Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19
- 1Cancer Center, Renmin Hospital of Wuhan University, Hubei Provincial Research Center for Precision Medicine of Cancer, Wuhan, China
- 2Cardiac Care Unit, Renmin Hospital of Wuhan University, Wuhan, China
- 3Department of Clinical Laboratory, Renmin Hospital of Wuhan University, Wuhan, China
- 4Institute of Cancer, Xinqiao Hospital, Army Medical University, Chongqing, China
- 5Department of Oncology, The First Affiliated Hospital of Shandong First Medical University, Jinan, China
Introduction: A recently emerging respiratory disease named coronavirus disease 2019 (COVID-19) has quickly spread across the world. This disease is initiated by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and uncontrolled cytokine storm, but it remains unknown as to whether a robust antibody response is related to clinical deterioration and poor outcome in COVID-19 patients.
Methods: Anti-SARS-CoV-2 IgG and IgM antibodies were determined by chemiluminescence analysis (CLIA) in COVID-19 patients at a single center in Wuhan. Median IgG and IgM levels in acute and convalescent-phase sera (within 35 days) for all included patients were calculated and compared between severe and non-severe patients. Immune response phenotyping based on the late IgG levels and neutrophil-to-lymphocyte ratio (NLR) was characterized to stratified patients into different disease severities and outcomes.
Results: A total of 222 patients were included in this study. IgG was first detected on day 4 of illness, and its peak levels occurred in the fourth week. Severe cases were more frequently found in patients with high IgG levels, compared to those with low IgG levels (51.8 vs. 32.3%; p = 0.008). Severity rates for patients with NLRhiIgGhi, NLRhiIgGlo, NLRloIgGhi, and NLRloIgGlo phenotype were 72.3, 48.5, 33.3, and 15.6%, respectively (p < 0.0001). Furthermore, severe patients with NLRhiIgGhi, NLRhiIgGlo had higher inflammatory cytokines levels including IL-2, IL-6 and IL-10, and decreased CD4+ T cell count compared to those with NLRloIgGlo phenotype (p < 0.05). Recovery rates for severe patients with NLRhiIgGhi, NLRhiIgGlo, NLRloIgGhi, and NLRloIgGlo phenotype were 58.8% (20/34), 68.8% (11/16), 80.0% (4/5), and 100% (12/12), respectively (p = 0.0592). Dead cases only occurred in NLRhiIgGhi and NLRhiIgGlo phenotypes.
Conclusions: COVID-19 severity is associated with increased IgG response, and an immune response phenotyping based on the late IgG response and NLR could act as a simple complementary tool to discriminate between severe and non-severe COVID-19 patients, and further predict their clinical outcome.
Introduction
Since December 2019, coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has quickly spread across the world (Wu et al., 2020). Approximately 20–30% of cases would develop severe illness, and some need further intervention in intensive care unit. Organ dysfunction including acute respiratory distress syndrome, shock, acute cardiac injury, and acute renal injury, could occur in severe cases with COVID-19, which lead to poor clinical outcome (Huang et al., 2020; Wang et al., 2020). Following SARS-CoV-2 infection, a high viral load and overexuberant host immune response involving innate and acquired immunity, simultaneously contributes to the pathogenesis of COVID-19 and organ injury (Huang et al., 2020; Wang et al., 2020; Xu et al., 2020). The activated host immunity is characterized as lymphopenia, cytokine release storm (CRS), and dysfunctional immune responses to virus-specific antigen. Increasing clinical data indicated that the neutrophil-to-lymphocyte ratio (NLR) was identified as a powerful predictive and prognostic indicator for severe COVID-19. However, the dynamic of anti-SARS-CoV-2 antibody upon virus infection and their relation to disease status and outcome remain to be determined (Liu et al., 2020a).
Here, we evaluated antibody response within 35 days after symptom onset in laboratory-confirmed cases with COVID-19 as one component of an overall exaggerated immune activation in severe SARS-CoV-2 infection, and developed an immune phenotyping based on the late IgG response and NLR that could help determine disease severity and clinical outcome for COVID-19 patients.
Methods
All included patients with COVID-19 had been admitted to the Renmin Hospital of Wuhan University, from January 13, 2020 to March 1, 2020. A total of 222 laboratory-confirmed COVID-19 patients were included in this study. The confirmed diagnosis of COVID-19 was defined as a positive result by using real-time reverse-transcriptase polymerase-chain-reaction (RT-PCR) detection for routine nasal and pharyngeal swab specimens or anti-SARS-CoV-2 antibody assay. Serum samples were collected at admission or convalescent-phase and were dated from the day of initial symptom onset. We retrospectively evaluated their anti-SARS-CoV-2 antibody response, clinical disease severity, and clinical outcome. This study received approval from the Research Ethics Committee of the Renmin Hospital of Wuhan University, Wuhan, China (approval number: WDRY2020-K094). The Research Ethics Committee waived the requirement informed consent before the study started because of the urgent need to collect epidemiological and clinical data. We analyzed all the data anonymously.
The clinical features, including clinical symptoms, signs, laboratory analyses, treatment, and outcomes, were obtained from the hospital's electronic medical records according to previously designed standardized data collection forms. The date of symptom onset, initial diagnosis of COVID-19, and death were recorded accurately. To increase the accuracy of collected data, two researchers independently reviewed the data collection forms. We also directly communicated with patients or their family members to ascertain the epidemiological and symptom data.
Anti-IgG and anti-IgM antibodies were detected using Human SARS-CoV-2 IgG and IgM Chemiluminescence Analysis (CLIA) Assays panel (Shenzhen YHLO Biotech Co.,Ltd., Shenzhen, China) and the high-speed CLIA system iFlash 3000 (Shenzhen YHLO Biotech Co.,Ltd., Shenzhen, China). Inflammatory cytokines including interleukin (IL)-2, IL-4, IL-6, IL-10, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) were detected using Human Cytokine Standard Assays panel (ET Healthcare, Inc., Shanghai, China) and the Bio-Plex 200 system (Bio-Rad, Hercules, CA, USA) according to the manufacturer's instructions. NLR was calculated by dividing the absolute neutrophil count by the lymphocyte count. For flow cytometric analysis, peripheral blood samples from patients were processed in the Department of Clinical Laboratory, Renmin Hospital of Wuhan University, to isolate peripheral blood mononuclear cells (PBMCs) by density gradient centrifugation, then isolated PBMCs were stained with a BD multitest IMK Kit (Cat340503, BD Biosciences, CA) and measured by a BD FACSCalibur flow cytometer (BD FACSCalibur, BD Biosciences, CA) to analyze the cell number of CD3+, CD4+, and CD8+ T cells.
Descriptive analyses were used to determine the patients' epidemiological and clinical features. Continuous variables were presented as median and interquartile range (IQR), and categorical variables were expressed as the percentages in different categories. Means for continuous variables were compared using independent group t-tests when the data were normally distributed; otherwise, the Mann-Whitney test was used. The Chi-squared test or Fisher's exact test was adopted for category variables. Statistical analyses in this study were performed with use of STATA 15.0 software (Stata Corporation, College Station, TX, USA). A two-sided p < 0.05 was considered statistically significant.
Results
A total of 222 patients with a diagnosis of laboratory-confirmed COVID-19 recorded in the Renmin Hospital of Wuhan University were analyzed. Median age was 62 years (IQR; range from 52 to 69 years), and 48.2% of patients were male. 39.2% of patients were severe at the time of sampling. As of March 12, 2020, five patients (2.3%) died. A total of 121 patients (54.5%) required supplemental oxygen at some stage of illness. A total of 111 patients were administrated with high-dose corticosteroid. The number of patients receiving mechanical ventilation and administration of intravenous immunoglobin were 31 (14.0%) and 123 (55.4%), respectively. One hundred ninety-four patients recovered from this infected disease, and 59 severe patients recovered by anti-viral and supported therapy.
All patients had convalescent-phase sera for analysis. Of these, 98.6% of patients had anti-SARS-CoV-2-IgG detected in sera, and 82.0% had anti-SARS-CoV-2-IgM detected in sera. As shown in Figure 1A, IgG was first detected on day 4 of illness, and its peak levels occurred in the fourth week, whereas IgM was first detected on day 3 of illness, and its peak levels occurred in the second week. Median IgG and IgM levels in convalescent-phase sera (within 35 days) for all included patients were compared between severe and non-severe patients. Higher IgM levels were detected in patients with severe disease compared to those with non-severe disease at early stage (<14 days), whereas higher IgG levels were detected at late stage (≥21 days) (Figures 1B,C). We used median as cut-off value to stratify high and low levels of IgM and IgG. Interestingly, severe cases were more frequently occurred in patients with low IgM levels (<34.1 AU/mL) than those with high IgM levels (≥3.04 AU/mL) (81.3 vs. 40%; p = 0.024) (Figure 1D). Severe cases were more frequently found in patients with high IgG levels (≥116.9 AU/mL), compared to those with low IgG levels (<116.9 AU/mL) (51.8 vs. 32.3%; p = 0.008) (Figure 1E).
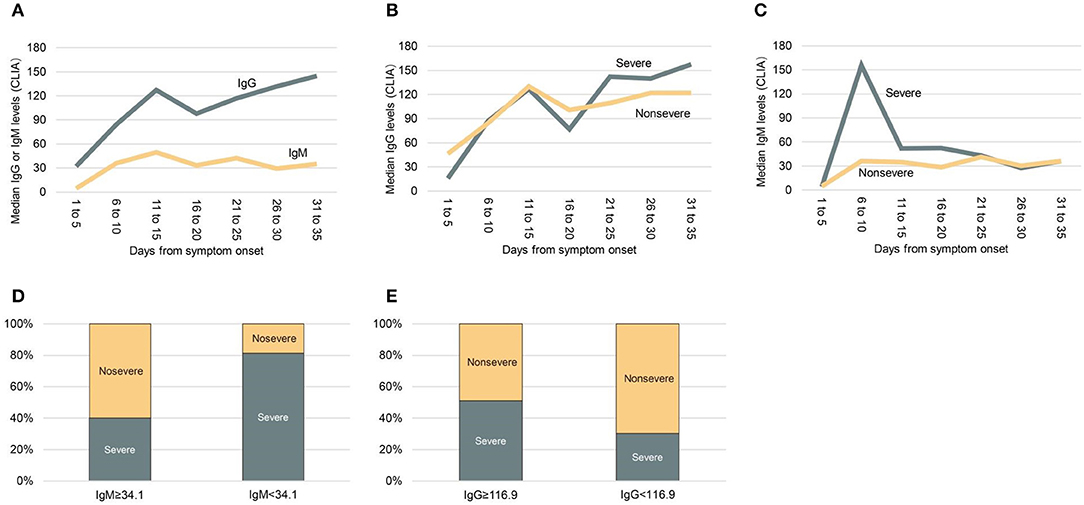
Figure 1. Median anti-SARS-CoV-2 IgG and IgM levels in patients with severe or non-severe illness within 35 days after symptom onset. (A) Median IgG and IgM levels in all patients. (B) Comparing median IgG levels between severe and non-severe patients. (C) Comparing median IgM levels between severe and non-severe patients. (D) Comparing the frequency of severity and non-severity between patients with low IgM levels (<34.1 AU/mL) or high IgM levels (≥3.04 AU/mL). (E) Comparing the frequency of severity and non-severity between patients with low IgG levels (<116.9 AU/mL) or high IgG levels (≥116.9 AU/mL). CLIA, chemiluminescence analysis.
Considering NLR is linked to innate immunity, and anti-IgG response is an indicator of acquired immunity, we stratified patients at late stage into four different immune response phenotypes: high NLR and high IgG levels (NLRhiIgGhi), high NLR and low IgG levels (NLRhiIgGlo), low NLR and high IgG levels (NLRloIgGhi), and low NLR and high IgG levels (NLRloIgGlo), according to NLR (cutoff: 3.04) and detected IgG levels (cutoff: 116.9 AU/mL). Severity rates for patients with NLRhiIgGhi, NLRhiIgGlo, NLRloIgGhi, and NLRloIgGlo phenotypes were 72.3% (34/47), 48.5% (16/33), 33.3% (12/36), and 15.6% (5/32), respectively (p < 0.0001) (Figure 2A).
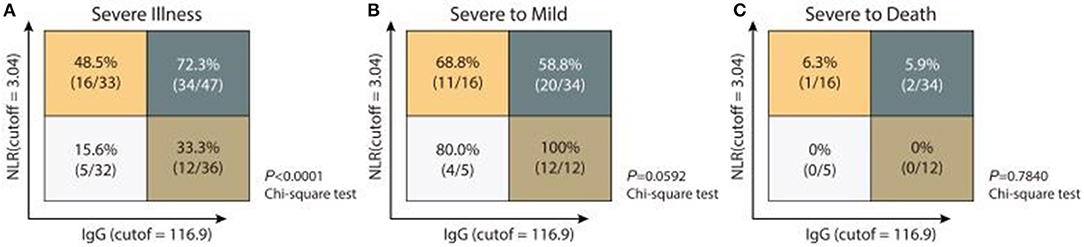
Figure 2. Immune response phenotyping with diverse disease severity according to NLR and IgG levels. Analyzing the frequencies of severe illness (A), severe to mild (B), or severe to death (C) in patients with four individual immune response phenotyping based on low or high IgG and IgM levels.
We next asked whether inflammatory cytokines levels and T cell count were consistent with the above immune phenotyping. As shown in Table 1, severe patients had higher inflammatory cytokines levels including IL-2, IL-6, and IL-10 than non-severe patients, especially in the NLRhiIgGhi or NLRhiIgGlo phenotype (p < 0.05). Furthermore, severe patients with the NLRhiIgGhi or NLRhiIgGlo phenotype had higher inflammatory cytokines levels including IL-2, IL-6, and IL-10, and decreased CD4+ T cell count, compared to those with the NLRloIgGlo phenotype (p < 0.05). In particular, only IgG and IgM were higher in severe patients with the NLRloIgGhi phenotype than those in the NLRloIgGlo phenotype (p < 0.05).
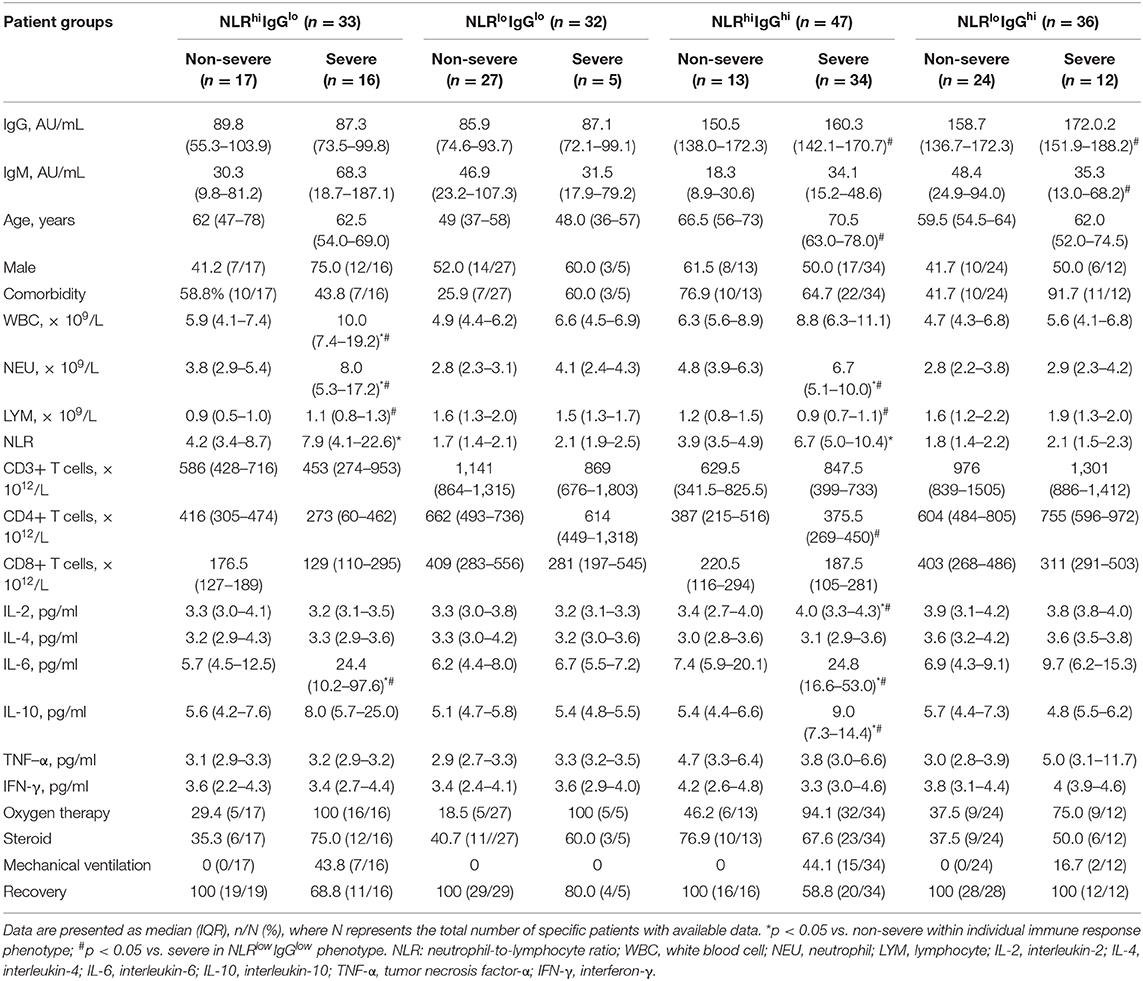
Table 1. Clinical characteristics, treatment, and outcome of COVID-19 patients with different immune response phenotypes.
We also analyzed the treatment and clinical outcome for patients with different combined immune response phenotypes. Follow up the patients so far, 44.1% of patients with NLRhiIgGhi phenotype, 43.8% of patients NLRhiIgGlo phenotype, 16.7% of patients with NLRloIgGhi phenotype received mechanical ventilation, but no patients with NLRloIgGlo phenotype were treated with mechanical ventilation. All non-severe patients did not develop severe disease. Recovery rates for severe patients with NLRhiIgGhi, NLRhiIgGlo, NLRloIgGhi, and NLRloIgGlo phenotype were 58.8% (20/34), 68.8% (11/16), 80.0% (4/5), and 100% (12/12), respectively (p = 0.0592) (Figure 2B). Dead cases only occurred in the population with the NLRhiIgGhi or the NLRhiIgGlo phenotypes (Figure 2C). However, too few severe cases at early stage being collected convalescent sera to allow separate analysis of anti-IgM response.
Discussion
In this study, we found enhanced IgM levels at early stage, and high IgM levels were more frequently found in patients with severe disease. IgG levels increased at late stage, whereas high levels of IgG were frequently found in patients with severe disease. These results indicated that beside the antiviral efficacy, the antibody response might be associated with secondary antibody-mediated organ damage. Using two immune response-related indicators NLR and IgG levels detected in sera at late stage, we developed a combined immune response phenotype, which could predict disease severity and the outcome of COVID-19 patients. To our knowledge, this is the first in the literatures to combine indicators from innate and acquired immunity to predict disease severity and outcome.
Anti-SARS-CoV-2-IgG could be detected at day 4 after onset, and it had peak levels at the fourth week, which was similar to the Anti-SARS-CoV-IgG response profile (Chan et al., 2004). Previous data showed severe SARS was associated more robust serological responses including early seroconversion (< day 16) and higher IgG levels (Lee et al., 2006). Low antibody titres were observed in patients with mild SARS or diseases with other viral infection (Zhang et al., 2006; Monsalvo et al., 2011; To et al., 2012). Be consistent with findings, present study supported that a robust humoral response to SARS-CoV-2 correlated with disease severity. Furthermore, we believe that this robust acquired anti-IgG response not only contributes to viral clearance, but also leads to robust immune-mediated tissue damage.
Lymphopenia, neutrophilia, and high NLR are commonly presented and associated with more severe viral infection (Zhu, 2004; Liu et al., 2020b; Wang et al., 2020). Recent data indicated that the NLR was identified as a powerful predictive and prognostic factor for severe COVID-19 (Liu et al., 2020a). Thus, we developed simple combined immune response phenotypes using NLR, an indicator of innate immunity, and IgG, an indicator of acquired immunity. These four individual immune response phenotypes could be useful to distinguish the severe from the non-severe cases and predict their clinical outcome. We observed more severe cases occurred in NLRhiIgGhi or NLRhiIgGlo phenotype, with only three dead cases. Patients with NLRhiIgGhi or NLRhiIgGlo appeared to be difficult to recover from severe SARS-CoV-2 infection, compared to NLRloIgGhi or NLRloIgGlo phenotype. A third of patients with NLRloIgGhi had critical illness, suggesting that IgG response alone could lead to disease deterioration. Furthermore, high Th1 cytokine IFN-γ, inflammatory cytokines proinflammatory cytokines IL-6 and TNF-α, and anti-inflammatory cytokine IL-10 were noted in NLRhiIgGhi or NLRhiIgGlo phenotype. In fact, proinflammatory and an opposing anti-inflammatory response occur concomitantly in virus infection as well as sepsis (Rivers et al., 2001; Zhang C. et al., 2020). The concentrations of the potent anti-inflammatory cytokine IL-10 were also increased. A high ratio of IL-10 to TNF-α correlated with mortality in patients with community acquired infection (Rivers et al., 2001). Accordingly, we speculated that anti-IgG humoral response directly contributed to tissue damage. In SARS-CoV/macaque models, Liu et al. found anti-spike IgG, the presence of high anti-spike IgG prior to viral clearance, abrogated wound-healing responses, and promoted inflammatory cytokines monocyte chemotactic protein 1 and IL-8 production and monocyte/macrophage recruitment and accumulation, eventually cause fatal acute lung injury during SARS-CoV infection (Liu et al., 2019).
Thus, we proposed potential mechanisms associated with different immune response phenotypes and presented specific treatment recommendations which would be helpful in guiding clinical decision (Figure 3). For example, in the NLRhiIgGlo group, both viruses directly mediated tissue damage and CRS are responsible for disease development. In this scenario, anti-virus, tocilizumab, and serum from convalescent should be considered. However, serum from convalescent should only be used in patients with low IgG levels and should not be used in the patients with high IgG levels since its use might aggravate the disease especially in the patients with NLRhiIgGhi phenotype (Greenough et al., 2005). Tocilizumab treatment might not be beneficial for patients with low NLR, because these patients are in the immunosuppression stage rather than in CRS stage (Singer et al., 2016).
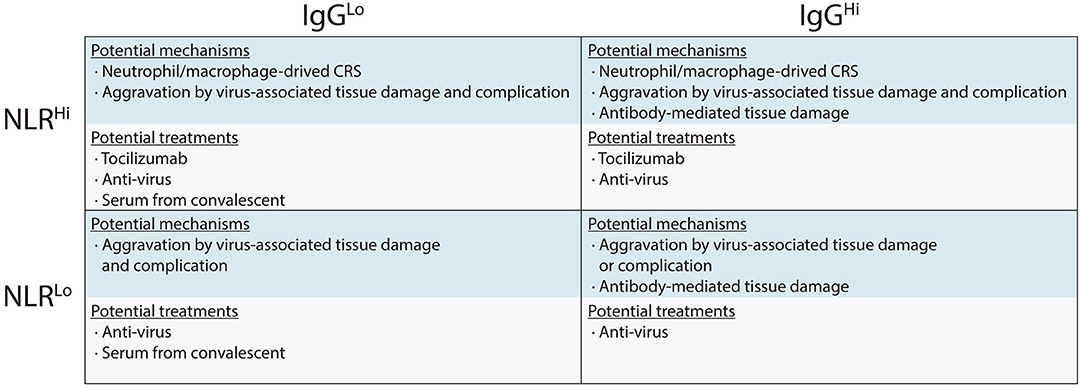
Figure 3. Immune response phenotyping with different immunological mechanisms associated with organ damage, and potential therapeutic strategies against severe COVID-19.
Our retrospective investigation had some limitations. Firstly, virus titers were not monitored during SARS-CoV-2 infection and patient recovery. Higher IgG levels, however, were previously detected in patients who had negative pre-discharge fecal RT-PCR results and SARS-CoV-infected rhesus macaques that had markedly reducing virus titers (Lee et al., 2006). Secondly, it is unknown whether the change or increase of IgM or IgA is related to disease severity. In this study, too few severe cases at early stage being collected convalescent sera to allow separate analysis of anti-IgM response. The precise mechanism responsible for the immunopathologic reaction of IgG remains elusive. Finally, the IgG response and its correlation to the severity of COVID-19 in patients without high-dose corticosteroid intervention have not been addressed. The reason was that the number of patients without intravenous high-dose corticosteroid intervention were limited in the subgroup with different immune phenotyping, especially in severe patients. Nevertheless, our findings indicate that severe COVID-19 was associated with a more robust IgG response that can be developed as an acquired immunity-related marker to predictive disease severity, along with other innate immunity-related makers such as NLR. Further study on the immunopathogenesis of SARS-CoV-2 infection is warranted.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
JW, BZhu, and QS had the idea for and designed the study and take responsibility for the integrity of the data and the accuracy of the data analysis. BZha, XZ, and CZ contributed to writing of the report. BZha and YS contributed to critical revision of the report. YQ, FF, JF, and QJ contributed to the statistical analysis and the data analysis portion of the manuscript. All authors contributed to data acquisition, data analysis, or data interpretation, and reviewed and approved the final version.
Funding
This work was funded by a grant from the National Natural Science Foundation of China (Grant No. 81572875) and the Fundamental Research Funds for the Central Universities, China (Grant No. 2042020kfxg07). None of the funding was involved in the design, conduct or analysis of this project.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This manuscript has been released as a pre-print at medRxiv (Zhang B. et al., 2020). We acknowledge all health-care workers involved in the diagnosis and treatment of patients in Eastern Campus, Renmin Hospital of Wuhan University. We thank Prof. Hong Zhou and Jiang Zheng for guidance in manuscript preparation.
References
Chan, P. K., Ng, K. C., Chan, R. C., Lam, R. K., Chow, V. C., Hui, M., et al. (2004). Immunofluorescence assay for serologic diagnosis of SARS. Emerg. Infect. Dis. 10, 530–532. doi: 10.3201/eid1003.030493
Greenough, T. C., Babcock, G. J., Roberts, A., Hernandez, H. J., Thomas, W. D. Jr., Coccia, J. A., et al. (2005). Development and characterization of a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody that provides effective immunoprophylaxis in mice. J Infect. Dis. 191, 507–514. doi: 10.1086/427242
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506. doi: 10.1016/S0140-6736(20)30183-5
Lee, N., Chan, P. K., Ip, M., Wong, E., Ho, J., Ho, C., et al. (2006). Anti-SARS-CoV IgG response in relation to disease severity of severe acute respiratory syndrome. J. Clin. Virol. 35, 179–184. doi: 10.1016/j.jcv.2005.07.005
Liu, J., Li, S., Liu, J., Liang, B., Wang, X., Wang, H., et al. (2020b). Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 18:102763. doi: 10.1016/j.ebiom.2020.102763
Liu, J., Liu, Y., Xiang, P., Pu, L., Xiong, H., Li, C., et al. (2020a). Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. J. Transl. Med. 18:206. doi: 10.1186/s12967-020-02374-0
Liu, L., Wei, Q., Lin, Q., Fang, J., Wang, H., Kwok, H., et al. (2019). Anti-spike IgG scauses severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight 4:e123158. doi: 10.1172/jci.insight.123158
Monsalvo, A. C., Batalle, J. P., Lopez, M. F., Krause, J. C., Klemenc, J., Hernandez, J. Z., et al. (2011). Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat. Med. 17, 195–199. doi: 10.1038/nm.2262
Rivers, E., Nguyen, B., Havstad, S., Ressler, J., Muzzin, A., Knoblich, B., et al. (2001). Early goal-directed therapy collaborative group. N. Engl. J. Med. 345, 1368–1377. doi: 10.1056/NEJMoa010307
Singer, M., Deutschman, C. S., Seymour, C. W., Shankar-Hari, M., Annane, D., Bauer, M., et al. (2016). The third international consensus definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801–810. doi: 10.1001/jama.2016.0287
To, K. K., Zhang, A. J., Hung, I. F., Xu, T., Ip, W. C., Wong, R. T., et al. (2012). High titer and avidity of nonneutralizing antibodies against influenza vaccine antigen are associated with severe influenza. Clin. Vaccine Immunol. 19, 1012–1018. doi: 10.1128/CVI.00081-12
Wang, D., Hu, B., Hu, C., Zhu, F., Liu, X., Zhang, J., et al. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323, 1061–1069. doi: 10.1001/jama.2020.1585
Wu, F., Zhao, S., Yu, B., Chen, Y. M., Wang, W., Song, Z. G., et al. (2020). A new coronavirus associated with human respiratory disease in China. Nature 579, 265–269. doi: 10.1038/s41586-020-2008-3
Xu, Z., Shi, L., Wang, Y., Zhang, J., Huang, L., Zhang, C., et al. (2020). Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 8, 420–422. doi: 10.1016/S2213-2600(20)30076-X
Zhang, B., Zhou, X., Zhu, C., Feng, F., Qiu, Y., Feng, J., et al. (2020). Immune phenotyping based on neutrophil-to-lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID-19. medRxiv. doi: 10.1101/2020.03.12.20035048
Zhang, C., Wu, Z., Li, J. W., Zhao, H., and Wang, G. Q. (2020). Cytokine release syndrome in severe COVID-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents 55:105954. doi: 10.1016/j.ijantimicag.2020.105954
Zhang, L., Zhang, F., Yu, W., He, T., Yu, J., Yi, C. E., et al. (2006). Antibody responses against SARS coronavirus are correlated with disease outcome of infected individuals. J. Med. Virol. 78, 1–8. doi: 10.1002/jmv.20499
Keywords: COVID-19, neutrophil-to-lymphocyte ratio, IgG, disease severity, clinical outcome
Citation: Zhang B, Zhou X, Zhu C, Song Y, Feng F, Qiu Y, Feng J, Jia Q, Song Q, Zhu B and Wang J (2020) Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Front. Mol. Biosci. 7:157. doi: 10.3389/fmolb.2020.00157
Received: 18 May 2020; Accepted: 22 June 2020;
Published: 03 July 2020.
Edited by:
Pier Paolo Piccaluga, University of Bologna, ItalyReviewed by:
Jing Yuan, Children's Hospital of Capital Institute of Pediatrics, ChinaAmit Prasad, Indian Institute of Technology Mandi, India
Copyright © 2020 Zhang, Zhou, Zhu, Song, Feng, Qiu, Feng, Jia, Song, Zhu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qibin Song, qibinsong@163.com; Bo Zhu, bo.zhu@tmmu.edu.cn; Jun Wang, ggjun2005@126.com
†These authors have contributed equally to this work
 Bicheng Zhang
Bicheng Zhang Xiaoyang Zhou2†
Xiaoyang Zhou2†  Bo Zhu
Bo Zhu Jun Wang
Jun Wang