
Four-arm robotic lung resection versus muscle-sparing mini-thoracotomy: retrospective experience
Introduction
Robotic surgery is a novel approach to thoracic surgery introduced in the early 2000s (1). Since the introduction of the first generation of robotic systems with three arms (Standard system three-arm, 1999) there has been considerable improvement in devices. In the early days, mini-thoracotomy (2) was required to assist the surgeon at the computer console, so no capnothorax was used. Since 2003, robotic surgery has progressed using a number of different approaches: three vs. four arms, totally robotic vs. robot-assisted. Appropriate instruments for thoracic surgery were developed slowly (3) and to date only one robotic system has been available to perform the type of delicate surgery required for hilum dissection or node harvesting in anatomical lung resection.
Currently, the use of robotic surgery remains limited. There are two major reasons for this: no surgical recommendations or standards have been described and its costs are huge. Nowadays, three systems are available from only one company, Si (since 2009), Xi (since 2014) and, recently, the X system [2017].
The clinical advantage of robotic surgery is non-traumatic penetration of the intercostal space (less pain, fewer respiratory complications) (4). The muscle-sparing and non-rib breaking approach is shared with video-assisted thoracoscopy (VATS). However, robotic surgery is superior for several reasons: (I) non-traumatic pressure on the intercostal nerve with movement of the arms; (II) excellent visual conditions (3D and 10 times magnification); (III) movement of the instruments close to the human hand allowing very precise and delicate manipulations such as those required for vessel dissection, suture or node harvest; (IV) the fourth arm adds an additional arm for instruments or a camera. This allows difficult procedures to be carried out (large tumours, N+ disease, pleurodesis patients, etc.).
The system requires specific training to acquire the skills necessary in the hands and feet. After this training, it is less challenging to use than VATS for sharp dissection and multisite visual control. In our practice, before the arrival of the robotic system we performed lung cancer surgery by muscle-sparing video-assisted mini-thoracotomy (MSMT).
Several teams worldwide have described their robotic surgery techniques and results (4-7) using either three or four arms, with or without capnothorax, with different tools, mostly stapling the vessels and bronchus. Here, we describe a comparison of the first consecutive 185 robotic four-arm procedures carried out in our institution vs. MSMT, our previous minimally invasive approach.
Methods
Study design, aim and setting
This was a retrospective, monocentric, controlled, comparative study with two arms: (I) 185 consecutive patients undergoing surgery using the four-arm robotic technique between February 2014 (start date of the robotic program in our institution) and December 2016; and (II) control group, consisting of a historical series of 136 consecutive patients undergoing surgery by MSMT between January 2011 and January 2014, in the same institution. All surgical procedures, whether MSMT or robotic surgery, were carried out by the same senior surgeon. The aim was to compare the two surgical techniques in terms of length of hospital stay (LOS), adverse events and/or surgical complications.
Patient selection
All consecutive patients undergoing lung resection and node harvest since the beginning of our robotic program were included retrospectively. Thus, the learning curve for the technique forms part of the analysis.
Ethics approval
All patients gave their written informed consent before undergoing the MSMT or robotic procedure. The procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2008. Ethics approval for the study was not required as it was part of routine surgical care of the patients.
Surgical procedure
Patients were intubated with a double lumen tracheal tube in the lateral position placing a tissue roll under the chest to avoid the hip. No arterial blood line or central venous access was used systematically in either procedure.
MSMT approach
The patient was placed in the lateral position with a surgeon behind his/her back. Two 10 mm axillary ports were placed on the fourth and seventh intercostal space (ICS) on the medial axillary line. The ports were used for either the endoscope or any thoracoscopic instrument. MSMT was performed through the fifth intercostal space and a small rib retractor was used to spare the ribs. The endoscope used was a 10 mm EndoCAMeLeon® (Karl Storz, Germany). A camera holder was used to stabilise vision (Endoboy™, Condor®; Gmbh Medicaltechnik, Germany). Dissection was performed using direct or indirect vision depending on local conditions. The surgical approach was a “fissure first” dissection. Vessels were sutured manually and the bronchus was stapled using a linear stapler or sutured manually. Radical mediastinal and hilar node harvest was performed in each patient. Chest drainage was performed via axillary thoracoport access. Usually two chest tubes were inserted in a crossed fashion, going up and down in the chest cavity.
Robotic approach
The Da Vinci Surgical Si System (Intuitive Surgical, California) was used for all procedures. The patients were placed in a lateral position. The settings of the cart and port were rapidly defined in the simplest way possible for all procedures. The cart was in the same axis as the scapula line. The position of the cart in the operating room was switched with the respiratory system depending on the side of surgery in order to leave the patient’s mouth access free for intubation if required. The ports were place as below:
Right side: (I) 12 mm 30-degree camera in the eighth ICS on the scapula line; (II) arm 1 through the sixth ICS on the posterior axillary line; (III) arm 2 through the ninth ICS on the posterior scapula line; (IV) arm 3 through the seventh ICS 5 cm above the vertebral bone in relation to the scapula end.
Left side: (I) 12 mm 30-degree camera in the eighth ICS on the scapula line; (II) arm 2 through the sixth ICS on the posterior axillary line; (III) arm 1 through the ninth ICS on the posterior scapula line; (IV) arm 3 through the seventh ICS 5 cm above the vertebral bone in relation to the scapula end.
The camera was inserted first and the other ports were placed by eye. This positioning allows control of any space within the thorax. In the case of pleurodesis, pneumolysis was performed through port access until the ICS needed to place the ports was freed and the robot was then docked to start the procedure. Capnothorax was started gently (pressure 5 mmHg, flow 10 L/min). The 30-degree camera was raised while placing the port or carrying out pneumolysis and then lowered to perform the lobectomy.
The instruments used by a right-handed surgeon are as follows: (I) right hand: permanent cautery spatula (ref 420184); (II) left hand: fenestrated bipolar forceps (ref 420205); (III) assistant arm: ProGraspTM forceps (ref 420093).
For each procedure, lung resection and radical node harvest were performed. A fissure first technique and sharp dissection were performed in order to carry out ligation of the artery before the vein. Arterial ligation was performed either by sewing knots with linen 0 with the needle holder SutureCutTM (ref 420296) for small vessels or stapled with white 35 mm endo GIA staplers or Echelon Flex 35 mm, tip up. Hilar and mediastinal node harvest were performed during the procedure. Finally, the bronchus was stapled or sutured and knotted (Vicryl 2/0). The specimen was extracted via port access in an endobag®.
The steps of the procedure at the surgical console can be summarised as follows: (I) pneumolysis as required; (II) triangular ligament section, node 9/8 harvest; (III) visualisation of the inferior vein; (IV) zone 7 harvest and opening of the inter-bronchial zone (right side) or atrial vascular pleura and zone 10 and 4L node harvest (left side); (V) fissure exposure, visualisation of the artery, opening of the posterior part of the fissure and ligation of the arteries, node harvest 11, 12; (VI) control and section of the vein and proximal arteries if upper lobectomy (stapling with load); (VII) opening of the remaining fissures if required; (VIII) freeing of the bronchus from surrounding tissues and stapling or cut and suturing; (IX) checking that the bronchial stump is waterproof; (X) one 4R (right side) 5/6 (left side) node harvest; (XI) placing the specimen in the bag; (XII) extraction through the port access port (enlarged as needed) and closing with one chest drain.
Data analysis
Quantitative data are described as number of observed (and missing) values, mean ± standard deviation (SD), median, range (min–max), and qualitative data are described as number of observed (and missing) values, number and percentage of patients per class. Comparisons between the two groups were performed using the Chi2 test for qualitative criteria and the Mann-Whitney, Wilcoxon or Student’s t-test for quantitative data. No adjustment for multiplicity was considered.
A P<0.05 was considered to be statistical significant. All statistical analyses were performed using SAS® 9.4 software.
Results
Study population
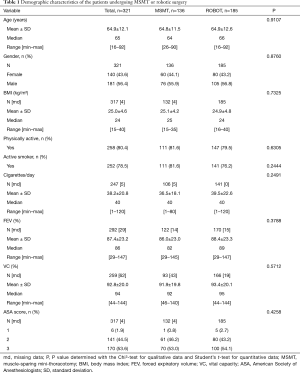
A total of 321 patients were analysed in this study (MSMT =136; ROBOT =185). Mean ± SD age of the patients was 64.9±12.1 years and 43.6% were female. There were no significant differences in the demographic characteristics of the two surgical groups (Table 1).
Full table
Preoperative diagnosis and tumour stage
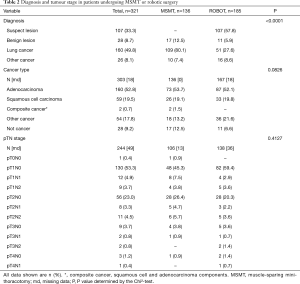
Preoperative diagnosis and tumour stage are shown in Table 2. Over half of the patients (57.5%) in the robotic group had a preoperative diagnosis of “suspect” lesion whereas patients in the MSMT group had a more definitive preoperative diagnosis of a benign lesion, lung cancer or some other type of cancer (P<0.0001). There was a wide range of tumour stages from T1 to T4 and N0 to N1 disease treated by the robotic or MSMT approach.
Full table
The most common surgical procedures performed were right upper lobe resection (30.3%), lung segmentectomy (16.2%), right lower lobe resection (15.1%), left upper lobe resection (14.1%), left lower lobe resection (11.9%), median lobe resection (10.3%) and other (2.2%). The type of procedures performed was almost similar in the two groups (Table 3).

Full table
Postoperative findings
Table 4 shows the postoperative characteristics of the study population. Median (range: min–max) LOS was significantly shorter (by 2 days) in the robotic group vs. MSMT {7 days [3–63] vs. 9 days [5–63], respectively; P<0.0001}. The duration of chest tube drainage was similar in the two groups. This latter finding should be interpreted with care as data were missing for 35% of the MSMT group.

Full table
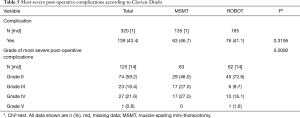
Clavien-Dindo post-operative complications are summarised in Table 5. The overall number of complications was similar in the two groups, but the complications appeared to be less severe in the robotic group (P<0.0092). This should also be interpreted with care as data were missing for 18.4% of the patients in this group.
Full table
For the robotic procedure, a mean of 5.5±2.0 staples (median: 5; range, 1–11) was used per procedure, mainly for repair of parenchymal section. Pneumolysis was carried out in 93 patients (50.3%). The overall conversion rate was 4.3% (8/185); this included 3/185 emergency conversions (1.6%). Mean (SD) duration of robotic surgery was 131.3±39.0 min (median 121 min; range, 62–320 min).
Discussion
This study of our first 185 consecutive patients undergoing four-arm robotic surgery shows a significantly better outcome in terms of LOS (−2 days) and less severe complications. The switch to robotic surgery in a senior practice was safe and relatively quick (no impact of the learning curve). Median (range) LOS was significantly shorter in the robotic group vs. the MSMT group {7 days [3–63] vs. 9 days [5–63], respectively; P<0.0001}. This LOS was quite long but was comparable to the national average for this type of procedure and included the day of admission (1 day before surgery) and the day of surgery itself. The standard deviations are quite large, which perhaps shows the impact of complications on LOS; however, the patients were discharged home in the majority of cases. In France, hospital stays are longer than in other countries for extra-medical reasons: cultural (patient and family fears, lack of extra hospital care organisation), economic (no incentive for the hospital to shorten LOS), geographic (distance of care structures, remoteness of the home, little availability of appropriate structures for convalescence).
The overall complication rate was similar in the two groups (Table 5). However, in terms of Clavien-Dindo classification (8) there was a switch from mainly grade III and IV complications in the MSMT group to grade II complications in the robotic group (grades III and IV, 54% vs. 25.8%, grade II, 46% vs. 72.6%, respectively; P=0.0092). The grade III complications (n=34) included: 21 cases of atelectasia requiring bronchoscopy, 13 lung infections requiring bacteriological sampling and antibiotherapy; and Grade IV complications (n=27) included 24 transfers to the ICU of which 16 were due to respiratory distress requiring invasive or non-invasive ventilation and antibiotherapy, two bronchopleural fistulas requiring redo surgery for muscle flap covering and one pleuropneumopathy requiring thoracoscopic cleaning of the pleura. Forty-one (82%) of the patients who suffered grade III or IV complications were men. One grade V complication occurred in the robot group on post-operative day 2 and was due to massive brain stroke (proximal thrombus of the carotid artery, despite preventative treatment with low molecular weight heparin and uninterrupted antiplatelet therapy). A dedicated meeting of medical experts external to the medical team in charge of this patient concluded that this was not directly due to the surgical approach. The complication rate for robotic surgery should be interpreted with care due to the large proportion of missing data (14%) in this group.
Our patients had stages I to IV cancer and underwent all types of lobectomy. There was a significantly higher proportion of right upper lobectomies in the robot group, but there was no significant difference in the proportion of radical node harvests, segmentectomies or other anatomical lung resections between the two groups (Table 3). As we do not get a good diagnostic yield from CT biopsy in our institution and do not have micro needle biopsy tools or endobronchial ultrasound there was a high rate of patients without a pre-op diagnosis. All of these the patients were discussed in a multidisciplinary staff meeting to validate the surgical procedure. For, these patients (unless there was a central tumour not eligible for wedge resection or per-op needle biopsy) frozen section analysis was performed per-operatively to ensure the cancer diagnosis before performing anatomical lung resection and node harvest. This led to a longer procedure time. Median duration of surgery for the robotic group was 121 min (range: 62–320). In all patients, we followed the oncological pathway of care, which means that a patient with pre-op N2 disease received neoadjuvant chemotherapy. We only proceeded with surgery if the tumour had stabilized or had responded to treatment (unless it was N2 disease requiring pneumonectomy).
The American Association of Thoracic Surgeons recently proposed a definition of a robotic thoracic operation as “a minimally invasive surgical procedure that does not spread, lift or remove any part of the chest or abdominal wall and is characterized by: the surgeon and the assistant’s vision of the operative field is via a monitor only and the patient’s tissue is manipulated by robotic instruments that follow a slave like mimic of human hands or thoughts via a computerized system” (9). There are significant variations in approach and use of the tools; three- vs. four-arm approach, totally endoscopic or with open access (mini-thoracotomy, capnothorax). The purpose of the tools is to enhance the surgeon’s capability, with the benefits of less parietal and dissection trauma for the patients. From this perspective, it appears more relevant to compare open surgery with robotic surgery than to compare robotic surgery with VATS. This is why it was important to use the full capacity of the machine (four arms, enhanced surgeon) without open access (use of capnothorax, one surgeon at the console not at the bedside). It also seemed more relevant to compare this technique with another less invasive technique preserving surgical skills, namely MSMT.
In our series, we had a low rate of conversions (4.3%) and emergency conversions (1.6%), including during the learning curve. No dramatic perioperative events occurred. The emergency conversions were due to problems of arterial bleeding. However, none of these bleeds were uncontrolled at the time of conversion, due to compression. Repair of the bleed could not be performed using the robotic system and required conversion via posterolateral thoracotomy, keeping the robotic camera. None of these three cases resulted in haemodynamic instability. The other five conversions were due to technical limitations of the robotic tool to perform the procedure (stone nodes, pneumonectomy). Emergency thoracotomy was performed by the surgeon who quickly rejoined the operating team with her gown and gloves ready, and a nursing team helping to dock out some of the arms.
In 2014, Lee et al. (10), compared 35 robotic procedures vs. 34 VATS without any significant differences between the two. The conversion rate was 3%. Veronesi et al. (7) reported a propensity score match analysis of 54 robotic procedures and open surgery, showing an impact of the learning curve on LOS. The patient series included early stages only (N0). The robotic procedure time was 213 min at best vs. 150 min for open surgery. They also used mini-thoracotomy as a bedside assistant, so the procedure was not fully robotic. Melfi et al. (11) reported 229 procedures, comparing the two systems (standard and Si). Their results were better with the new system in terms of surgery duration, complication rate and conversion rate. They were also better in terms of morbidity, mortality and LOS (decrease of 2 days). However, they also had 26 wedge resections in the second group, which limited the interpretation of the results.
Nasir et al. (12) focused on the cost-effectiveness of lobectomy and segmentectomy in a series (n=394) of stage I and II patients. The global conversion rate was 10% and 4% in an emergency. Cerfolio et al. (4) described a study including 122 segments and lobes. They conducted a propensity score match with 318 muscle-sparing nerve-sparing thoracotomies. This study is probably the closest to ours as the technique was comparable (fully robotic, insufflation, four arms). In another study in 2016, these authors also reported a series of cases (n=632) with a wide range of disease stages (I to IV). In this series, the conversion rate was 6% and 2% in an emergency (13).
In VATS and RATS many techniques are mixed. In full robotic surgery for stage I to III patients, there are still not enough data. Here we compare MSMT, which is close to open surgery in terms of surgical technique, and robotic surgery, which retains the surgical technique, thus we observe the benefit of sparing parietal trauma for very similar surgical procedures. Our robotic approach is not VATS-based as Melfi et al. have suggested (14). In VATS surgery, 2D or even 3D vision combined with a low range motion instrument gives the surgeon front to back vision, like working in a tunnel (15). The 3D perspective and immersive experience of robotic surgery allows the surgeon to move everywhere in the thorax and to have depth control. Therefore, starting from a VATS point of view might not give the surgeon the full benefits of the instrument. This also minimises the risk of mis-visualisation of the vessels, which can lead to per- and postoperative complications (16).
The fourth arm is probably more complex to integrate during the switch to robotic surgery. Its use enables more complex surgery to be performed. Our study included the first 185 patients treated with the four-arm robotic system. Those who did not undergo robotic surgery had apical parietal invasion, pneumonectomies and vascular invasion. So far, we do not have appropriate tools to perform safe vascular clamping for arterial sleeve procedures.
Analysis of the Premier database reached the same conclusions regarding the benefits of robotic vs. VATS or open surgery (17,18). Despite the fact that this database is administrative, contains a mixture of three-arm and four-arm full robotic or mixed surgery and focuses on early disease stages, the large number of cases analysed shows a trend. The first retrospective study shows a lower conversion rate and equivalent results in terms of postoperative course for robotic surgery vs. VATS. The second is an open comparison of VATS vs. robot-assisted thoracoscopic surgery (RATS) and open surgery. Again these authors found a lower conversion rate with RATS than with VATS, similar morbidity and shorter LOS for the robotic group suggesting that robotic surgery might be less traumatic. This makes sense as magnification of vision, plus the indirect traction/exposure that can be achieved, plus the lack of haptic feedback might make the surgeon more careful than with VATS.
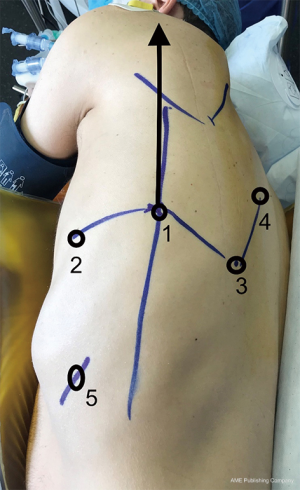
The other aim of this paper was to describe our technique, which is a modified approach of the four-arm technique described previously. As shown in Figure 1, the shape of the robotic port is a partial W. Our aim was to standardise the approach quickly to make it simple, reproducible and safe. We use a systematic approach irrespective of the type of resection (upper middle or lower lobes, left or right side). The idea is that whatever the resection performed, the surgeon needs access to the same thoracic area for node harvest and hilum control. In our centre, a single senior surgeon handled the development of the procedure. The bedside assistant was a nurse trained in stapling. The stapling delegation was controlled by voice communication from the surgeon. In our private hospital structure we had no resident and did not think it necessary to have a second surgeon to share the lead. Thus, the bedside nurse had the job of a physician assistant without the title, as there is no such title in France. This requires good team management and effective communication.
In this paper, we report a mean number of five staples per procedure in 133 patients, mainly for parenchymal section. We also routinely stitch small vessels. Thus, we would like to highlight the benefits of robotic surgery in terms of surgical hard skills. As the wrist motion of the robotic instruments is similar to hand motion, this ensures that suturing and dissection is similar to that in open surgery. If an open thorax is required, particularly in an emergency and stress conditions, the surgeon has his/her habitual suturing skills. With VATS, the use of automatic cutting-suturing devices reduces the surgeon’s hard skills. Surgeons need to be able to dissect and stitch without an automatic device; this can be better achieved with a robotic system and is why robotic surgery is the surgical future.
The modern era of robotic surgery has begun. Our study shows that the Da Vinci system for thoracic surgery can be implemented in a safe and rapid manner in a senior surgical practice. The results with this approach offer a better outcome than MSMT in terms of morbidity and mortality due to reduced chest trauma and sharp dissection. The technique provides excellent technical conditions similar to those of open surgery to allow dissection in a broad range of patients (N+, large tumours, bronchial sleeve, etc.). These results should be confirmed in a prospective, comparative study if such a study is ethically possible.
Acknowledgements
The authors thanks Capionis (Bordeaux, France) for performing the statistical analysis, Newmed Publishing for providing medical writing services and Mélanie Dehais for data collection. No funding was obtained for this study.
Footnote
Conflicts of Interest: Marion Durand is an official proctor for Intuitive Surgical. The other authors have no conflicts of interest to declare.
Ethical Statement: All patients gave their written informed consent before undergoing the MSMT or robotic procedure. The procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2008. Ethics approval for the study was not required as it was part of routine surgical care of the patients.
References
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Morgan JA, Ginsburg ME, Sonett JR, et al. Advanced thoracoscopic procedures are facilitated by computer-aided robotic technology. Eur J Cardiothorac Surg 2003;23:883-7; discussion 887. [Crossref] [PubMed]
- Bodner J, Wykypiel H, Wetscher G, et al. First experiences with the da Vinci operating robot in thoracic surgery. Eur J Cardiothorac Surg 2004;25:844-51. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36-42. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Cerfolio R, Louie BE, Farivar AS, et al. Consensus statement on definitions and nomenclature for robotic thoracic surgery. J Thorac Cardiovasc Surg 2017;154:1065-9. [Crossref] [PubMed]
- Lee BE, Korst RJ, Kletsman E, Rutledge JR. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: are there outcomes advantages? J Thorac Cardiovasc Surg 2014;147:724-9. [Crossref] [PubMed]
- Melfi FM, Fanucchi O, Davini F, et al. Robotic lobectomy for lung cancer: evolution in technique and technology. Eur J Cardiothorac Surg 2014;46:626-30; discussion 630-1. [Crossref] [PubMed]
- Nasir BS, Bryant AS, Minnich DJ, et al. Performing robotic lobectomy and segmentectomy: cost, profitability, and outcomes. Ann Thorac Surg 2014;98:203-8; discussion 208-9. [Crossref] [PubMed]
- Cerfolio RJ, Bess KM, Wei B, et al. Incidence, results, and our current intraoperative technique to control major vascular injuries during minimally invasive robotic thoracic surgery. Ann Thorac Surg 2016;102:394-9. [Crossref] [PubMed]
- Melfi FM, Fanucchi O, Davini F, et al. VATS-based approach for robotic lobectomy. Thorac Surg Clin 2014;24:143-9. [Crossref] [PubMed]
- Decaluwe H, Sokolow Y, Deryck F, et al. Thoracoscopic tunnel technique for anatomical lung resections: a 'fissure first, hilum last' approach with staplers in the fissureless patient. Interact Cardiovasc Thorac Surg 2015;21:2-7. [Crossref] [PubMed]
- Decaluwe H, Petersen RH, Hansen H, et al. Major intraoperative complications during video-assisted thoracoscopic anatomical lung resections: an intention-to-treat analysis. Eur J Cardiothorac Surg 2015;48:588-98; discussion 599. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term survival based on the surgical approach to lobectomy for clinical stage I nonsmall cell lung cancer: comparison of robotic, video-assisted thoracic surgery, and thoracotomy lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-assisted, video-assisted thoracoscopic and open lobectomy: propensity-matched analysis of recent Premier data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]


