Video-assisted thoracic surgery for thymoma: long-term follow-up results and prognostic factors—single-center experience of 150 cases
Introduction
Thymomas account for approximately 95% of thymic neoplasms. Most thymoma patients are between 40 and 60 years of age, and a similar incidence is noted between men and women (1). Surgery is indicated as initial treatment for patients whose tumor can be resected completely with remarkable results. Kondo et al. (2) reported that R0 resection rates of Masaoka I, II, III, and IV were 100%, 100%, 85% and 42%, respectively, and recurrence rates were 0.9%, 4.1%, 28.4% and 34.3%, respectively. For stage III and IV thymoma, 5-year overall survival (OS) rates of complete resection, incomplete resection and non-resection were 92.9%, 64.4% and 35.6%, respectively. However, the current standard of care is still open surgical approach via median sternotomy. The application of video-assisted thoracic surgery (VATS) remains controversial without large sample sized report of long term results, even with clearer view as well as minimal-invasive advantage.
Besides, given the relatively low morbidity and slow progression of thymoma, studies to evaluate the thoracoscopic treatment of thymoma with large sample sizes and long-term follow-up (>10 years) results are still lacking. The aim of this study was to evaluate safety and effectiveness of thoracoscopic treatment of thymoma and, more importantly, to report long-term follow-up results of a large single-center cohort.
Methods
Patients
Patients who received resections for thymoma at Peking University People’s Hospital from April 2001 to November 2014 were retrospectively reviewed. Patients who received VATS procedure and were pathologically diagnosed with thymoma were included. Only patients whose follow-up periods were longer than 2 years after surgery were analyzed. A total of 150 patients were enrolled finally.
Surgical techniques
The thoracoscopic approach was performed as a priority, and the procedure was converted to thoracotomy if necessary. Thymectomy was performed for most patients without myasthenia gravis (MG). Extended thymectomy was performed for thymoma accompanied with MG. Tumor resection was performed for patients with heavy adhesion or other reasons that caused them unable to undergo thymectomy. Palliative resection (non-R0 resection) was performed for patients whose tumor could not be completely resected to reduce tumor burden.
Follow-up
Post-operative patients received chest CT scan every 6 months in the first 2 years then annually thereafter. Survival and recurrence information was investigated by telephone or outpatient record. Recurrence was divided into three categories according to the definition of International Thymic Malignancy Interest Group (ITMIG) (3). For patients who had thymoma accompanied with MG, outcome was evaluated according to the thymectomy outcome measurements by Remes-Troche et al. (4).
Statistical analysis
Categorical data were presented as frequency and percent. Continuous data were presented as mean and standard deviation or median and interquartile range depending on data distribution. OS and recurrence free survival (RFS) were calculated by the Kaplan-Meier method. Regarding prognostic factors of RFS, univariable and multivariable Cox’s regression analyses were performed. A P value <0.05 was considered statistically significant. All statistically analysis was performed using SPSS program version 23.0 (SPSS Inc., Chicago, IL, USA).
Result
Patient characteristics
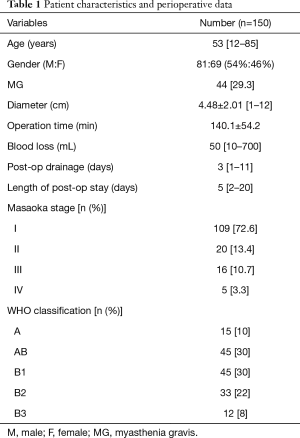
Patient characteristics are listed in Table 1. In total, 69 males (46%) and 81 females (54%) among 150 patients were enrolled in the study. The median age was 53 years (range, 12–85 years); 44 patients (29.3%) were diagnosed with MG prior to operation. In addition, there were 21 type I, 13 type IIa, 8 type IIb and 2 type III MG cases according to the Osserman classification.
Full table
Perioperative data
Perioperative data, Masaoka stage and WHO classification are provided in Table 1. Ninety-six cases of thymectomy (64.0%), 41 cases of extended thymectomy (27.3%), 11 cases of thymoma resection (7.3%) and 2 cases of palliative resection (1.3%) were performed. Seven cases were converted to thoracotomy (4.7%), and the main reason for conversion was tumor invasion to great vessels. R0 resection rates of Masaoka stage I, II, III and IV were 100%, 100%, 93.7% and 80%, respectively.
Eleven cases experienced post-operative complications (7.3%), including 3 cases of myasthenic crisis, 2 cases of fever, 1 case of atrial fibrillation (AF) accompanied with myasthenic crisis, 1 case of AF, 1 case of heavy aerodermectasia, 1 case of pulmonary infection, 1 case of delirium and 1 case of uroschesis. All patients with complications were treated successfully and discharged. No perioperative death occurred.
Survival and recurrence
By November 2016, 134 of 150 patients (89.3%) were successfully investigated, and median follow-up period was 59.5 months (range, 2–187 months).
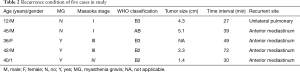
Five cases of recurrence were observed, including four cases of local recurrence and one cases of pulmonary metastases (Table 2). Thirteen cases of death were observed with no thymoma-related cause. The main causes of death were lung cancer, aplastic anemia, cardiovascular disease, breast cancer, pulmonary infection and virus infection.
Full table
Five- and 10-year OS rates were 91.4% and 84.8%, respectively for the whole group. Five-year OS rates of Masaoka stage I, II and III were 90.3%, 88.2% and 86.7%, respectively. Ten-year OS rates were 85%, 88.2% and 77%, respectively. All five Masaoka stage IV cases were followed up, and no death occurred. Five- and 10-year RFS rates of the entire cohort were 96.5% and 94.4%, respectively. Five- and 10-year RFS rates for Masaoka stage I + II were 98.1% and 98.1%, respectively. Five- and 6-year RFS rates for Masaoka stage III were 90% and 60%, respectively. One case of recurrence was noted in five Masaoka stage IV patients, and the 4-year RFS was 80%.
Prognostic factors
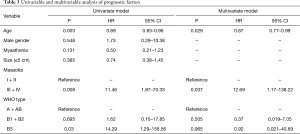
Masaoka stage was divided into early (stage I, II) and late (stage III, IV) here in order to investigate its prognostic effect. Thymomas were divided into three categories: thymoma including neoplastic epithelial cells having spindle or oval shape (types A and AB), thymoma exhibiting organoid pattern (types B1 and B2), and atypical thymoma (type B3) (5). Univariable analysis demonstrated that age, Masaoka stage and histologic type (WHO type) were prognostic factors of recurrence. Multivariable analysis indicated that recurrence tended to occur in Masaoka stages III + IV patients (P=0.037, HR =12.69, 95% CI: 1.17–138.22) and older patients had a lower risk of recurrence (P=0.029, HR =0.87, 95% CI: 0.77–0.99) (Table 3).
Full table
Treatment outcome of MG
Thirty-six of 44 thymoma cases also exhibiting MG (81.8%) were followed up. Nine patients achieved clinical sustained remission, 19 patients achieved improvement, and 8 patients deteriorated. The overall response rate of MG was 77.8% (28/36).
Discussion
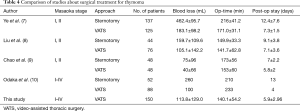
A recent meta-analysis comparing minimally invasive surgery (MIS) with open approach for thymic malignancies showed that MIS group had significantly less blood loss and shorter length of stay (6). Compared with past published results of open surgery, our study also demonstrates that the VATS approach could achieve similar even better results regarding blood loss, operation time, post-operative drainage time and post-operative hospitalization with a more minimally invasive approach (Table 4). The complications rates (7.3%) and conversion rates (4.7%) were low, and no perioperative death was observed. Four cases of severe complications, including myasthenic crisis, were recorded, and patients were treated well and discharged. Based on these data, VATS is a safe approach for thymoma, especially in well-experienced centers.
Full table
Kondo et al. (2) reported that R0 resection rates of Masaoka stage I, II, III and IV by open procedure were 100%, 100%, 85% and 42%, and recurrence rates were 0.9%, 4.1%, 28.4% and 34.3%, respectively. In our study, R0 resection rates for Masaoka stage I, II, III, and IV were 100%, 100%, 94.1% and 80%, respectively. Only two patients underwent incomplete resection due to great vessel invasion and pleural metastasis. Recurrence rates were 2.1%, 0%, 20% and 20%, respectively. Here we could say R0 resection and recurrence rates were not worse, or even improved in our study.
Detterbeck et al. (11) reported that for patients with thymoma, the average recurrence time of Masaoka stage I and other stages was 10 and 5 years, respectively. Most causes of death are non-thymoma related. Thus, 5-year OS is not an adequate measurement to evaluate the long-term therapeutic efficacy of thymoma. We think 5- and 10-year RFS rates may be more applicable. Maniscalco et al. (12) reported long-term follow-up results of 27 patients. Fourteen patients received open surgery, and 13 patients received VATS. The median follow-up period was 123 months, and no recurrence and thymoma-related death were observed. Ye et al. (7) reported 5-year follow-up results of 137 cases with open surgery and 125 cases with thoracoscopic surgery, and no differences in OS and RFS rates were noted between two groups. Xu et al. (13) also reported long term results of 331 cases with open surgery, and the 5-year RFS rates for Masaoka stage I, II, III, and IV were 99.4%, 88.3%, 81.5% and 57.1%, respectively. The 10-year RFS rates were 97.9%, 76.5%, 57.4% and 57.1%, respectively. Compared with past VATS studies, our study had a larger sample size and longer follow-up period. Only five cases of recurrence were observed in our study. Five- and 10-year RFS rates for early stage thymoma (Masaoka I and II) were 98.1% and 98.1%, respectively. Five- and 6-year RFS rates for Masaoka stage III thymoma were 90% and 52.5%, respectively. Masaoka stage IV thymoma was not calculated given the insufficient number of patients. Follow up results were comparable with previous VATS and open surgery studies.
Masaoka stage is widely accepted as a prognostic factor of recurrence (10,13-15). We also demonstrated that advanced Masaoka stage was associated with poor RFS (P=0.037, HR = 12.69, 95% CI: 1.17–138.22).
There is no conclusion whether age affects RFS. Age was usually switched to a categorical variable and was considered not associate with recurrence in most studies (13,15). In our study, age was analyzed as a continuous variable. Multivariable analysis suggested that older patients have a lower risk of recurrence (P=0.029, HR =0.87, 95% CI: 0.77–0.99). However, considering there were only five recurrences observed in our study, we could not exclude bias from retrospective study.
Most reports indicated that complete resection was an independent risk factor (14). But complete resection was not an independent predictor in our analysis. Also, possibly because of the small number of patients with incomplete resection in our study.
Most previous studies reported WHO histological classification as a significant recurrence factor (14). But a consensus is lacking. Six histologic types of thymic tumors were usually divided into different groups based on the invasiveness, survival, and recurrence features in different studies, while the criterion was not in full agreement (13,15-17). D’Angelillo et al. considered the relationship of WHO classification with prognosis only when combined with other factors, such as Masaoka staging and resection margin status (18). In our study, WHO types were an independent prognostic factor of recurrence in univariable analysis, but not in multivariable analysis. This study could not prove the correlation between WHO classification and recurrence.
Up to one-half of patients with thymoma have symptoms consistent with MG (1). Orsini et al. (19) reported that there was no convincing data demonstrating superior efficacy or long-term remission rates in MG between transsternal and thoracoscopic approaches. Heng et al. (20) reported that the transsternal approach with complete removal of the thymus gland was performed in 20 patients with thymoma and MG. Thirteen patients achieved complete remission, and seven patients achieved partial remission. The response rate was 100%. Siwachat et al. (21) reported 98 thymoma patients with MG. The response rate was 88.7% in the thoracoscopic group (n=53) and 88.9% in the transsternal group (n=45). The thoracoscopic approach exhibited comparable neurological outcomes to the trans-sternal approach. In our study, 36 patients were interviewed. Nine patients achieved clinically sustained remission, 19 patients achieved improvement and 8 patients deteriorated. The overall response rate was 77.8% (28/36). The result was also comparable to previous reports.
Compared with other retrospective studies, our study had a relatively larger sample size and longer follow-up period. The limitation of this study is the retrospective nature of data collection. Some selection bias or confounders may be present. As the majority of patients in this study were in early stages, a median follow-up period of 59.5 months might not be sufficiently long to evaluate the therapeutic efficacy of VATS in early stage thymoma. Thus, a longer follow-up period is required.
Conclusions
VATS is a safe and effective procedure for the treatment of thymoma with minimal invasion and satisfactory prognosis. MG with thymoma treated by VATS exhibited comparable neurological outcomes to those associated with the trans-sternal approach.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: As the study was a retrospective study and did not involve human subjects, approval for the study was exempted by the Institutional Review Board of Peking University People’s Hospital. The study was waived the requirement of obtaining patient informed consent because the patients remained anonymous in the study.
References
- Safieddine N, Liu G, Cuningham K, et al. Prognostic factors for cure, recurrence and long-term survival after surgical resection of thymoma. J Thorac Oncol 2014;9:1018-22. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [Crossref] [PubMed]
- Huang J, Detterbeck FC, Wang Z, et al. Standard outcome measures for thymic malignancies. Zhongguo Fei Ai Za Zhi 2014;17:122-9. [PubMed]
- Remes-Troche JM, Tellez-Zenteno JF, Estanol B, et al. Thymectomy in myasthenia gravis: response, complications, and associated conditions. Arch Med Res 2002;33:545-51. [Crossref] [PubMed]
- Kondo K, Yoshizawa K, Tsuyuguchi M, et al. WHO histologic classification is a prognostic indicator in thymoma. Ann Thorac Surg 2004;77:1183-8. [Crossref] [PubMed]
- Friedant AJ, Handorf EA, Su S, et al. Minimally Invasive versus Open Thymectomy for Thymic Malignancies: Systematic Review and Meta-Analysis. J Thorac Oncol 2016;11:30-8. [Crossref] [PubMed]
- Ye B, Tantai JC, Ge XX, et al. Surgical techniques for early-stage thymoma: video-assisted thoracoscopic thymectomy versus transsternal thymectomy. J Thorac Cardiovasc Surg 2014;147:1599-603. [Crossref] [PubMed]
- Liu TJ, Lin MW, Hsieh MS, et al. Video-assisted thoracoscopic surgical thymectomy to treat early thymoma: a comparison with the conventional transsternal approach. Ann Surg Oncol 2014;21:322-8. [Crossref] [PubMed]
- Chao YK, Liu YH, Hsieh MJ, et al. Long-term outcomes after thoracoscopic resection of stage I and II thymoma: a propensity-matched study. Ann Surg Oncol 2015;22:1371-6. [Crossref] [PubMed]
- Odaka M, Shibasaki T, Kato D, et al. Comparison of oncological results for early- and advanced-stage thymomas: thoracoscopic thymectomy versus open thymectomy. Surg Endosc 2017;31:734-42. [Crossref] [PubMed]
- Detterbeck FC, Parsons AM. Thymic tumors. Ann Thorac Surg 2004;77:1860-9. [Crossref] [PubMed]
- Maniscalco P, Tamburini N, Quarantotto F, et al. Long-term outcome for early stage thymoma: comparison between thoracoscopic and open approaches. Thorac Cardiovasc Surg 2015;63:201-5. [Crossref] [PubMed]
- Xu C, Feng QF, Fan CC, et al. Patterns and predictors of recurrence after radical resection of thymoma. Radiother Oncol 2015;115:30-4. [Crossref] [PubMed]
- Weis CA, Yao X, Deng Y, et al. The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol 2015;10:367-72. [Crossref] [PubMed]
- Wright CD, Wain JC, Wong DR, et al. Predictors of recurrence in thymic tumors: importance of invasion, World Health Organization histology, and size. J Thorac Cardiovasc Surg 2005;130:1413-21. [Crossref] [PubMed]
- Hamaji M, Allen MS, Cassivi SD, et al. The role of surgical management in recurrent thymic tumors. Ann Thorac Surg 2012;94:247-54; discussion 254. [Crossref] [PubMed]
- Lucchi M, Mussi A. Surgical treatment of recurrent thymomas. J Thorac Oncol 2010;5:S348-51. [Crossref] [PubMed]
- D'Angelillo RM, Trodella L, Ramella S, et al. Novel prognostic groups in thymic epithelial tumors: assessment of risk and therapeutic strategy selection. Int J Radiat Oncol Biol Phys 2008;71:420-7. [Crossref] [PubMed]
- Orsini B, Santelmo N, Pages PB, et al. Comparative study for surgical management of thymectomy for non-thymomatous myasthenia gravis from the French national database EPITHOR. Eur J Cardiothorac Surg 2016;50:418-22. [Crossref] [PubMed]
- Heng HS, Lim M, Absoud M, et al. Outcome of children with acetylcholine receptor (AChR) antibody positive juvenile myasthenia gravis following thymectomy. Neuromuscul Disord 2014;24:25-30. [Crossref] [PubMed]
- Siwachat S, Tantraworasin A, Lapisatepun W, et al. Comparative clinical outcomes after thymectomy for myasthenia gravis: Thoracoscopic versus trans-sternal approach. Asian J Surg 2018;41:77-85. [Crossref] [PubMed]


