Minimally invasive and robotic esophagectomy: state of the art
Introduction
Esophageal cancer is the eight most common cancer in the world and surgical resection remains the gold standard not only in providing the optimal chance for cure but also the best palliation for dysphagia. Esophagectomy is a complex operation and is associated with significant morbidity and mortality that are reported as 23–50% and 2–8% in western country (1). In the early nineties surgeons from all over the world started to have an interest in minimally invasive esophagectomy (MIE) and in finding a way to reduce the rate of complications (2). The results of these experiences were affected by the use of different surgical techniques but is now widely clear that mini-invasive approach reduces morbidity and mortality after esophagectomy. Patients operated with MI techniques reported better global quality of life, physical function, fatigue and pain at 3 months after surgery (3). Furthermore, in the last fifteen years, we have witnessed the rising of the robotic approach. However, if in some cases, such as prostatectomy, minimally invasive techniques are routinely used and the robotic approach is mandatory, in other cases the situation is completely different; this is the case of esophagectomy: only 15% of cases of esophagectomy worldwide are performed by using the conventional thoraco-laparoscopic or robotic approach (1). The aim of this paper is to review the available literature on MIE and robotic assisted minimally invasive esophagectomy (RAMIE) and check the advances in these techniques.
Literature search
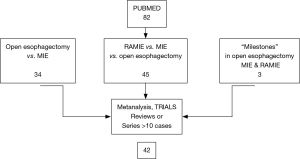
PubMed database was searched for “minimally invasive”, “esophagectomy” and “robotic assisted” and their synonyms and abbreviations. No additional search software or special features were used. The search was limited to papers describing original patient data series >10 patients, written in English, ongoing or completed trials, reviews and meta-analyses. Three “milestone” studies have been quoted because they proposed innovative surgical techniques in open, mini-invasive and robotic assisted esophagectomy (2,4,5). The final search was performed on July 1st 2017. The investigators (M Taurchini and A Cuttitta) independently performed article and articles selection procedures. The results of the search and the selection process were summarized in a flow chart (Figure 1). Eighty-two studies were collected, out of which 42 were analyzed.
Open esophagectomy and minimally invasive techniques
At moment no gold standard technique exists for esophagectomy. The choice of the technique depends on several factors; location of tumor and surgeon’s experience are probably the most relevant. Transthoracic esophagectomy (TTE) (Ivor-Lewis and McKeown TTE) remains the most used approach for the surgical management of resectable localized esophageal cancer. It provides a transthoracic en bloc esophagectomy with an extensive mediastinal lymph node dissection. Transhiatal esophagectomy (THE) has been proposed to reduce the high incidence of morbidity (4). Results from the comparison between TTE and THE have been controversial. Some reports have argued that transhiatal approach have a lower rate of complications whereas other scientific studies have demonstrated similar postoperative results and 5 years of survival rate (6,7) for the two approaches. In a published review by Verhage dated 2009 (8), ten case-controlled studies and one systematic review were retrieved. Data collection was grouped by surgical approach. Overall MIE data showed a decrease in blood loss (577 mL for conventional open surgery versus 312 mL for MIE) and reduction of hospital and ICU stay (19.6 and 7.6 versus 14.9 and 4.5 days, respectively). Total rates of complication were 60.4% for open esophagectomy and 43.8% for MIE. Pulmonary complications occurred in 22.9% and 15.1%. Mean lymph node retrieval was higher in MIE (23.8 versus 20.2). In 2012, 1,011 patients who underwent MIE for esophageal cancer, enrolled in a period of 15 years, were retrospectively considered by Luketich et al. to evaluate the postoperative outcome (9). He concluded that MIE is a feasible technique that reduces the incidence of perioperative complications and provide a quicker recovery, than open approaches, with a mortality rate of 1.6%. Nevertheless, he stressed the concept that MIE is a challenging surgical technique and that better results depend on each individual surgeon’s experience and surgical volume. To date we have a couple of completed multicentric and randomized trials comparing MIE and open esophagectomy. The MIRO trial started in 2011. Two hundred patients were enrolled and randomly divided into a MIE group and an open esophagectomy group (the operation was an Ivor-Lewis esophagectomy). The results showed a significant reduction in the rate of perioperative pulmonary complications in the MIE group (10). Biere et al. (11) randomly assigned 56 patients to the open esophagectomy group and 59 to the MIE group. In the first group 16 patients (29%) versus five patients (9%) in the second group developed pulmonary infection in the first 2 weeks; in the open esophagectomy group 19 patients (34%) against seven in the minimally invasive group, had pulmonary in hospital infection. One patient in the open esophagectomy group and two in the minimally invasive group died from aspiration and mediastinitis after anastomotic leakage. Other published works reported similar results (12,13). Furthermore, a few randomized trials are ongoing: the ROMIO trial is a three arm trial which aims at comparing the outcomes of total MIE versus hybrid MIE versus conventional open esophagectomy (Ivor-Lewis technique) (14). In the other two ongoing trials the Mc Keown procedure is being used (NCT02188615 and NCT 02017002). To date none of them has showed any results (15,16). In his paper published in 2015, Sihag et al. (17) retrospectively evaluated data on 3,780 data on 3,780 open esophagectomy and MIE, taken from the Society of Thoracic Surgeon Database. Comparable rates of morbidity and mortality between the two different approaches were reported but also a longer operation time and a higher rate of reoperation in the MIE group was observed. This rate of reoperation may reflect a learning curve. In fact, Osugy et al. in 2002 while he was examining from his experience, has determined that at least a number of 17 operations are required for a surgeon to acquire basic skills in thoracoscopic esophagectomy and 34 cases to obtain a similar or better postoperative outcome than that obtained through the open esophagectomy (18). A large meta-analysis (19) in 2015 of 13 studies with a total of 1,549 patients with resectable esophageal cancer was conducted and found that MIE does not compromise the long-term curative effect. In fact, the 2-year survival rate following the MIE is better than that following open esophagectomy. Incidence of anastomosis leakage was similar in the two groups, while greater operative blood loss was found in the open esophagectomy group. A very interesting survey was published by Haverkamp et al. in 2016 (20): the authors sent the survey to 1,118 members of the International Society for Diseases of Esophagus (ISDE), the World Organization for Specialized Studies on Diseases of the Esophagus (OESO), the International Gastric Cancer Association (IGCA). The questionnaire was filled out by 42% surgeons. Most of them (65%) worked in a University Hospital and in high volume centers (72%). The minimally invasive transthoracic approach was preferred in 43% of cases (especially in high volume centers). This percentage represents a three-fold increase in the number of respondents favoring minimally invasive techniques after the previous survey that was held in 2007.
RAMIE
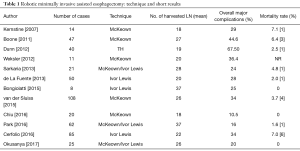
The addition of the robotic technique to MIE is relatively new. Robot assisted thoraco-laparoscopic esophagectomy has been introduced to overcome the limitations of conventional MIE. In fact, especially the thoracoscopic step of the esophagectomy is really uncomfortable due to the 2D planar vision, the fulcrum effect through the thoracic wall and the use of non-articulated instruments. Magnified 3D vision, great maneuverability of “endo wrist” instruments, motion scaling, tremor filtration and a more comfortable surgeon position could be helpful in such as complex surgical procedure. Horgan et al. first described robotic assisted THE in 2003 (5). Since this first report, robotic assisted esophagectomy has being gained wider acceptance and wider consensus., In fact, Kumar and Bin Asa (21) in a personal review, published in 2014, collected 26 articles on the topic “robotic assisted esophagectomy” with total reported experiences of 295 procedures. The same indications and contraindications that are conventionally used for MIE are applicable to RAMIE. In the published literature are reported studies on all three types of esophagectomy: we may have robotic assisted transhiatal esophagectomy (RATE), robotic assisted mini invasive McKeown esophagectomy (RAMIME) and robotic assisted Ivor-Lewis esophagectomy (RILE) but the procedures are usually performed with hybrid approaches and the different techniques are difficult to compare. The abdominal step is often performed by means of the classic laparoscopic approach; in fact, the large area in the upper abdomen that needs to be dissected cannot often be reached with a robotic single docking position. The robot is usually very helpful during lymphadenectomy. In the thoracic phase of the operation, the patient is positioned in the left lateral decubitus, tilted 45° toward the prone position. The semiprone decubitus is usually preferred since conversion to thoracotomy is more easily performed than with the completely prone position. The robotic cart is brought to the table from the dorso cranial side of the patient. Some case reports or limited series in RAMIE (22-24) showed that robotic assisted esophagectomy was safe and feasible. These and further experiences in terms of number of cases, techniques, number of harvested lymph nodes, overall major perioperative complications rate and mortality rate are resumed in Table 1. Kernstine et al. in 2007 (25) showed the first series of 14 RAMIME with a morbidity rate of 29%. In a 3-year single center experience by Dunn et al. (26) in 2012, 40 patients underwent RATE with an anastomotic leak rate of 25% (10/40) and anastomotic stricture rate of 67% (27/40) This high rate of complications improved in the last 20 cases with the growing experience acquired by the surgeon in performing RAMIE. Nowadays RAMIME and RAILE, as in standard MIE, replaced RATE in most cases. The transhiatal approach may be performed in critical patients considered too ill to undergo single lung ventilation (22,27,28). In 2012, Boone et al. (29) reported his personal experience in 47 RAMIME in a prospective study started in 2003 with an overall major morbidity of 46.5%. She showed a significative reduction in respiratory postoperative complications from 57% of the personal open esophagectomy series to 33% of the robotic assisted procedures. Subsequently other authors started to compare robotic approaches to conventional MIE. To date some comparative studies between RAMIE and conventional MIE are available. Weksler et al. (30) showed few differences in short terms outcome. In fact, postoperative morbidity rate, length of hospital stay and number of harvested lymph nodes were comparable in both groups of patients. Also, Yerokun et al. (31) has recently failed to find any clear advantage related to the use of the robot-assisted esophagectomy. He compared perioperative outcomes and 3-years oncological results obtained after open (2,958 procedures), conventional MIE [1,077] and RAMIE [231] for T1–3 N0–3 M0 cancer of the middle or distal esophagus. Patients undergoing standard mini-invasive or RAMIE had shorter hospital stay and more harvested lymph nodes than patients who received open surgery. However, no significant differences were observed in resection margins involvement and 3-year survival. In a prospective study by van der Sluis et al., 108 patients with resectable esophageal cancer underwent RAMIE between 2007 and 2011 with a median follow up of 58 months. RAMIE was shown to be oncologically effective with a high percentage of R0 resection (95%) and allowed an adequate lymphadenectomy with a median number of 26 harvested lymph nodes. This is quite interesting especially if we consider that 78% of patients presented with T3–T4 disease and 65% of them had received neoadjuvant treatment. Conversion rate was 19% and 5-year overall survival was 42% (32). A randomized controlled trial to assess the real benefit of RAMIE versus open esophagectomy on long term outcome is absolutely needed. For this reason, the Utrecht group started the ROBOT trial (33) in 2012. As many as 112 patients with resectable T1–T4 N0–3 M0 intrathoracic esophageal cancer are to be enrolled in the study. As many as 56 patients will undergo RALE esophagectomy and 56 the open three-stage TTE. The primary endpoint is the rate of overall complications as stated by the modified Clavien-Dindo classification of surgical complications (perioperative blood loss, postoperative complications, QOL). The secondary endpoint is recognized the length of ICU stay, length of hospital stay, mortality within 30 and 60 days and R0 resection rate. Definitive results of the study should be available by the end of 2017. Another interesting study was published by Okusanya et al. (34) who reported on 25 RAMIE cases enrolled since 2014 to 2016. Here, 72% of patients had previously undergone neoadjuvant therapy, 72% were adenocarcinomas and Ivor-Lewis esophagectomy was performed in 23 cases meanwhile in the other two a Mc Keown procedure was carried out. An R0 resection was obtained in 96% of cases and patients had a similar 30-day mortality, an anastomotic leakage rate, number of harvested lymph nodes and a conversion rate to conventional MIE patients previously reported. With the growing experience in this procedure, RAMIE can be helpful especially in patients with tumors in the upper mediastinum and with paratracheal lymph node metastasis. Okamura et al. (35) revealed the influence of anatomical factors on the difficulty of the thoracic procedure in MIE. A narrow upper mediastinum is an element predicting a thoracic procedural difficulty in MIE. The thoracic inlet is normally difficult to reach with an open or thoracoscopic conventional approach whereas the robotic system can reach this area without limitations and extends the operative and potentially curative options to these groups of patients. An adequate paratracheal lymphadenectomy along the laryngeal nerves is feasible during RAMIE according to the experience of a Korean group (36,37). The authors report the operative outcomes of robot-assisted thoracoscopic esophagectomy with extensive mediastinal lymphadenectomy for intrathoracic esophageal cancer. As many as 114 consecutive patients who underwent RAMIE with lymph node dissection along recurrent laryngeal nerve (RLN) followed by cervical esophagogastrostomy were enrolled in the study. The mean number of RLN nodes was 9.7±0.7. The most common complication was RLN palsy (26.3%), followed by anastomotic leakage (14.9%) and pulmonary complications (9.6%). The 90-day mortality was observed in three patients (2.5%). At multivariate analysis, preoperative concurrent chemoradiation was a risk factor for pulmonary complications. In extensive mediastinal lymphadenectomy, Broussard et al. (38) advises avoiding a monopolar electrocautery near the left/right mainstem bronchus. Unrecognized airway injury can lead to esophagotracheal fistula that is a devastating complication. In a recent retrospective study, Cerfolio et al. (39) analyzed personal experience in 85 Ivor Lewis esophagectomy (laparoscopic or robotic abdominal and robotic chest) from 2011 to 2015 and recognized a major morbidity rate of 36.4% and a 10.6% (9/85) overall operative mortality rate. Causes for these high rates were identified in anastomotic complications and wrong selection of patients. Therefore, corrective actions were taken. First, a stapled anastomosis was done in the following experience instead of the previous hand sewed one. Moreover, more attention was given to preoperative selection of the patients especially after neoadjuvant chemotherapy. Nutritional status and cardiopulmonary performance were carefully evaluated before surgery. In patients with an history of significative abuse of alcohol, a hepatic biopsy was always performed at the start of the operation and the planned surgery was aborted in case of cirrosis. However, the technical aspects of intrathoracic gastroesophageal anastomosis are quite difficult to explain. In fact, during Ivor-Lewis esophagectomy, it is very uncomfortable and difficult to perform a hand sewed anastomosis with conventional thoracoscopic technique even for very skilled surgeons. In RAMIE, the utilization of EndoWrist instruments, the deeper high definition 3D vision, the motion scaling and tremor filtration make a hand-sewn intrathoracic anastomosis possible and easier to accomplish. Trugeda and more recently Bongiolatti (40,41), published personal series of RAILE with hand-sewn intrathoracic esophagogastric anastomosis without increased incidence of leakage, stenosis or prolonged operative time. A published survey by Haverkamp et al. (20) shows that nowadays the preferred technique of anastomosis is to stapler the thoracic anastomosis and to hand-sew the cervical ones.
Full table
Conclusions
Esophagectomy is a complex and time-consuming procedure and it is associated with significant morbidity and mortality. As with other major procedures, surgeons have strived to increase the safety and efficacy of the procedure by employing minimally invasive techniques. Over the past 20 years, numerous papers regarding safety and efficacy of MIE were published, and it is clear nowadays that postoperative short and long-term results of conventional thoraco-laparoscopic MIE are similar to open esophagectomy. The role of robotic assistance is not well established, as this is more controversial. While direct clinical benefits to the patient may be difficult to clarify, benefits to the surgeon in terms of ease of the surgical performance and potential decrease in chronic work-related trauma and injuries may be significant. Another major challenge is to define what the robotic assistance is, since most of the reports on RAMIE use hybrid techniques using the robot only for some part of the procedure. It is therefore very difficult to compare the outcomes. In conclusion, robot-assisted resection for esophageal cancer is feasible, but a real benefit has not yet been demonstrated due to the limited number of randomized trials about RAMIE and lack of long-term oncological data (42). Despite its limitations and disadvantages there is little doubt that robotic assistance is here to stay. However, as esophagectomy is a challenging procedure teaching programs and proctoring are mandatory. As an example, a systematic teaching program in RAMIE at the Memorial Sloan Kettering Cancer Center in Pittsburgh started in 2014 and has reported excellent outcomes with increasing proficiency over the course of the surgeons’ learning curve (34).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mu J, Gao S, Mao Y, et al. Open three stage transthoracic esophagectomy versus minimally invasive thoracolaparoscopic esophagectomy for esophageal cancer. BMJ Open 2015;5:e008328. [Crossref] [PubMed]
- Cuschieri A, Shimi S, Banting S. Endoscopic esophagectomy trough a right thoracoscopic approach. J R Coll Surg Edinb 1992;37:7-11. [PubMed]
- Kauppila JH, Xie S, Johar A, et al. Meta-analysis of health-related quality of life after minimally invasive versus open oesophagectomy for oesophageal cancer. Br J Surg 2017;104:1131-40. [Crossref] [PubMed]
- Orringer MB, Marshall B, Stirling MC. Transhiatal esophagectomy for benign and malignant disease. J Thorac Cardiovasc Surg 1993;105:265. [PubMed]
- Horgan S, Berger RA, Elli E, et al. Robotic-assisted minimally invasive transhiatal esophagectomy. The American Surgeon 2003;69:624-6. [PubMed]
- Braghetto I, Csendes A, Cardemil G, et al. Open transthoracic or transhiatal esophagectomy versus minimally invasive esophagectomy in terms of morbidity, mortality and survival. Surg Endosc 2006;20:1681-6. [Crossref] [PubMed]
- DePaula AL, Hashiba K, Ferreira EB, et al. Laparoscopic transhiatal esophagectomy with esophagogasttroplasty. Surg Laparosc Endosc 1995;5:1-5. [PubMed]
- Verhage RJ, Hazebroek EJ, Boone J, et al. Minimally invasive surgery compared to open procedures in esophagectomy for cancer: a systematic review of the literature. Minerva Chir 2009;64:135-46. [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy. Review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Mariette C, Meunier B, Pezet D, et al. Hybrid minimally invasive versus open oesophagectomy for patients with oesophageal cancer: A multicenter, open-label, randomized phase III controlled trial, the MIRO trial. J Clin Oncol 2015;33:abstr 5.
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomized controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Smithers BM, Gotley DC, Martin I, et al. Comparison of the outcomes between open and minimally invasive esophagectomy Ann Surg 2007;245:232-40. [Crossref] [PubMed]
- Nguyen NT, Roberts P, Rivers R, et al. Thorachoscopic and laparoscopic esophagectomy for benign and malign disease: lessos learned from 46 consecutive procedures. J Am Coll Surg 2003;197:902-913. [Crossref] [PubMed]
- Avery KN, Metcalfe C. The feasibility of a randomized controlled trial of esophagectomy for esophageal cancer - the ROMIO study: protocol for a randomized controlled trial. Trials 2014;15:200. [Crossref] [PubMed]
- Zhu C. Study of Neo-adjuvant Chemoradiotherapy followed by minimally invasive esophagectomy for squamous cell esophageal cancer (NACRFMIE). Available online: NCT02188615.https//clinicaltrials.gov.
- Lee JM. Comparison of Ivor Lewis and tri incision approaches for patients with esophageal cancer. Available online: NCT02017002.https//clinicaltrials.gov.
- Sihag S, Kosinski S, Henning A, et al. Minimally invasive vs open esophagectomy for esophageal cancer: a comparison of early outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg 2016;101:1281-8. [Crossref] [PubMed]
- Osugi H, Takemura M, Higashino M, et al. Learning curve of video assisted thoraoscopic esophagectomy n d extensive limphadenectomy for sqaumous cell cancer of the thoracic esophagus and results. Surg Endosc 2003;17:515-9. [Crossref] [PubMed]
- Guo W, Ma X, Yang S, et al. Combined thoracoscopic-laparoscopic esophagectomy versus open esophagectomy: a meta-analysis of outcomes. Surg Endosc 2016;30:3873-81. [Crossref] [PubMed]
- Haverkamp L, Seesing MF, Ruurda JP, et al. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal cancer. Dis Esophagus 2017;30:1-7. [PubMed]
- Kumar A, Bin Asa B. Robotic thoracic surgery: the state of art. J minim Access Sur 2015;11:60-6.
- Giulianotti PC, Coratti A, Angelmini B, et al. Robotics in general surgery. Arch Surg 2003;138:777-84. [Crossref] [PubMed]
- Bodner JC, Zitt M, Mott H, et al. Robotic assisted thoracoscopic surgery (RATS) for benign and malignant esophageal tumors. Ann Thorac Surg 2005;80:1202-6. [Crossref] [PubMed]
- Gutt CN, Bintintan VV, Koninger J. Robot assisted transhiatal esophagectomy. Langenbecks Arch Surg 2006;391:428-34. [Crossref] [PubMed]
- Kernstine KH, DeArmond DT, Shamoun DN, et al. The first series of completely robotic esophagectomies with three-field lymphadenectomy: initial experience. Surg Endosc 2007;21:2285-92. [Crossref] [PubMed]
- Dunn DH, Johnson EM, Morphew JA, et al. Robot-assisted transhiatal esophagectomy: a 3 years single-center experience. Dis Esophagus 2013;26:159-66. [Crossref] [PubMed]
- de la Fuente SG, Weber J, Hoffe SF, et al. Initial experience from a large referral center with robotic assisted Ivor-Lewis esophagogastrectomy for oncologic purpose. Surg Endosc 2013;27:3339-47. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP. Robotic assisted minimally invasive esophagectomy: the Ivor Lewis approach. Thorac Surg Clin 2014;24:211-22. [Crossref] [PubMed]
- Boone J, Schipper ME, Moojen WA, et al. Robot-assisted thoracoscopic oesophagectomy for cancer. Br J Surg 2009;96:878-86. [Crossref] [PubMed]
- Weksler B, Sharma P, Moudgill N, et al. Robot assisted minimally invasive esophagectomy is equivalent to thoracoscopic mininvasive esophagectomy. Dis Esophagus 2012;25:403-9. [Crossref] [PubMed]
- Yerokun BA, Sun Z, Jeffrey Yang CF, et al. Minimally invasive vs open esophagectomy for esophageal cancer: a population based analysis. Ann Thor Surg 2016;102:403-9. [Crossref]
- van der Sluis PC, Ruurda JP, Verhage RJ, et al. Oncologic long term result of RAMIE with two field lymphadenectomy for esophageal cancer Ann Surg Oncol 2015;22:S1350-6. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, van der Horst S, et al. Robot assisted minimally invasive thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: a randomized controlled trial (ROBOT). Trials 2012;13:230. [Crossref] [PubMed]
- Okusanya OT, Sarkaria IS, Nicholas R, et al. Robotic assisted minimally invasive esophagectomy (RAMIE): the University of Pittsburgh Medical Center initial experience. Ann Cardiothorac Surg 2017;6:179-85. [Crossref] [PubMed]
- Okamura A, Watanabe M, Mine S, et al. Factors influencing difficulty of the thoracic procedure in minimally invasive surgery. Surg Endosc 2016;30:4279-85. [Crossref] [PubMed]
- Kim DJ, Park SY, Lee S, et al. Feasibility of a robotic assisted thoracoscopic lymphadenectomy along the recurrent laringeal nerves in radical esophagectomy for esophageal squamous carcinoma. Surg Endosc 2014;28:1866-73. [Crossref] [PubMed]
- Park SY, Kim DJ, Yu WS, et al. Robot-assisted thoracoscopic esophagectomy with extensive mediastinal lymphadenectomy: experience with 114 consecutive patients with intrathoracic esophageal cancer. Dis Esophagus 2016;29:326-32. [Crossref] [PubMed]
- Broussard B, Evans J, Wei B, et al. Robotic esophagectomy. J Vis Surg 2016;2:139-47. [Crossref]
- Cerfolio RJ, Wei B, Hawn MT, et al. Robotic esophagectomy for cancer: early results and lesson learned. Semin Thorac Cardiovasc Surg 2016;28:160-9. [Crossref] [PubMed]
- Trugeda S, Fernandez Diaz MJ, Gomez Fleitas M, et al. Initial result of Ivor Lewis oesophagectomy with intrathoracic hand sewn anastomosis in the prone position. Int J Med Robot 2014;10:397-403. [Crossref] [PubMed]
- Bongiolatti S, Annecchiarico M. Robotic-sewn Ivor Lewis anastomosis: preliminary experience and technical details. Int J Med Robot 2016;12:421-6. [Crossref] [PubMed]
- Falkenback D, Lehane CW, Lord RV. Robotic assisted gastrectomy band oesophagectomy for cancer. ANZ J Surg 2014;84:712-21. [Crossref] [PubMed]
Cite this article as: Taurchini M, Cuttitta A. Minimally invasive and robotic esophagectomy: state of the art. J Vis Surg 2017;3:125.