliminary revision. YZ and JH: Study supervision and final approval. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Key Technologies R&D Program (2018YFC1106800), the Natural Science Foundation of China (82070644, 82002572, 82002967, 81972747, 81872004, 81800564, 81770615, 81700555, and 81672882).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the Outcomes of Pancreatic Cancer Surgery. Nat Rev Clin Oncol (2019) 16(1):11–26. doi: 10.1038/s41571-018-0112-1
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.2149
3. Cuschieri A, Jakimowicz JJ, van Spreeuwel J. Laparoscopic Distal 70% Pancreatectomy and Splenectomy for Chronic Pancreatitis. Ann Surg (1996) 223(3):280–5. doi: 10.1097/00000658-199603000-00008
4. Melvin WS, Needleman BJ, Krause KR, Ellison EC. Robotic Resection of Pancreatic Neuroendocrine Tumor. J Laparoendosc Adv Surg Tech A (2003) 13(1):33–6. doi: 10.1089/109264203321235449
5. Koughnett J, Jayaraman S, Eagleson R, Quan D, van Wynsberghe A, Schlachta CM. Are There Advantages to Robotic-Assisted Surgery Over Laparoscopy From the Surgeon's Perspective? J Robot Surg (2009) 3(2):79–82. doi: 10.1007/s11701-009-0144-8
6. Guerrini GP, Lauretta A, Belluco C, Olivieri M, Forlin M, Basso S, et al. Robotic Versus Laparoscopic Distal Pancreatectomy: An Up-to-Date Meta-Analysis. BMC Surg (2017) 17(1):105. doi: 10.1186/s12893-017-0301-3
7. Raoof M, Nota CLMA, Melstrom LG, Warner SG, Woo Y, Singh G, et al. Oncologic Outcomes After Robot-Assisted Versus Laparoscopic Distal Pancreatectomy: Analysis of the National Cancer Database. J Surg Oncol (2018). doi: 10.1002/jso.25170
8. Najafi N, Mintziras I, Wiese D, Albers MB, Maurer E, Bartsch DK. A Retrospective Comparison of Robotic Versus Laparoscopic Distal Resection and Enucleation for Potentially Benign Pancreatic Neoplasms. Surg Today (2020). doi: 10.1007/s00595-020-01966-z
9. De Pastena M, Esposito A, Paiella S, Surci N, Montagnini G, Marchegiani G, et al. Cost-Effectiveness and Quality of Life Analysis of Laparoscopic and Robotic Distal Pancreatectomy: A Propensity Score-Matched Study. Surg Endoscopy 2020(1):1–9. doi: 10.1007/s00464-020-07528-1
10. Moher D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Int J Surg (2010) 8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007
11. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: Comparing Reviewers' to Authors' Assessments. BMC Med Res Methodol (2014) 14:45. doi: 10.1186/1471-2288-14-45
12. Hozo SP, Djulbegovic B, Hozo I. Estimating the Mean and Variance From the Median, Range, and the Size of a Sample. BMC Med Res Methodol (2005) 5:13. doi: 10.1186/1471-2288-5-13
13. Daouadi M, Zureikat AH, Zenati MS, Choudry H, Tsung A, Bartlett DL, et al. Robot-Assisted Minimally Invasive Distal Pancreatectomy Is Superior to the Laparoscopic Technique. Ann Surg (2013) 257(1):128–32. doi: 10.1097/SLA.0b013e31825fff08
14. Lee SY, Allen PJ, Sadot E, D'Angelica MI, DeMatteo RP, Fong Y, et al. Distal Pancreatectomy: A Single Institution's Experience in Open, Laparoscopic, and Robotic Approaches. J Am Coll Surg (2015) 220(1):18–27. doi: 10.1016/j.jamcollsurg.2014.10.004
15. Qu L, Zhiming Z, Xianglong T, Yuanxing G, Yong X, Rong L, et al. Short- and Mid-Term Outcomes of Robotic Versus Laparoscopic Distal Pancreatosplenectomy for Pancreatic Ductal Adenocarcinoma: A Retrospective Propensity Score-Matched Study. Int J Surg (London England) (2018) 55:81–6. doi: 10.1016/j.ijsu.2018.05.024
16. Lyman WB, Passeri M, Sastry A, Cochran A, Iannitti DA, Vrochides D, et al. Robotic-Assisted Versus Laparoscopic Left Pancreatectomy at a High-Volume, Minimally Invasive Center. Surg Endoscopy (2019) 33(9):2991–3000. doi: 10.1007/s00464-018-6565-6
17. Hong S, Song KB, Madkhali AA, Hwang K, Yoo D, Lee JW, et al. Robotic Versus Laparoscopic Distal Pancreatectomy for Left-Sided Pancreatic Tumors: A Single Surgeon’s Experience of 228 Consecutive Cases. Surg Endosc (2020) 34:2465–73. doi: 10.1007/s00464-019-07047-8
18. Lof S, van der Heijde N, Abuawwad M, Al-Sarireh B, Boggi U, Butturini G, et al. Robotic Versus Laparoscopic Distal Pancreatectomy: Multicentre Analysis. Br J Surg (2021). doi: 10.1093/bjs/znaa039
19. Stauffer JA, Rosales-Velderrain A, Goldberg RF, Bowers SP, Asbun HJ. Comparison of Open With Laparoscopic Distal Pancreatectomy: A Single Institution's Transition Over a 7-Year Period. HPB (2013) 15(2):149–55. doi: 10.1111/j.1477-2574.2012.00603.x
20. Jarufe N, Soto P, Ahumada V, Pacheco S, Salinas J, Galindo J, et al. Laparoscopic Versus Open Distal Pancreatectomy: Comparative Analysis Ofclinical Outcomes at a Single Institution. Surg Laparosc Endosc Percutan Tech (2017) 28(1):1. doi: 10.1097/sle.0000000000000494
21. Sahakyan MA, Kim SC, Kleive D, Kazaryan AM, Song KB, Ignjatovic D, et al. Laparoscopic Distal Pancreatectomy for Pancreatic Ductal Adenocarcinoma: Long-Term Oncologic Outcomes After Standard Resection. Surgery (2017) 802. doi: 10.1016/j.surg.2017.06.009
22. Gavriilidis P, Lim C, Menahem B, Lahat E, Salloum C, Azoulay D. Robotic Versus Laparoscopic Distal Pancreatectomy – The First Meta-Analysis. HPB (2016) 18(7):567–74. doi: 10.1016/j.hpb.2016.04.008
23. Kamarajah SK, Sutandi N, Robinson SR, French JJ, White SA. Robotic Versus Conventional Laparoscopic Distal Pancreatic Resection: A Systematic Review and Meta-Analysis - ScienceDirect. HPB (2019) 21(9):1107–18. doi: 10.1016/j.hpb.2019.02.020
24. Guerrini GP, Lauretta A, Belluco C, Olivieri M, Forlin M, Basso S, et al. Robotic Versus Laparoscopic Distal Pancreatectomy: An Up-to-Date Meta-Analysis. BMC Surg (2017) 17(1):105. doi: 10.1186/s12893-017-0301-3
Keywords: distal pancreatectomy, pancreatic ductal adenocarcinoma, robotic, laparoscopic, meta-analysis
Citation: Feng Q, Jiang C, Feng X, Du Y, Liao W, Jin H, Liao M, Zeng Y and Huang J (2021) Robotic Versus Laparoscopic Distal Pancreatectomy for Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 11:752236. doi: 10.3389/fonc.2021.752236
Received: 02 August 2021; Accepted: 31 August 2021;
Published: 20 September 2021.
Edited by:
Damiano Caputo, Campus Bio-Medico University, ItalyReviewed by:
Alessandro Esposito, Verona Integrated University Hospital, ItalyFrancesco Coratti, Careggi University Hospital, Italy
Copyright © 2021 Feng, Jiang, Feng, Du, Liao, Jin, Liao, Zeng and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiwei Huang, huangjiweimd@hotmail.com
†These authors have contributed equally to this work and share first authorship
 Qingbo Feng
Qingbo Feng Chuang Jiang1†
Chuang Jiang1† Xuping Feng
Xuping Feng Wenwei Liao
Wenwei Liao Hongyu Jin
Hongyu Jin Yong Zeng
Yong Zeng Jiwei Huang
Jiwei Huang