Abstract
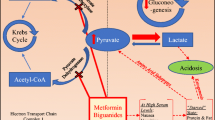
The biguanide drugs metformin and phenformin have been linked in the past to lactic acidosis, a metabolic condition associated with high rates of mortality. Although concern over the hyperlactataemic effect of phenformin led to the withdrawal of this drug from clinical practice in the 1970s, the situation with metformin has been less clear. Retrospective data indicate that, in metformintreated patients with lactic acidosis, neither the degree of hyperlactataemia nor accumulation of metformin is of prognostic significance. Furthermore, the lowest rates of mortality were seen in patients with high plasma concentrations of metformin, which has led to the hypothesis that the drug may confer some benefit, linked to an increase in vasomotility, in such cases.
Overall, it appears that mortality in patients receiving metformin who develop lactic acidosis is linked to underlying disease rather than to metformin accumulation, and that metformin can no longer be considered a toxic drug in this respect. These findings are likely to be of considerable relevance to the management of patients with type 2 (non-insulin-dependent) diabetes mellitus, especially where such patients are elderly.
Similar content being viewed by others
References
Assan R, Heuclin C, Ganeval D, et al. Metformin-induced lactic acidosis in the presence of acute renal failure. Diabetologia 1977; 13: 211–7
Fulop M, Hoberman HD. Phenformin-associated metabolic acidosis. Diabetes 1976; 25: 292–6
Sirtori CR, Pasik C. Re-evaluation of a biguanide, metformin: mechanism of action and tolerability. Pharmacol Res 1994; 30: 187–228
Luft D, Schmulling RM, Eggstein M. Lactic acidosis in biguanide-treated diabetics. A review of 330 cases. Diabetologia 1978; 14: 75–87
Krentz AJ, Ferner RE, Bailey CJ. Comparative tolerability profiles of oral antidiabetic agents. Drug Saf 1994; 11: 223–41
Lalau JD, Westeel PF, Debussche X, et al. Bicarbonate haemodialysis: an adequate treatment for lactic acidosis in diabetics treated by metformin. Intensive Care Med 1987; 13: 383–7
Weil MH, Afifi AA. Experimental and clinical studies on lactate and pyruvate as indicators of the severity of acute circulatory failure (shock). Circulation 1970; 41: 989–1001
Cady LD, Weil MH, Afifi AA, et al. Quantitation of severity of critical illness with special reference to blood lactate. Crit Care Med 1973; 1: 75–80
Lalau J-D, Race J-M. Lactic acidosis in metformin-treated patients: prognostic value of arterial lactate levels and plasma metformin concentrations. Drug Saf 1999; 20: 377–84
Misbin RI, Lanh Green RPh, Stadel BV, et al. Lactic acidosis in patients treated with metformin [letter]. N Engl J Med 1998; 338: 265–6
Lalau JD, Lacroix C, Compagnon P, et al. Role of metformin accumulation in metformin-associated lactic acidosis. Diabetes Care 1995; 18: 779–84
Lalau JD, Mourlhan C, Bergeret A, et al. Consequences of metformin intoxication [letter]. Diabetes Care 1998; 21: 2036–7
Lalau JD, Andrejak M, Moriniére Ph, et al. Hemodialysis in the treatment of lactic acidosis in diabetics treated by metformin: a study of metformin elimination. Int J Clin Pharmacol Ther Toxicol 1989; 27: 285–8
Nattrass M, Todd PG, Hinks L, et al. Comparative effects of phenformin, metformin and glibenclamide in metabolic rhythms in maturity-onset diabetes. Diabetologia 1977; 13: 145–52
Jackson RA, Hawa MI, Jaspan JV, et al. Mechanism of metformin action in non-insulin-dependent diabetes. Diabetes 1987; 36: 632–40
Oates NS, Shah RR, Idle JR, et al. Genetic polymorphism of phenformin 4-hydroxylation. Clin Pharmacol Ther 1982; 32: 81–9
Bailey CJ, Turner RC. Metformin. N Engl J Med 1996; 334: 574–9
Cusi K, Consoli A, De Fronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1996; 81: 4059–67
Bailey CJ, Wilcock C, Day C. Effect of metformin on glucose metabolism in the splanchnic bed. Br J Pharmacol 1992; 105: 1009–13
Bailey CJ. Metformin — an update. Gen Pharmacol 1993; 24: 1299–309
Arieff AI, Gertz EW, Park R, et al. Lactic acidosis and the cardiovascular system in the dog. Clin Sci 1983; 64: 573–80
Sirtori CR, Franceschini G, Gianfranceschi G, et al. Metformin improves peripheral vascular flow in nonhyperlipidemic patients with arterial disease. J Cardiovasc Pharmacol 1984; 6: 914–23
Montanari G, Bondioli A, Rizzato G, et al. Treatment with low dose metformin in patients with peripheral vascular disease. Pharmacol Res 1992; 25: 63–73
Bouskela E, Wiernsperger N. Effects of metformin on hemorrhagic shock, blood volume, and ischemia/reperfusion on nondiabetic hamsters. J Vasc Med Biol 1993; 4: 41–6
King P, Parkin H, Macdonald IA, et al. The effect of intravenous lactate on cerebral function during hypoglycaemia. Diabetic Med 1997; 14: 19–28
King P, Kong M-F, Parkin H, et al. Intravenous lactate prevents cerebral dysfunction during hypoglycaemia in insulin-dependent diabetes mellitus. Clin Sci 1998; 94: 157–63
Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II noninsulin-dependent diabetes. Obes Res 1998; 6: 47–53
Smits P, Thien T. Cardiovascular effects of sulfonylurea derivatives: implications for the treatment of NIDDM? Diabetologia 1995; 38: 116–22
Leibowitz G, Cerasi E. Sulfonylurea treatment of NIDDM patients with cardiovascular disease: a mixed blessing? Diabetologia 1996; 39: 503–15
UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352: 854–65
Lalau JD, Vermersch A, Mary L, et al. Type 2 diabetes in the elderly: an assessment of metformin (metformin in the elderly). Int J Clin Pharmacol Ther Toxicol 1990; 28: 329–32
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lalau, JD., Race, JM. Lactic Acidosis in Metformin Therapy. Drugs 58 (Suppl 1), 55–60 (1999). https://doi.org/10.2165/00003495-199958001-00013
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199958001-00013