- 1Department of Neurology 2, Kepler University Hospital GmbH, Linz, Austria
- 2Medical Faculty, Johannes Kepler University Linz, Linz, Austria
- 3Department of Neurology, Medical University of Graz, Graz, Austria
- 4Department of Neurology, Medical University of Vienna, Vienna, Austria
- 5Department of Neurology, Academic Teaching Hospital Wels-Grieskirchen, Wels, Austria
- 6Clinical Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria
Objectives: To analyze safety and impact of natalizumab (NTZ) exposure on the disease course, pregnancy, and newborn outcomes of relapsing-remitting multiple sclerosis (RRMS) patients from the Austrian Multiple Sclerosis Treatment Registry (AMSTR).
Materials and Methods: Twelve pregnancies of 11 women with RRMS exposed to treatment with NTZ were identified from the AMSTR. Exposure to NTZ was defined as treatment with NTZ from 8 weeks prior to the start of the last menstrual period and onward. All patients completed a standardized questionnaire regarding pregnancy and newborn outcomes until the postpartum period for up to 12 months.
Results: NTZ was stopped on average 46 days after the last menstrual period. There were 11 live births and one elective termination due to ectopic pregnancy. Mean gestational age of live born individuals was 39.0 weeks [standard deviation (SD) ± 1.1]. Mean birth weight and length were 3,426 g (SD ± 348) and 51.9 cm (SD ± 1.9), respectively. Apgar scores 1 min after birth were normal, with 9.2 points on average. One child displayed hip dysplasia as the only congenital malformation documented in this cohort. Three patients experienced relapses during pregnancy and three patients in the postpartum period, resulting in confirmed Expanded Disability Status Scale (EDSS) progression in four of them.
Conclusion: In this cohort, there was no increased risk concerning pregnancy and newborn outcomes due to NTZ exposure. However, relapses occurring during pregnancy and postpartum period resulted in confirmed disability.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system diagnosed predominantly in females mostly during the reproductive age (1). Female patients thus need to be counseled accordingly to balance the potential benefits and risks of drug exposure when considering treatment options before or during pregnancy (2). Importantly, there is a high degree of variability in the course of disease and its response to treatment. Natalizumab (NTZ), a therapeutic monoclonal antibody, is approved for the treatment of relapsing-remitting MS (RRMS) and is widely used in the treatment of RRMS. However, limited data are available on the outcome of exposure to NTZ during pregnancy (3–9). On the other hand, there is also limited information concerning the course of MS during pregnancy after NTZ cessation (6, 9, 10), which, however, would be needed for optimizing patient management in this particular scenario.
Our aim therefore was to analyze the impact of NTZ exposure and cessation on disease course and pregnancy outcome of pregnant MS patients documented in the Austrian Multiple Sclerosis Treatment Registry (AMSTR).
Materials and Methods
Data Collection
The AMSTR had been established in 2006 as part of MS network activities in Austria, consisting of all MS clinics and some specially qualified neurological practices (“MS-centres,” in total comprising ~4,300 patients). It maintains quality control and compliance with reimbursement regulations including treatment indications of the Austrian sick funds and contains the clinical profiles of the included patients and MRI and safety data (11–13). In Austria, prescriptions of disease-modifying therapies (DMTs) for MS are exclusively reserved for MS centers. Thus, one can expect data to be evenly collected all over Austria. The AMSTR is compliant with Austrian laws on bioethics, and it also was approved by the ethical committee of the Medical University of Vienna (EC number 2096/2013). The present study on pregnant women exposed to NTZ had been approved by the ethical committee of the State of Upper Austria (EC application number K-66-15).
The AMSTR anonymously documents baseline data such as MS onset and duration, relapses during the 12 months before baseline, the Expanded Disability Status Score [EDSS], gross MRI activity, and use of previous DMTs. Follow-up data (relapses, EDSS, adverse events, change or discontinuation of treatment) are required to be documented every 3–6 months.
Each suspected relapse is confirmed by a specialized neurologist from an MS center and documented in the AMSTR. Relapses are defined as new or worsening neurological symptoms lasting for at least 24 h in the absence of fever. Documentation requires relapse onset, EDSS, and use/dose of intravenous (i.v.) methylprednisolone treatment. Confirmed disability progression is defined as an increase from baseline of at least 1.0 point in the EDSS (or at least 0.5 points for patients with a baseline EDSS higher than 5.5) persisting for at least 24 weeks.
Besides the fact that registration in the AMSTR is mandatory for reimbursement of therapy costs, independent external data monitoring concerning completeness and plausibility of documented data represents a quality-related feature of the AMSTR.
In 2006, the European Medicines Agency (EMA) approved NTZ for RRMS patients. Reimbursement for NTZ in Austria adheres to this approval. Thus, MS patients to be treated with NTZ have to have either a minimum of one relapse in the prior 12 months under treatment with interferon beta or glatiramer acetate and at least 9 T2 lesions or one gadolinium-enhancing lesion on a recent brain MRI (“indication A”) or, if the person has been previously untreated, two or more severe relapses in the preceding 12 months and one or more gadolinium-enhancing lesions on brain MRI or a significant increase in T2 lesion load as compared to a previous recent MRI (“indication B”).
For the present analyses, we selected all female MS patients treated with NTZ within the AMSTR with confirmed pregnancy between 2006 and the end of the year 2016. Exposure to NTZ was defined as treatment with NTZ from 8 weeks prior to the start of the last menstrual period (LMP) onward. Patients were followed until 12 months postpartum. A standardized questionnaire on patient- and pregnancy-related outcome as well as child-specific data was completed by the treating neurologist (Table 1).

Table 1. Questionnaire on patient- and pregnancy-related data as well as child-specific data and postpartum period.
The primary outcome measures were (a) safety and impact of NTZ exposure on pregnancy and newborn outcomes of RRMS patients and (b) the impact on disease course during pregnancy and postpartum period.
Statistical Methods
Descriptive statistics, including mean ± standard deviation (SD), as well as range were used to describe the data.
Results
Eleven women with 12 pregnancies under NTZ treatment had been registered between 2006 and the end of December 2016. One patient became pregnant twice, and both pregnancies were included in this analysis.
In this cohort, mean age at diagnosis of MS was 25 years (SD ± 5.5) and 30 years (SD ± 5.4) at LMP. All women had received NTZ as second- or third-line therapy (“indication A”), with an average of 22 (SD ± 9.6) and a minimum of seven administrations before pregnancy. Treatment before NTZ included beta-interferons, glatiramer acetate, and fingolimod. A stable course of disease had been achieved with NTZ in all women prior to pregnancy, without an increase in the EDSS score at pregnancy onset (mean 1.3, SD ± 1.1) compared to the EDSS 1 year before (mean 1.3, SD ± 1.1). NTZ infusions were terminated after a mean interval of 46 days (SD ± 38) after LMP. In one patient, treatment was continued after confirmed pregnancy until the 20th week of pregnancy (Day 137) because of high disease activity before NTZ start and suspected rebound after NTZ cessation.
Maternal baseline data are summarized in Table 2.
Eight women were primiparous. Eleven live births and one elective termination/miscarriage due to ectopic pregnancy occurred. There was no stillbirth or neonatal death. Vaginal delivery was performed in seven (63.6%) compared to cesarean section in four women (36.4%). A total of five patients (45.5%) received epidural anesthesia. Mean gestational age of live births was 39.0 weeks (SD ± 1.1).
Most children were delivered as early terms (between 37 weeks/0 days and 38 weeks/6 days). There was no preterm delivery (before 37 weeks/0 days).
Three patients suffered from an MS relapse during pregnancy. NTZ was terminated in two cases 43 and 51 days after the LMP; in one case, the relapse occurred during NTZ treatment (neutralizing antibodies against NTZ negative). All relapses occurred at the end of the first trimester. One of these relapses was treated with standard high-dose methylprednisolone and another one with i.v. immunoglobulins to avoid adverse events with high-dose methylprednisolone. Two of these three patients showed confirmed EDSS progression (from EDSS 1.0–2.0 and EDSS 1.5–2.5, respectively) (Figures S1, S2).
Apart from treatment of relapses, there was no further documented exposure to any other potential toxin. Pregnancy outcomes are shown in Table 3.
Six newborns were female. Mean birth weight was 3,426 g (SD ± 348) and length was 51.9 cm (SD ± 1.9). Apgar score 1 min after birth was within the normal range, with a minimum score of 8 points and 9.2 points on average. One child displayed hip dysplasia, which was the only documented congenital malformation in this cohort.
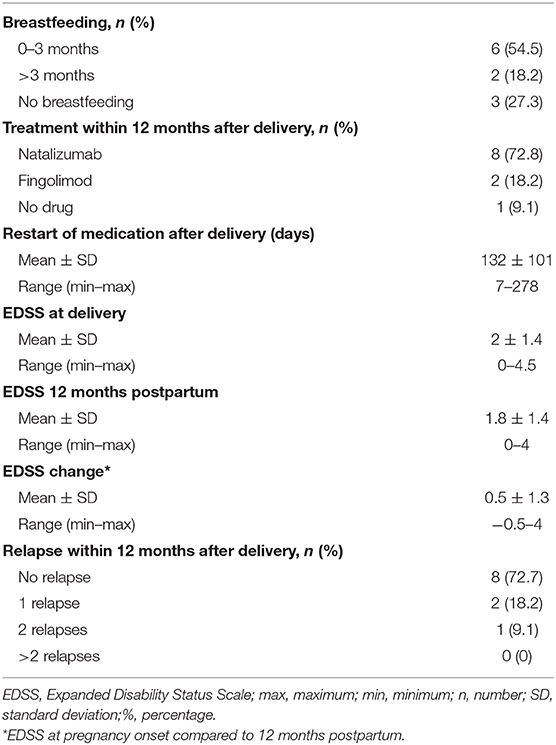
Eight of 11 women decided to breastfeed at least for 3 months; two of them continued breastfeeding until the second control postpartum.
Eight patients received NTZ for an average of 113 (SD ± 103, range 7–278) days after delivery (as primary choice of therapy), while two patients started with fingolimod 180 and 240 days after delivery, respectively.
The mean EDSS score 12 months postpartum compared to the EDSS score at pregnancy onset showed a deterioration of 0.5 (SD ± 1.3). Three patients suffered from relapses in the postpartum period before restarting NTZ, resulting in a sustained EDSS progression in two of them. The first patient experienced a relapse 3 months after delivery and deteriorated from EDSS 0.0 to 4.0; the other patient had a relapse at 6 months postpartum with an EDSS worsening from 1.0 to 3.0 (Figures S3, S4).
In contrast, two patients experienced an EDSS improvement (Table 4).
Discussion
So far, limited data are available on pregnancies in MS patients with NTZ (3–10) Ebrahimi et al. (4) documented 102, Friend et al. (7) 355, and Portaccio et al. (8) 92 pregnancies under NTZ treatment. The spontaneous abortion rate of MS women who received NTZ during pregnancy was similar to that of the general population; however, birth defect rates seemed slightly higher than that in the general population and disease-matched groups (6).
In our study, NTZ was continued for a mean of 46 days after LMP with no further negative impact on pregnancy and delivery. This fact corroborates previous publications showing similar results. Out of 12 pregnancies, there was one ectopic pregnancy and hip dysplasia, respectively.
In comparison to healthy controls, the observed birth weight was lower in this cohort, which has been reported previously among women who became pregnant under NTZ (4, 6, 7, 14–16). There was no newborn with a low birth weight (<2,500 g) nor was there a preterm delivery in our cohort.
The EDSS score remained stable in all patients who had no relapses during pregnancy or postpartum period. In contrast, four of six patients who had suffered from relapses during pregnancy or the postpartum period showed a confirmed EDSS progression. Also in this respect, our study further affirms previous publications (4, 9, 10, 17). This underlines the importance to shorten the treatment gap to reduce the maternal risk of experiencing disease activity during pregnancy or thereafter.
Our study has several limitations. Apart from the retrospective nature of the study, the sample size of women was small. However, this study contributes to the still limited number of reported pregnancies with prior exposure to NTZ with further 12 cases. In a recent review regarding NTZ exposure during pregnancy in multiple sclerosis, several studies with a similar or even smaller number of pregnancies as in our work were included and added valuable information to the review (6). Clearly, higher sample sizes would allow to draw firmer conclusions. Therefore, our study should stimulate further investigations into this area, e.g., by collaborative efforts across academic centers and countries.
In conclusion, NTZ seems to be safe in early pregnancy; however, termination of NTZ in patients with active RRMS may harbor the risk of reoccurrence of disease activity with potential disability.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by ethical committee of the State of Upper Austria (EC application number K-66-15). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MG contributed patients, performed the statistic, and wrote the manuscript. GR performed the statistic and revised the manuscript. TB, FD, DO, FL, CE, and DL-P contributed patients and revised the manuscript. MD contributed patients and performed the statistic. GT performed the statistic and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
MG received support and honoraria for research, consultation, lectures and education from Almirall, Bayer, Biogen, Celgene, Genzyme, MedDay, Merck, Novartis, Octapharma, Roche, Sanofi Aventis, Shire and TEVA ratiopharm. GT received support and honoraria for research, lectures and education from Bayer, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, EVER Pharma, Genzyme, Merz Pharma, Novartis, Sanofi-Aventis, Pfizer and TEVA. DL-P received support and honoraria for research, lectures and education from Roche and Sanofi-Aventis. CE has received funding for travel and speaker honoraria from Biogen, Bayer, Merck Serono, Novartis, Shire, Genzyme and Teva Pharmaceutical Industries Ltd./sanofi-aventis; research support from Merck Serono, Biogen, and Teva Pharmaceutical Industries Ltd./sanofi-aventis; and has served on scientific advisory boards for Bayer Schering, Biogen, Celgene, Merck Serono, Novartis, Roche and Teva Pharmaceutical Industries Ltd./sanofi-aventis. FL received support and honoraria for research, consultation, lectures and education from Bayer, Biogen, Celgene, Genzyme, MedDay, Merck, Novartis, Octapharma, Roche, Sanofi Aventis, and TEVA ratiopharm. DO received honoraria for lectures and congress support by Biogen, Genzyme and Novartis. FD has participated in meetings sponsored by, received honoraria (lectures, advisory boards, consultations), or travel funding from Bayer, Biogen, Merck, Novartis, Sanofi-Genzyme, Teva, Celgene and Roche. TB has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for multiple sclerosis: Almirall, Bayer, Biogen, Biologix, Bionorica, Genzyme, MedDay, Merck, Novartis, Octapharma, Roche, Sanofi/Genzyme, TG Pharmaceuticals, TEVA-ratiopharm and UCB. His institution has received financial support in the last 12 months by unrestricted research grants (Biogen, Bayer, Merck, Novartis, Sanofi/Genzyme, and TEVA ratiopharm) and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi/Genzyme, and TEVA. GR received consultation honoraria and congress support by Biogen, Genzyme, Novartis, Roche and Teva.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The study group wishes to thank all Austrian MS centers for contributing data to the registry and the patients for providing written informed consent.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.00676/full#supplementary-material
References
1. Alcalde-Cabero E, Almazán-Isla J, García-Merino A, de Sá J, de Pedro-Cuesta J. Incidence of multiple sclerosis among European economic area populations, 1985-2009: the framework for monitoring. BMC Neurol. (2013) 13:58. doi: 10.1186/1471-2377-13-58
2. Coyle PK. Management of women with multiple sclerosis through pregnancy and after childbirth. Ther Adv Neurol Disord. (2016) 9:198–210. doi: 10.1177/1756285616631897
3. Hellwig K, Haghikia A, Gold R. Pregnancy and natalizumab: results of an observational study in 35 accidental pregnancies during natalizumab treatment. Mult Scler. (2011) 17:958–63. doi: 10.1177/1352458511401944
4. Ebrahimi N, Herbstritt S, Gold R, Amezcua L, Koren G, Hellwig K. Pregnancy and fetal outcomes following natalizumab exposure in pregnancy. A prospective, controlled observational study. Mult Scler. (2015) 21:198–205. doi: 10.1177/1352458514546790
5. Hellwig K, Brune N, Haghikia A, Müller T, Schimrigk, Schwödiauer V, et al. Reproductive counselling, treatment and course of pregnancy in 73 German MS patients. Acta Neurol Scand. (2008) 118:24–8. doi: 10.1111/j.1600-0404.2007.00978.x
6. Peng A, Qiu X, Zhang L, Zhu X, He S, Lai W, et al. Natalizumab exposure during pregnancy in multiple sclerosis: a systematic review. J Neurol Sci. (2019) 396:202–5. doi: 10.1016/j.jns.2018.11.026
7. Friend S, Richman S, Bloomgren G, Cristiano LM, Wenten M. Evaluation of pregnancy outcomes from the Tysabri® (natalizumab) pregnancy exposure registry: a global, observational, follow-up study. BMC Neurol. (2016) 16:150. doi: 10.1186/s12883-016-0674-4
8. Portaccio E, Annovazzi P, Ghezzi A, Zaffaroni M, Moiola L, Martinelli V, et al. Pregnancy decision-making in women with multiple sclerosis treated with natalizumab: I: fetal risks. Neurology. (2018) 90:e823–31. doi: 10.1212/WNL.0000000000005067
9. Portaccio E, Moiola L, Martinelli V, Annovazzi P, Ghezzi A, Zaffaroni M, et al. Pregnancy decision- making in women with multiple sclerosis treated with natalizumab: II: maternal risks. Neurology. (2018) 90:e832–9. doi: 10.1212/WNL.0000000000005068
10. Bsteh G, Algrang L, Hegen H, Auer M, Wurth S, Di Pauli F, et al. Pregnancy and multiple sclerosis in the DMT era: a cohort study in Western Austria. Mult Scler. (2020) 26:69–78. doi: 10.1177/1352458518816614
11. Guger M, Enzinger C, Leutmezer F, Kraus J, Kalcher S, Kvas E, et al. Real-life use of oral disease-modifying treatments in Austria. Acta Neurol Scand. (2019) 140:32–9. doi: 10.1111/ane.13097
12. Guger M, Enzinger C, Leutmezer F, Kraus J, Kalcher S, Kvas E, et al. Real-life clinical use of natalizumab and fingolimod in Austria. Acta Neurol Scand. (2018) 137:181–7. doi: 10.1111/ane.12864
13. Guger M, Enzinger C, Leutmezer F, Kraus J, Kalcher S, Kvas E, et al. Switching from natalizumab to fingolimod treatment in multiple sclerosis: real life data from the Austrian MS treatment Registry. J Neurol. (2019) 266:2672–7. doi: 10.1007/s00415-019-09464-0
14. Voigt M, Fusch C, Olbertz D, Hartmann K, Rochow N, Renken C, et al. Analyse des Neugeborenenkollektivs der Bundesrepublik Deutschland. 12. Mitteilung: vorstellung engmaschiger Perzentilwerte (-kurven) für die Körpermaße Neugeborener. Geburtsh Frauenheilk. (2006) 66:956–70. doi: 10.1055/s-2006-924458
15. Dahl J, Myhr KM, Daltveit AK, Gilhus NE. Planned vaginal births in women with multiple sclerosis: delivery and birth outcome. Acta Neurol Scand Suppl. (2006) 183:51–4. doi: 10.1111/j.1600-0404.2006.00616.x
16. Dahl J, Myhr KM, Daltveit AK, Gilhus NE. Pregnancy, delivery and birth outcome in different stages of maternal multiple sclerosis. J Neurol. (2008) 255:623–7. doi: 10.1007/s00415-008-0757-2
Keywords: breastfeeding, fetal safety, multiple sclerosis, natalizumab, pregnancy outcome
Citation: Guger M, Traxler G, Drabauer M, Leitner-Pohn D, Enzinger C, Leutmezer F, Oel D, Di Pauli F, Berger T and Ransmayr G (2020) Pregnancy Outcomes in Patients With Multiple Sclerosis Exposed to Natalizumab—A Retrospective Analysis From the Austrian Multiple Sclerosis Treatment Registry. Front. Neurol. 11:676. doi: 10.3389/fneur.2020.00676
Received: 06 March 2020; Accepted: 05 June 2020;
Published: 04 August 2020.
Edited by:
Valentina Tomassini, University of Studies G. d'Annunzio Chieti and Pescara, ItalyReviewed by:
Alessandra Lugaresi, IRCCS Institute of Neurological Sciences of Bologna (ISNB), ItalyEmanuele D'Amico, University of Catania, Italy
Copyright © 2020 Guger, Traxler, Drabauer, Leitner-Pohn, Enzinger, Leutmezer, Oel, Di Pauli, Berger and Ransmayr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Guger, michael.guger@kepleruniklinikum.at
 Michael Guger
Michael Guger Gerhard Traxler1
Gerhard Traxler1 Christian Enzinger
Christian Enzinger Fritz Leutmezer
Fritz Leutmezer Thomas Berger
Thomas Berger Gerhard Ransmayr
Gerhard Ransmayr