Robotic central pancreatectomy
Introduction
Historically, surgeons have faced a predicament when tackling centrally located pancreatic lesions in the neck or genu. Such lesions pose the problem of needing to resect sufficient parenchyma to minimize the risk of recurrence while simultaneously preserving enough parenchyma to maintain pancreatic endocrine function (1). Central pancreatectomy (CP) is a parenchyma-sparing procedure that can be utilized in the resection of tumors of the neck or the proximal body of the pancreas (2). However, after the operation two transected surfaces of the pancreas remain, which leaves the patient with an increased risk of developing a pancreatic fistula or leak at both ends of the exposed and divided pancreatic duct, a major drawback (3). Other terms used to refer to the CP include: medial, median, segmental, limited conservative, middle segment, intermediate pancreatectomy, and pancreatic isthmusectomy (1).
Oskar Ehrhardt first described the CP in 1908, when he published on segmental pancreatic neck resection (4). Finney, the first president of the American College of Surgeons, followed in 1910 and described the segmental pancreatic neck resection of a cystic tumor (5). Additionally, Takada et al. credited the first CP to Honjyo in 1950. However, Honjyo performed a central resection, and the distal pancreatic stump was not reconstructed (6). Guillemin and Bessot first described the concept of CP followed by reconstruction in 1957 (7). The authors’ patient presented with calcific chronic pancreatitis, and by attempting to visualize the main pancreatic duct they inadvertently transected the entire pancreatic neck and thus decided to drain the two pancreatic stumps with an omega jejunal loop. In 1984, Dagradi and Serio performed the first planned CP and reconstruction in order to resect an insulinoma of the pancreatic neck (8). This landmark operation marked the first use of CP (9).
Indications for CP include benign tumors between two and five cm in size, which typically involve the pancreatic duct, benign or low grade lesions at low risk of local regional recurrence, low grade malignant lesions such as neuroendocrine tumors, cystic lesions not suitable for enucleation especially in young patients, cysts that display indeterminate characteristics such as branch-duct-type IPMNs, symptomatic serous or mucinous cystadenomas, pseudopapillary tumors, focal chronic pancreatitis with isolated and short stenosis of Wirsung’s duct and solitary metastases in the pancreatic neck. Contraindications to this procedure include malignant tumors such as ductal adenocarcinomas, neoplastic involvement of other adjacent organs such as the stomach or colon, diffuse chronic pancreatitis, large lesions where it is impossible to preserve at least five cm of the left or distal pancreatic stump and distal body-tail atrophy (1,10,11).
Technique
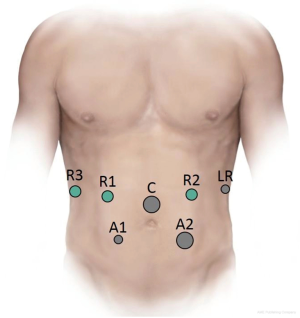
The approach adopted by our experts at the University of Pittsburgh Medical Center (UPMC) is as follows. The patient is placed on a split-leg table in the supine position with the left arm extended at 90° on the arm board. An orogastric tube, a Foley catheter and monitoring lines are inserted. An optical separator is first inserted in the left midclavicular line to access the peritoneal cavity. Next, a 12-mm camera port is placed in the supraumbilical midline, and a 5-mm port is placed in the left anterior axillary line for the liver retractor. Port placement is then continued with the insertion of two robotic 8-mm ports in the right upper quadrant, a 5-mm port in the right lower quadrant and 12-mm assistant port in the left lower quadrant (Figure 1). The patient is then placed in reverse Trendelenburg with left side up. The 12-mm ports are closed with a 0-polysorb suture on a Carter-Thompson needle in figure-of-eight fashion.
Subsequently, the robot is docked over the patient’s head with two arms on the patient’s right side. Of note, two robot arms can also be placed in the left upper quadrant as well. If there is a high chance of performing a distal pancreatectomy (DP) then this is the preference, but both are equally feasible. The standard practice at UPMC is using a Hook monopolar (Intuitive Surgical, Sunnyvale, CA, USA) in console surgeon’s right hand and a Fenestrated bipolar in the left hand. The third arm typically has a Cadiere or ProGrasp. The laparoscopic assistant typically has a blunt tipped 5-mm Ligasure (Covidien, Boulder, CO, USA) in the right hand and a suction irrigator in the left hand.
Entry to the lesser sac is achieved through the gastrocolic omentum below the gastroepiploic pedicle. Elevation of the posterior wall of the stomach and exposure of the anterior surface of the pancreas is done using the Mediflex (Mediflex Surgical Products, Islandia, NY, USA) liver retractor. Afterwards, an ultrasound assists in identifying the tumor (Figure 2A), determining the lesion’s borders and marking the margins of resection. The extent of the right border is the gastroduodenal artery. This can be mobilized 1–2 mm; however, going any more to the right risks injury to the common bile duct. The left has no anatomic border, but the only worth risk is of the pancreatic anastomosis if adequate parenchyma is spared.
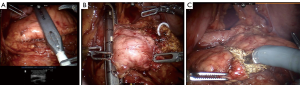
After resection, the inferior border of the pancreas is mobilized to elevate the pancreatic neck from the splenic vein-superior mesenteric vein confluence. Exposure to the common hepatic artery, gastroduodenal artery and portal vein at the superior border of the pancreas is easily achieved with removal of the hepatic artery (8A) lymph node. Following removal of this node, the superior pancreatic neck is dissected off the portal vein. A tunnel is created under the neck of the pancreas from the superior mesenteric vein to the portal vain. The pancreatic neck is then divided with a vascular stapler (Figure 2B). The neck is usually thin, allowing for this method of transection. Depending on the thickness and consistency of the pancreas, it may be necessary to use a larger stapler or transect with scissors. If the latter is necessary, the pancreatic duct is ligated with a 4-0 polydioxanone suture and the pancreatic parenchyma is oversewn with 3-0 vloc (Covidien, Boulder, CO, USA) horizontal mattress sutures.
Taking precaution to identify and control the left gastric vein as necessary, the central pancreas is then elevated from the splenic vein and artery origin. The dissection between the pancreatic remnant and splenic vein is continued distally using the ultrasound to mark its boundaries in order to ensure adequate tumor surgical margins. Afterwards, the pancreas is transected with monopolar scissors (Figure 2C). The transverse pancreatic arteries are easily controlled using the bipolar, as sutures are not necessary along the transverse pancreatic vessels. Using a 12-mm EndoCatch (Covidien, New Haven, CT, USA) bag in the left lower quadrant port, the specimen is removed and frozen section examination of the margins is performed if necessary. If the consistency of the gland is very high risk: soft, friable, tiny duct or if the remnant is small, the CP would be aborted in favor of the DP.
Several options for pancreatic reconstruction exist. In our early experience, we performed a pancreaticogastrostomy (PG). We have subsequently switched to a pancreaticojejunostomy (PJ). Early in the experience the PG was technically easier. However, the downside to a PG is that when a patient leaks, feeding can be problematic, thus necessitating parenteral nutrition. With our more robust experience performing robotic pancreatoduodenectomies, we have transitioned to the PJ.
For the PG, we initially mobilize the greater curvature of the stomach. Next, the anterior surface of the pancreatic remnant is anchored to the posterior wall of the stomach using interrupted horizontal mattress sutures. The pancreatogastrostomy is created using a modified Blumgart technique. First, a small gastrotomy is created using cautery scissors and a pancreatic duct-to-gastric mucosa anastomosis is produced using interrupted 5-0 PDS and bridged with a 7 French Zimmon pancreatic duct stent (Winston-Salem, NC, USA). The posterior surface of the anastomosis is then completed using the transfixion sutures already in place. In the end, two closed-suction drains are placed around the surgical field (1).
Before commencement of the PJ, we first bring up a roux-limb and perform a jejunojejunostomy. We then lift up the transverse mesocolon, identify the ligament of Treitz and measure 40 cm distal. At this point, we make a window in the mesentery and transect the bowel with a linear stapler. Using the Ligasure (Covidien, Boulder, CO, USA), we take a couple bites in the mesentery, sparing the arcade. We measure another 40–50 cm for the roux-limb and pull this antecolic or retrocolic depending on body habitus, making sure it meets the pancreas without tension. The stapled end of the roux-limb is sutured by the pancreas temporarily to hold it in place. A stitch is then placed by the staple line of the pancreaticobiliary limb and sutured to the bottom portion of the roux-limb. The third hand then pulls this stich cranially. After, using the monopolar, a hole is made in the anti-mesenteric surface of both loops of bowel. A 60-mm stapler is then inserted and fired. The common enterotomy is closed using a 6-inch 4-0 vloc suture in running fashion with lembert stitches.
We perform the PJ using a modified Blumgart technique with three 2-0 silk stitches on a V-20 cut to eight inches and six 5-0 PDS stitches on a CV-23 cut to five inches. We place full-thickness mattress stitches through the pancreas and seromuscular through the bowel. These are tied and the needles left intact. A small enterotomy is made in roux-limb jejunum. Two posterior duct-to-mucosa PDS stitches are placed. A 4 or 5 French Hobbs Stent (Hobbs Medical, Stafford Springs, CT) is placed into duct and bowel. Then four anterior duct-to-mucosa stitches are placed. For these three rows of stitches, our standard practice is to place each row, then tie each row. The last row is a buttress layer using previously placed silk stitches. These are placed seromuscular in bowel and tied as they are placed.
Video clinical vignette
This video depicts a 40-year-old male with a newly discovered well-differentiated pancreatic neuroendocrine tumor (Figure 3). He underwent an endoscopic ultrasound that showed a round 12 mm × 11 mm mass in the genu of the pancreas, with well-defined borders. Immunostains were positive for synaptophysin and chromogranin, and weakly positive for CKAE1/AE3. A Ki-67 stain shows a proliferative index of less than 1%. This was initially surveyed with an MRI that demonstrated a stable ovoid mass lesion in the central portion of the pancreas at the junction of the head and neck, measuring 12 mm × 7 mm, consistent with the EUS findings. Given the patient’s young age in face of long term surveillance, the patient underwent a robotic CP without any complications. The pathology results demonstrated a well-differentiated pancreatic neuroendocrine tumor, 1.4 cm, WHO grade 1. The Ki-67 index was 1.5%, and zero mitoses per HPF. There was no angiolymphatic or perineural invasion. All margins were negative, and there was no nodal involvement. The patient did well postoperatively and was discharged on post-op day seven with no issues.

CP outcomes
There have been various published series on open CP (Table 1). Among 872 open CP reported since 1993, the mean rate of morbidity was 43.2% and mean rate of mortality was 0.24%. The mean pancreatic fistula rate was 28%. The rate of clinically significant pancreatic fistulas with ISGPF Grades B and C was 19%. However, ISGPF nomenclature was not used in all publications. The rate of development of post-operative diabetes mellitus was at 2% and the average incidence of exocrine insufficiency experienced by patients undergoing open CP was 4.4%. Finally, the mean length of hospital stay was around 15 days.
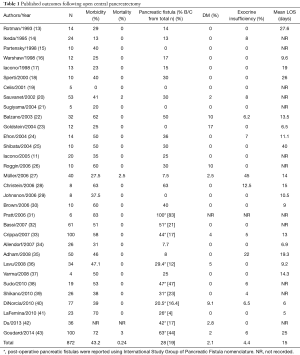
Full table
In 2003, Baca and Bokan performed the first laparoscopic CP on a patient with a pancreatic cystadenoma (9). Subsequently, there have been several published case series regarding laparoscopic CP since 2003 (Table 2). Recently, the addition of robotic assistance to laparoscopy has redefined minimally invasive surgery by adding the benefits of three dimensional binocular vision, scaling, stabilization of tremor, reduced operative fatigue and improved ergonomics from the console-surgeon interface. In 2004, Giulianotti et al. performed the first robot CP (2). In addition to laparoscopic case series, there have been a handful of published case series regarding robotic CP (Table 2). In total, 100 patients underwent either laparoscopic or robotic CP. Among those, the mean rate of morbidity was 37.3% and the mean rate of mortality was 0%. In addition, the mean rate of development of pancreatic fistula was 36.6%. This relatively high rate compared to the open case series may be due to the much lower sample size in series for minimally invasive surgery for CP. The rate of clinically significant pancreatic fistulas with ISGPF Grades B and C was 17%. However, ISGPF nomenclature was not used in all publications. The rate of development of post-operative diabetes mellitus was at 1.5%. None of the patients included in these series developed any postoperative exocrine insufficiency. The mean length of hospital stay was around 13 days. Ultimately, laparoscopic and robotic-assisted CP can be performed safely with oncologic outcomes comparable to published open series. In conclusion, these multiple series show that laparoscopic and robotic-assisted approaches to CP are safe and feasible and may offer better outcomes for patients.
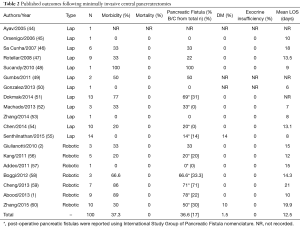
Full table
CP vs. DP
In recent times, the diagnosis of incidental pancreatic neoplasms has been on the rise due to the increased use of advanced cross-sectional imaging such as computed tomography (CT) and other imaging modalities to assess nonspecific abdominal symptoms (61). Surgeons may choose to approach such lesions differently by performing either a DP or a CP, among others procedures. Surgeons will weigh up whether to choose a parenchyma-sparing surgery that has a higher risk of postoperative leak and lower risk of long-term endocrine and exocrine insufficiency or a procedure with the opposite outcomes.
Ocuin et al. published a study in 2008 comparing 13 patients who underwent CP to 19 patients who underwent an extended left pancreatectomy or DP. It was found that CP patients were significantly more likely to experience complications than those undergoing DP (92% vs. 39%, P=0.003) (61). The likelihood of a major complication was only 21% greater in the CP group, but the rate of development of a pancreatic fistula was significantly higher (62% vs. 11%, P=0.003). Of those developing a pancreatic fistula, the rate of clinically significant fistulas (ISGPF grade B and C) was 38% in the CP group vs. 5.5% in the DP group (P=0.22). However, the DP group had a 17% higher rate of exocrine insufficiency requiring pancreatic enzyme supplementation and had a significantly higher incidence of new-onset diabetes mellitus (57% vs. 11%, P=0.04). The only case of new-onset diabetes in the CP group was managed with diet only compared to the patients in the DP group who required medical therapy (61).
Iacono et al. performed a meta-analysis of patients who underwent a CP vs. patients who underwent a DP. Their study included 359 patients treated by CP and 480 patients treated by DP. Similar to the study performed by Ocuin et al., the overall morbidity was significantly higher after a CP, as was the incidence of pancreatic fistula. However in the Iacono study, CP was found to be associated with a significantly reduced risk of reoperation. The study also found a significant reduction in the incidence of long term endocrine failure after CP, but the reduction in exocrine failure was of only marginal significance (62). Furthermore, Müller et al. compared patients undergoing CP to patients undergoing a standard pancreaticoduodenectomy (PD) in addition to patients undergoing a DP. The pancreatic fistula rates for patients undergoing CP, DP and PD were 7.5%, 10% and 2.5%, respectively, although these results were non-significant. However, their study showed a significantly lower rate of endocrine insufficiency in the CP group (15% vs. 42% for DP, 29% for PD, P<0.05) (27). This study gave proof to the safety and feasibility of CP in comparison to other procedures for selected patients with benign or low malignant lesions. Finally, Crippa et al. compared 100 patients undergoing CP over a 15-year period and found that CP was associated with higher morbidity (51% vs. 36%, P=NS) but a lower incidence of long-term endocrine and exocrine insufficiency when compared to patients who underwent DP (33).
Conclusions
In summary, the major motivation behind CP performance is parenchymal conservation. As a result, this procedure should reduce the risk of postoperative diabetes and exocrine insufficiency and allow for the preservation of the spleen and its immunological properties, thus providing good long-term quality of life. Standard procedures such as DP and PD are associated with lower rates of short-term morbidity such as pancreatic fistula development but are also accompanied with a higher rate of long-term endocrine and exocrine insufficiency due to the significant loss of normal pancreatic parenchyma when compared to CP. Minimally invasive surgery such as laparoscopy or robotic-assisted laparoscopy reduces the risk of postoperative morbidity usually encountered with open surgery. The robotic interface offers many technical advantages that overcome the limitations of laparoscopic surgery, such as 2-dimensional imaging, limited range of instrument motion and poor surgeon ergonomics, while permitting a meticulous dissection and reconstruction. It can be inferred, albeit from limited and small retrospective studies and case reports, that conventional and robotic-assisted laparoscopic approaches to CP are safe and feasible in highly specialized centers. However, robotic surgery imposes significant cost and a long learning curve for surgeons training to adopt this new technology. There is a need to further perform comparative studies on the efficacy of minimally invasive approaches with larger population samples.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Abood GJ, Can MF, Daouadi M, et al. Robotic-assisted minimally invasive central pancreatectomy: technique and outcomes. J Gastrointest Surg 2013;17:1002-8. [Crossref] [PubMed]
- Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic middle pancreatectomy. J Laparoendosc Adv Surg Tech A 2010;20:135-9. [Crossref] [PubMed]
- Winer J, Can MF, Bartlett DL, et al. The current state of robotic-assisted pancreatic surgery. Nat Rev Gastroenterol Hepatol 2012;9:468-76. [Crossref] [PubMed]
- Ehrhardt O. Ueber Resektionen am Pancreas. Dtsch med Wochenschr 1908;34:595-7. [Crossref]
- Finney JM. VII. Resection of the Pancreas: Report of a Case. Ann Surg 1910;51:818-29. [Crossref] [PubMed]
- Takada T, Yasuda H, Uchiyama K, et al. A proposed new pancreatic classification system according to segments: Operative procedure for a medial pancreatic segmentectomy. J Hepatobiliary Pancreat Surg 1994;1:322-5. [Crossref]
- Guillemin P, Bessot M. Chronic calcifying pancreatitis in renal tuberculosis: pancreatojejunostomy using an original technic. Mem Acad Chir (Paris) 1957;83:869-71. [PubMed]
- Dagradi A, Serio G. Pancreatectomia intermedia. Enciclopedia Medica Italiana, vol XI: pancreas. Firenze: USES Edizioni Scientifiche, 1984:850-1.
- Iacono C, Ruzzenente A, Bortolasi L, et al. Central pancreatectomy: the Dagradi Serio Iacono operation. Evolution of a surgical technique from the pioneers to the robotic approach. World J Gastroenterol 2014;20:15674-81. [Crossref] [PubMed]
- Ronnekleiv-Kelly SM, Javed AA, Weiss MJ. Minimally invasive central pancreatectomy and pancreatogastrostomy: current surgical technique and outcomes. J Vis Surg 2016;2:138. [Crossref]
- Iacono C, Bortolasi L, Serio G. Indications and technique of central pancreatectomy-early and late results. Langenbecks Arch Surg 2005;390:266-71. [Crossref] [PubMed]
- Hamad A, Novak S, Hogg ME. Technique for robotic-assisted central pancreatectomy. Asvide 2017;4:282. Available online: http://www.asvide.com/articles/1591
- Rotman N, Sastre B, Fagniez PL. Medial pancreatectomy for tumors of the neck of the pancreas. Surgery 1993;113:532-5. [PubMed]
- Ikeda S, Matsumoto S, Maeshiro K, et al. Segmental pancreatectomy for the diagnosis and treatment of small lesions in the neck or body of the pancreas. Hepatogastroenterology 1995;42:730-3. [PubMed]
- Partensky C, Apa D, Marchal F, et al. Medial pancreatectomy with pancreato-gastric anastomosis for resection of pancreatic tumors. Chirurgie 1998;123:363-7. [Crossref] [PubMed]
- Warshaw AL, Rattner DW, Fernandez-del Castillo C, et al. Middle segment pancreatectomy: a novel technique for conserving pancreatic tissue. Arch Surg 1998;133:327-31. [Crossref] [PubMed]
- Iacono C, Bortolasi L, Serio G. Is there a place for central pancreatectomy in pancreatic surgery? J Gastrointest Surg 1998;2:509-16; discussion 516-7. [Crossref] [PubMed]
- Sperti C, Pasquali C, Ferronato A, et al. Median pancreatectomy for tumors of the neck and body of the pancreas. J Am Coll Surg 2000;190:711-6. [Crossref] [PubMed]
- Celis J, Berrospi F, Ruiz E, et al. Central pancreatectomy for tumors of the neck and body of the pancreas. J Surg Oncol 2001;77:132-5. [Crossref] [PubMed]
- Sauvanet A, Partensky C, Sastre B, et al. Medial pancreatectomy: a multi-institutional retrospective study of 53 patients by the French Pancreas Club. Surgery 2002;132:836-43. [Crossref] [PubMed]
- Sugiyama M, Abe N, Ueki H, et al. Pancreaticogastrostomy for reconstruction after medial pancreatectomy. J Am Coll Surg 2004;199:163-5. [Crossref] [PubMed]
- Balzano G, Zerbi A, Veronesi P, et al. Surgical treatment of benign and borderline neoplasms of the pancreatic body. Dig Surg 2003;20:506-10. [Crossref] [PubMed]
- Goldstein MJ, Toman J, Chabot JA. Pancreaticogastrostomy: a novel application after central pancreatectomy. J Am Coll Surg 2004;198:871-6. [Crossref] [PubMed]
- Efron DT, Lillemoe KD, Cameron JL, et al. Central pancreatectomy with pancreaticogastrostomy for benign pancreatic pathology. J Gastrointest Surg 2004;8:532-8. [Crossref] [PubMed]
- Shibata S, Sato T, Andoh H, et al. Outcomes and indications of segmental pancreatectomy. Comparison with distal pancreatectomy. Dig Surg 2004;21:48-53. [Crossref] [PubMed]
- Roggin KK, Rudloff U, Blumgart LH, et al. Central pancreatectomy revisited. J Gastrointest Surg 2006;10:804-12. [Crossref] [PubMed]
- Müller MW, Friess H, Kleeff J, et al. Middle segmental pancreatic resection: An option to treat benign pancreatic body lesions. Ann Surg 2006;244:909-18; discussion 918-20. [Crossref] [PubMed]
- Christein JD, Smoot RL, Farnell MB. Central pancreatectomy: a technique for the resection of pancreatic neck lesions. Arch Surg 2006;141:293-9. [Crossref] [PubMed]
- Johnson MA, Rajendran S, Balachandar TG, et al. Central pancreatectomy for benign pancreatic pathology/trauma: is it a reasonable pancreas-preserving conservative surgical strategy alternative to standard major pancreatic resection? ANZ J Surg 2006;76:987-95. [Crossref] [PubMed]
- Brown KM, Shoup M, Abodeely A, et al. Central pancreatectomy for benign pancreatic lesions. HPB (Oxford) 2006;8:142-7. [Crossref] [PubMed]
- Pratt W, Maithel SK, Vanounou T, et al. Postoperative pancreatic fistulas are not equivalent after proximal, distal, and central pancreatectomy. J Gastrointest Surg 2006;10:1264-78; discussion 1278-9. [Crossref] [PubMed]
- Bassi C. Middle segment pancreatectomy: a useful tool in the management of pancreatic neoplasms. J Gastrointest Surg 2007;11:726-9. [Crossref] [PubMed]
- Crippa S, Bassi C, Warshaw AL, et al. Middle pancreatectomy: indications, short- and long-term operative outcomes. Ann Surg 2007;246:69-76. [Crossref] [PubMed]
- Allendorf JD, Schrope BA, Lauerman MH, et al. Postoperative glycemic control after central pancreatectomy for mid-gland lesions. World J Surg 2007;31:164-8; discussion 169-170. [Crossref] [PubMed]
- Adham M, Giunippero A, Hervieu V, et al. Central pancreatectomy: single-center experience of 50 cases. Arch Surg 2008;143:175-80; discussion 180-171; discussion 180-171.
- Lavu H, Knuth JL, Baker MS, et al. Middle segment pancreatectomy can be safely incorporated into a pancreatic surgeon's clinical practice. HPB (Oxford) 2008;10:491-7. [Crossref] [PubMed]
- Varma V, Gandhi V, Bheerappa N, et al. Central pancreatectomy for neoplasm of mid pancreas - a report of four cases. Indian J Surg 2008;70:237-40. [Crossref] [PubMed]
- Sudo T, Murakami Y, Uemura K, et al. Middle pancreatectomy with pancreaticogastrostomy: a technique, operative outcomes, and long-term pancreatic function. J Surg Oncol 2010;101:61-5. [Crossref] [PubMed]
- Shikano T, Nakao A, Kodera Y, et al. Middle pancreatectomy: safety and long-term results. Surgery 2010;147:21-9. [Crossref] [PubMed]
- DiNorcia J, Ahmed L, Lee MK, et al. Better preservation of endocrine function after central versus distal pancreatectomy for mid-gland lesions. Surgery 2010;148:1247-54; discussion 1254-6. [Crossref] [PubMed]
- LaFemina J, Vagefi PA, Warshaw AL, et al. Transgastric pancreaticogastric anastomosis: an alternative operative approach for middle pancreatectomy. Arch Surg 2010;145:476-81. [Crossref] [PubMed]
- Du ZY, Chen S, Han BS, et al. Middle segmental pancreatectomy: a safe and organ-preserving option for benign and low-grade malignant lesions. World J Gastroenterol 2013;19:1458-65. [Crossref] [PubMed]
- Goudard Y, Gaujoux S, Dokmak S, et al. Reappraisal of central pancreatectomy a 12-year single-center experience. JAMA Surg 2014;149:356-63. [Crossref] [PubMed]
- Ayav A, Bresler L, Brunaud L, et al. Laparoscopic approach for solitary insulinoma: a multicentre study. Langenbecks Arch Surg 2005;390:134-40. [Crossref] [PubMed]
- Orsenigo E, Baccari P, Bissolotti G, et al. Laparoscopic central pancreatectomy. Am J Surg 2006;191:549-52. [Crossref] [PubMed]
- Sa Cunha A, Rault A, Beau C, et al. Laparoscopic central pancreatectomy: single institution experience of 6 patients. Surgery 2007;142:405-9. [Crossref] [PubMed]
- Rotellar F, Pardo F, Montiel C, et al. Totally laparoscopic Roux-en-Y duct-to-mucosa pancreaticojejunostomy after middle pancreatectomy: a consecutive nine-case series at a single institution. Ann Surg 2008;247:938-44. [Crossref] [PubMed]
- Sucandy I, Pfeifer CC, Sheldon DG. Laparoscopic assisted central pancreatectomy with pancreaticogastrostomy reconstruction - An alternative surgical technique for central pancreatic mass resection. N Am J Med Sci 2010;2:438-41. [Crossref] [PubMed]
- Gumbs AA, Rodriguez-Rivera AM, Hoffman JP. Minimally invasive pancreatic surgery of the entire gland: initial experience. Minerva Chir 2011;66:269-80. [PubMed]
- Gonzalez F, Mesleh MG, Lukens FJ, et al. Laparoscopic central pancreatectomy and pancreaticogastrostomy for the management of a proximally migrated pancreatic stent. JOP 2013;14:273-6. [PubMed]
- Dokmak S, Aussilhou B, Fteriche FS, et al. Pure laparoscopic middle pancreatectomy: single-center experience with 13 cases. Surg Endosc 2014;28:1601-6. [Crossref] [PubMed]
- Machado MA, Surjan RC, Epstein MG, et al. Laparoscopic central pancreatectomy: a review of 51 cases. Surg Laparosc Endosc Percutan Tech 2013;23:486-90. [Crossref] [PubMed]
- Zhang RC, Xu XW, Zhou YC, et al. A rare case of mixed mucinous cystadenoma with serous cystadenoma of the pancreas treated by laparoscopic central pancreatectomy. World J Surg Oncol 2014;12:318. [Crossref] [PubMed]
- Chen XM, Zhang Y, Sun DL. Laparoscopic central pancreatectomy for solid pseudopapillary tumors of the pancreas: our experience with ten cases. World J Surg Oncol 2014;12:312. [Crossref] [PubMed]
- Senthilnathan P, Gul SI, Gurumurthy SS, et al. Laparoscopic central pancreatectomy: Our technique and long-term results in 14 patients. J Minim Access Surg 2015;11:167-171. [Crossref] [PubMed]
- Kang CM, Kim DH, Lee WJ, et al. Initial experiences using robot-assisted central pancreatectomy with pancreaticogastrostomy: a potential way to advanced laparoscopic pancreatectomy. Surg Endosc 2011;25:1101-6. [Crossref] [PubMed]
- Addeo P, Marzano E, Nobili C, et al. Robotic central pancreatectomy with stented pancreaticogastrostomy: operative details. Int J Med Robot 2011;7:293-7. [PubMed]
- Boggi U, Amorese G, De Lio N, et al. Central pancreatectomy with inframesocolic pancreatojejunostomy. Langenbecks Arch Surg 2012;397:1013-21. [Crossref] [PubMed]
- Cheng K, Shen B, Peng C, et al. Initial experiences in robot-assisted middle pancreatectomy. HPB (Oxford) 2013;15:315-21. [Crossref] [PubMed]
- Zhang T, Wang X, Huo Z, et al. Robot-Assisted Middle Pancreatectomy for Elderly Patients: Our Initial Experience. Med Sci Monit 2015;21:2851-60. [Crossref] [PubMed]
- Ocuin LM, Sarmiento JM, Staley CA, et al. Comparison of central and extended left pancreatectomy for lesions of the pancreatic neck. Ann Surg Oncol 2008;15:2096-103. [Crossref] [PubMed]
- Iacono C, Verlato G, Ruzzenente A, et al. Systematic review of central pancreatectomy and meta-analysis of central versus distal pancreatectomy. Br J Surg 2013;100:873-85. [Crossref] [PubMed]
Cite this article as: Hamad A, Novak S, Hogg ME. Robotic central pancreatectomy. J Vis Surg 2017;3:94.


