
PD-L1 immunohistochemistry comparison of 22C3 and 28-8 assays for gastric cancer
Introduction
Gastric cancer is the fifth most common cancer and the third most common cause of cancer-related deaths worldwide (1). Although advanced gastric cancer is treated with platinum and fluoropyrimidine doublet as first-line treatment, its prognosis remains poor at 8–15 months after initial treatment (2-4).
Recently, immune checkpoint inhibitors targeting programmed death-1 (PD-1) or programmed death ligand 1 (PD-L1) showed improved survival of patients with various solid tumors compared with standard treatment options (5,6). PD-L1 and its partner PD-L2, transmembrane proteins expressed by normal tissues, inhibit T-cell activation and prevent autoimmunity. The binding of PD-1/PD-L1 on tumor cells (TCs) or tumor-infiltrating immune cells (ICs) was reported to induce T-cell tolerance. Therefore, antibodies that block this interaction showed benefit in clinical trials of patients with refractory malignancies (7). A multicenter, double-blind, randomized phase III trial (ATTRACTION 2) reported nivolumab, a fully human IgG4 monoclonal antibody against PD-1, improved survival as a third-line treatment for advanced gastric cancer compared with placebo (8). The phase 2 non-randomized KEYNOTE 059 trial reported radiological response rates were improved in gastric cancer patients with overexpressed PD-L1 proteins in TCs and ICs who were treated with pembrolizumab, another anti-PD-1 monoclonal antibody (9). In the CheckMate 649 trial, nivolumab significantly improved the overall survival (OS) and progression-free survival (PFS) of patients with a positive PD-L1 combined proportion score (CPS) using the Dako PD-L1 immunohistochemistry 28-8 pharmDx assay (10). However, the KEYNOTE-061 and KEYNOTE-062 trials evaluated tumors expressing high levels of PD-L1 (CPS ≥10) by the PD-L1 IHC 22C3 pharmDx assay (11,12). Therefore, several PD-L1 immunohistochemistry IHC assays using scoring criteria, including the tumor proportion score (TPS), have been developed in parallel (13).
Recent genomic and molecular characterization studies of gastric adenocarcinoma (GAC), such as the Cancer Genome Atlas (TCGA), have characterized PD-L1 positivity and four different subtypes (14). PD-L1 expression is high in microsatellite instability (MSI) gastric cancer and Epstein-Barr virus (EBV)-positive gastric cancer, which are susceptible to treatment with immune checkpoint inhibitors (15).
Based on these results, we planned a single-center, non-intervention, retrospective, observational study to investigate the clinicopathological features of PD-L1 expression using three different tissue microarrays (TMA) in patients with esophagogastric cancer.
We present the following article in accordance with the REMARK reporting checklist (available at https://dx.doi.org/10.21037/jgo-21-505).
Methods
Case selection
The patient selection criteria were as follows: (I) histological diagnosis of esophagogastric adenocarcinoma (stage I–IV); (II) underwent gastrectomy at a single institution from 2009 to 2010; (III) sufficient tumor content in formalin-fixed paraffin-embedded (FFPE) samples; and (IV) no systemic chemotherapy before surgery. The clinicopathological patient characteristics, including age, sex, tumor location, tumor-node-metastasis (TNM) stage (the 7th edition of the American Joint Committee on Cancer staging manual), and tumor histology were collected. This study was approved by the Institutional Review Board (IRB) at Aichi Cancer Center Hospital (IRB reference number: 2020-1-496) and conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all individual participants.
Construction of TMA
Tumor gastric FFPE samples were collected from gastrectomy specimens. The representative tumor regions were selected and marked in hematoxylin and eosin (H&E)-stained slides of each case by two pathologists. Briefly, four representative cores (2-mm diameter) with sufficient tissue quality and amount of tissue were stamped out of the donor block and transferred to a recipient paraffin block. Serial 4-μm sections were used for H&E staining, IHC, and in situ hybridization (ISH).
Immunohistochemistry
The PD-L1 expression of TCs and ICs, mismatch repair (MMR) (MLH1/MSH2/MSH6/PMS2) and HER2 were evaluated by IHC. PD-L1 positivity was evaluated by the CPS, which was defined as the number of PD-L1 stained cells (TCs, lymphocytes, macrophages) as a proportion of the total number of TCs multiplied by 100 (16). The TPS was also evaluated by membrane staining as follows: score 3+, ≥50%; score 2+, ≥25, <50%; and score 1+, ≥1, <25%. Lacking MLH1, MSH2, MSH6, or PMS2 was defined as defective MMR (dMMR), and maintaining MLH1, MSH2, MSH6, and PMS2 was defined as MMR proficient (pMMR). EBV positive was defined by chromogenic encoded ribonucleic acid in situ hybridization (INFORM EBER probe, Ventana, Tucson, USA). HER2 positivity was defined as an IHC score of 3+ or an IHC score of 2+. ISH positivity was determined by fluorescence ISH or dual-color ISH. HER2 and ISH positivity are considered indications for using trastuzumab as in the ToGA trial (17,18). Antibodies used for IHC analysis were summarized in Table S1. IHC was performed with a Dako Autostainer Link48. ES and YY evaluated the immunostaining as board certified pathologists.
Statistical analysis
The statistical significance of differences in proportions and medians were compared using independent χ2 tests or Fisher’s exact test for categorical variables. Interobserver concordance was assessed to compare the dichotomized expression values between each assay using Cohen’s Kappa (non-weighted) method. Kappa scores of 0.9 or higher were considered near perfect, scores of 0.80 to 0.89 were considered strong, scores of 0.60 to 0.79 were considered moderate, and scores of 0.40 to 0.59 were considered weak (19). The OS was defined as the time from the date of gastrectomy until death from any cause or censored at the last follow-up date. Median OS was estimated by the Kaplan-Meier method. The hazard ratio (HR) and 95% confidence interval (CI) were estimated using the Cox proportional hazards model. Statistical analyses were performed using R software version 4.1.0 (R Project for Statistical Computing, Vienna, Austria). All tests were two-sided and P values <0.05 were considered statistically significant.
Results
Patient characteristics and prevalence of PD-L1 expression
Of 331 patients who underwent gastrectomy at Aichi Cancer Center Hospital during the study period, 105 were excluded for receiving neoadjuvant chemotherapy (N=26) or insufficient tumor content (N=79). Finally, 226 patients were selected for this study. Patient demographics are shown in Table 1. The median age was 65 years (range, 32–86 years), and the pathological TNM stage included 100 cases of stage I (44%), 39 cases of stage II (17%), 58 cases of stage III (26%), and 29 cases of stage IV (13%). EBV was detected in 13 patients (6%) and dMMR was observed in 29 cases (13%). Of 79 patients (35%) who received adjuvant chemotherapy, 69 were treated with S-1 (tegafur, gimeracil, and oteracil potassium) monotherapy.
Table 1
| Characteristics | Categories | N=226 | % |
|---|---|---|---|
| Age, years | Median [range] | 65 [32–86] | – |
| <65/≥65 | 113/113 | 50/50 | |
| Sex | Male/female | 162/64 | 72/28 |
| Tumor location | EGJ/U/M/L | 12/47/85/82 | 5/21/38/36 |
| Depth of invasion | T1/T2/T3/T4 | 94/25/32/75 | 42/11/14/33 |
| Lymph node metastasis | N0/N1/N2/N3 | 111/32/29/54 | 49/14/13/24 |
| TNM stage | I/II/III/IV | 100/39/58/29 | 44/17/26/13 |
| Tumor histology | Diffuse/intestinal | 87/139 | 39/61 |
| EBV | Positive/negative | 13/213 | 6/94 |
| MMR | pMMR/dMMR | 197/29 | 87/13 |
| HER2 | Positive/negative | 24/202 | 11/89 |
| Adjuvant chemotherapy | Yes/no | 79/147 | 35/65 |
| PD-L1 CPS (22C3) | ≥1/≥5/≥10 | 63/25/17 | 28/11/8 |
| PD-L1 CPS (28-8) | ≥1/≥5/≥10 | 45/22/16 | 20/10/7 |
| PD-L1 CPS (E1L3) | ≥1/≥5/≥10 | 79/33/23 | 35/15/10 |
CPS, combined positive score; dMMR, defective mismatch repair; EBV, Epstein-Barr virus; GEJ, esophagogastric junction; HER2, human epidermal growth factor 2; L, lower third; M, middle third; MMR, mismatch repair; PD-L1, programmed death ligand-1; pMMR, mismatch repair proficient; U, upper third.
The 22C3 pharmDx assay demonstrated the numbers of patients with PD-L1 expression CPS ≥1, ≥5, and ≥10 were 63 (28%), 25 (11%), and 17 (8%), respectively (Figure 1). Numbers of patients with PD-L1 expression CPS ≥1, ≥5, and ≥10 by the 28-8 pharmDx assay were 45 (20%), 22 (10%), and 16 (7%), respectively. Higher levels of PD-L1 expression at a PD-L1 CPS ≥5 by the 22C3 pharmDx assay were more frequently observed in older patients (P=0.032), those with a tumor in the upper or lower stomach (P=0.017), or who were EBV positive (P=0.008), or dMMR (P=0.001) (Table 2). In contrast, using the 28-8 pharmDx assay, PD-L1 expression with CPS ≥5 was significantly associated with a tumor location in the upper or lower stomach (P=0.017), stage II or III (P=0.049), EBV positive (P=0.004), and dMMR (P=0.012). The clinicopathological features obtained from the analysis of PD-L1 positivity evaluated by CPS in the E1L3 assay and examined by TPS in each assay are presented in Tables S2-S7. The PD-L1 positivity rate (range, 3–5%) of TPS was markedly lower than that of CPS in all three assays.
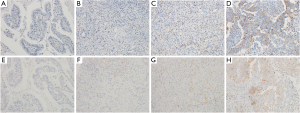
Table 2
| Characteristics | Categories | PD-L1, N [%] | ||||||
|---|---|---|---|---|---|---|---|---|
| 22C3 pharmDx | 28-8 pharmDx | |||||||
| CPS ≥5 (N=25) | CPS <5 (N=201) | P | CPS ≥5 (N=22) | CPS <5 (N=184) | P | |||
| Age, years | <65 | 7 [28] | 106 [53] | 0.032 | 7 [32] | 106 [52] | 0.115 | |
| ≥65 | 18 [72] | 95 [47] | 15 [68] | 98 [48] | ||||
| Sex | Male | 18 [72] | 144 [72] | 1.000 | 16 [73] | 146 [72] | 1.000 | |
| Female | 7 [28] | 57 [28] | 6 [27] | 58 [28] | ||||
| Tumor location | GEJ | 0 | 12 [6] | 0.017 | 0 | 12 [6] | 0.017 | |
| U | 10 [40] | 37 [18] | 9 [41] | 38 [19] | ||||
| M | 4 [16] | 81 [40] | 3 [14] | 82 [40] | ||||
| L | 11 [44] | 71 [35] | 10 [45] | 72 [35] | ||||
| Depth of invasion | T1 | 6 [24] | 88 [44] | 0.064 | 5 [23] | 89 [44] | 0.087 | |
| T2 | 1 [4] | 24 [12] | 1 [5] | 24 [12] | ||||
| T3 | 6 [24] | 26 [13] | 4 [18] | 28 [14] | ||||
| T4 | 12 [48] | 63 [31] | 12 [55] | 63 [31] | ||||
| Lymph node metastasis | Absent | 10 [40] | 101 [50] | 0.399 | 7 [32] | 104 [51] | 0.116 | |
| Present | 15 [60] | 100 [50] | 15 [68] | 100 [49] | ||||
| TNM stage | I | 7 [28] | 93 [46] | 0.053 | 6 [27] | 94 [46] | 0.049 | |
| II | 6 [24] | 33 [16] | 4 [18] | 35 [17] | ||||
| III | 11 [44] | 47 [23] | 11 [50] | 47 [23] | ||||
| IV | 1 [4] | 28 [14] | 1 [5] | 28 [14] | ||||
| Tumor histology | Intestinal | 11 [44] | 76 [38] | 0.664 | 11 [50] | 76 [37] | 0.257 | |
| Diffuse | 14 [56] | 125 [62] | 11 [50] | 128 [63] | ||||
| EBV | Positive | 5 [20] | 8 [4] | 0.008 | 5 [23] | 8 [4] | 0.004 | |
| Negative | 20 [80] | 194 [96] | 17 [77] | 196 [96] | ||||
| MMR | dMMR | 9 [36] | 20 [10] | 0.001 | 7 [32] | 22 [11] | 0.012 | |
| pMMR | 16 [64] | 182 [90] | 15 [68] | 182 [89] | ||||
| HER2 | Positive | 3 [12] | 21 [10] | 0.734 | 3 [14] | 21 [10] | 0.713 | |
| Negative | 22 [88] | 181 [90] | 19 [86] | 183 [90] | ||||
CPS, combined positive score; dMMR, defective mismatch repair; EBV, Epstein-Barr virus; GEJ, esophagogastric junction; HER2, human epidermal growth factor 2; L, lower third; M, middle third; MMR, mismatch repair; PD-L1, programmed death ligand-1; pMMR, mismatch repair proficient; U, upper third.
Comparison of 22C3 and 28-8 PD-L1 pharmDx assays
To quantify potential differences in PD-L1 expression, a pairwise comparison of the 22C3 and 28-8 pharmDx assays was plotted: 87% of pairs were concordant and 11% had a higher PD-L1 expression by the 22C3 assay (Table 3). With a CPS cutoff of 5, concordance between the 22C3 and 28-8 assays was strong (kappa score =0.881) (Table 4). However, various kappa scores were observed when using different cutoff points for CPS: kappa score =0.735 at a cutoff point of 1; and kappa score =0.837 at a cutoff point of 10. Conversely, the analysis of TPS within all three assays showed higher concordant rates (range, 94–97%) (Tables S8-S10).
Table 3
| 28-8 pharmDx | 22C3 pharmDx | |||
|---|---|---|---|---|
| <1 | ≥1, <5 | ≥5, <10 | ≥10 | |
| <1 | 161 | 19 | 1 | 0 |
| ≥1, <5 | 2 | 18 | 2 | 1 |
| ≥5, <10 | 0 | 1 | 3 | 2 |
| ≥10 | 0 | 0 | 2 | 14 |
Concordant, 0.87; 22C3 higher, 0.11; 28-8 higher, 0.02. CPS, combined positive score; PD-L1, programmed death ligand-1.
Table 4
| 28-8 pharmDx | 22C3 pharmDx, N (%) | Kappa value | |||||
|---|---|---|---|---|---|---|---|
| PD-L1 CPS <1 | PD-L1 CPS ≥1 | PD-L1 CPS <5 | PD-L1 CPS ≥5 | PD-L1 CPS <10 | PD-L1 CPS ≥10 | ||
| PD-L1 CPS <1 | 161 (71.2) | 20 (8.8) | 0.735 | ||||
| PD-L1 CPS ≥1 | 2 (0.9) | 43 (19.0) | |||||
| PD-L1 CPS <5 | 200 (88.5) | 4 (1.8) | 0.881 | ||||
| PD-L1 CPS ≥5 | 1 (0.4) | 21 (9.3) | |||||
| PD-L1 CPS <10 | 207 (91.6) | 3 (13.3) | 0.837 | ||||
| PD-L1 CPS ≥10 | 2 (0.9) | 14 (6.2) | |||||
CPS, combined positive score; and PD-L1, programmed death ligand-1.
Representative images of the PD-L1 immunohistochemical staining of the 22C3 and 28-8 pharmDx assays are shown in Figure 1. In most cases, the assays had equivalent relative staining. However, in a few cases, the 28-8 pharmDx assay had a weaker staining of TC and IC membranes compared with the 22C3 assay.
Survival analysis
During the median follow-up time of 60.3 months, 54 patients (24%) died (Figure 2). Using the 22C3 pharmDx assay, the median OS was not reached (NR) with PD-L1 CPS ≥5 vs. NR with PD-L1 CPS <5 (HR, 0.94; 95% CI: 0.40–2.20). Using the 28-8 pharmDx assay, no survival difference was observed with a CPS cutoff point of 5 (HR, 0.70; 95% CI: 0.22–2.25). In addition, PD-L1 expression was not a prognostic factor for OS when using other CPS cutoff points in the 22C3 pharmDx, 28-8 pharmDx, and E1L3 assays (Figure S1). Overall, 38 patients (44%) who were stage III or IV died, and patients who were PD-L1 positive tended to have a better prognosis than those who were PD-L1 negative by all three assays (Table S11). Furthermore, MMR and EBV status were not independent prognostic factors for OS (Figure S2).

Discussion
The present study of gastric cancer diagnostic samples demonstrated high concordance for PD-L1 expression between the 22C3 and 28-8 pharmDx assays when using a CPS cutoff of 5. In addition, the PD-L1 positivity rate evaluated by CPS was higher than that evaluated by TPS in all three assays.
The independent development of PD-L1 assays for the clinical use of pembrolizumab or nivolumab makes it difficult to determine whether the interchangeability of the 22C3 and 28-8 pharmDx assays is useful in clinical settings. In the present cohort, comparisons of PD-L1 CPS between the 22C3 and 28-8 pharmDx assays showed a relatively high concordance rate with a CPS cutoff point of 5 or 10. However, a lower concordant rate of 1 was also observed. These results are consistent with data for other cancer types including lung cancer, bladder cancer, urothelial cancer, and triple-negative breast cancer (20-22). Many studies have reported the PD-L1 expression of TPS but not that of CPS. The data of 1,930 patients with lung cancer or other malignancies demonstrated strong concordance (Cohen’s kappa of 0.90–0.95) between the PD-L1 IHC 22C3 and 28-8 assays when evaluating the percentage tumor-cell membrane PD-L1 expression (23). Regarding the PD-L1 CPS when comparing the 22C3 and 28-8 pharmDx assays, two small sample sized studies of breast cancer and gastric cancer reported the Kappa coefficient was moderate to strong (kappa values, 0.80–1.00) (21,24). These results provide evidence for the interchangeability of these two assays to determine PD-L1 expression levels in gastric cancer patients.
In our cohort, the PD-L1 expression using the three CPS cutoff values in the 22C3 pharmDx assay was higher than that in the 28-8 pharmDx assay, with a difference of 1.8–13.3%. These results are in contrast with previous reports. The PD-L1 positivity rate with a CPS cutoff of 1 using the 28-8 pharmDx assay was slightly higher than that of the 22C3 pharmDx assay for breast cancer (43% vs. 35%) (21). In addition, in gastric cancer, a CPS cutoff of 10 in the 28-8 pharmDx assay showed a modestly higher PD-L1 positivity rate than that in the 22C3 assay (25.5% vs. 21.8%) (24). According to our study, a PD-L1 CPS cutoff of 5 in the E1L3 assay had a higher PD-L1 positivity rate than the 22C3 (11% vs. 15%) and 28-8 (10% vs. 15%) pharmDx assays. The Blueprint IHC Assay Comparison Project of four PD-L1 IHC assays (22C3, 28-8, SP142, and SP263) in lung cancer reported the comparability of the 22C3, 28-8, and SP263 assays whereas the SP142 assay had the lowest levels of agreement, when evaluating the TPS. Based on these conflicting results, different IHC assays and scoring algorithms suggest that caution should be taken when selecting patients who will benefit from immune-checkpoint inhibitors. A practical next step will be to compare the staining patterns using the 22C3 and 28-8 pharmDx assays in a larger cohort of gastric cancer patients.
In the current study, the PD-L1 positivity rate determined by CPS was higher than that by TPS (10–15% vs. 3–5%). Our results are in accord with a previous study reporting that PD-L1 expression on TCs and ICs was positive in 8.4% and 65.3% of cases (25). The prospective CheckMate 649, KEYNOTE-061, and KEYNOTE-062 clinical trials evaluating the efficacy of immune checkpoint inhibitors as chemotherapy reported that as the CPS cutoff increased, the HR tended to improve (10-12). However, in the ATTRACTION-2 trial, PD-L1 positivity examined by TPS was not a predictive marker for nivolumab therapy. These findings suggest that the PD-L1 CPS is more useful than PD-L1 TPS in clinical settings when selecting cases that are likely to derive benefit from immune checkpoint inhibitor treatment.
It is well known that dMMR and EBV positive gastric cancers are associated with the overexpression of PD-L1 (14,15,25,26). In addition, the impact of PD-L1 expression, MMR status, and EBV status on prognosis remains controversial in gastric cancer (26-28). Our results are in line with these previous reports. Furthermore, dMMR and EBV positive are associated with responses to immune checkpoint inhibitors. A prestigious meta-analysis reported that MSI was a robust prognostic marker for resectable gastric cancer (29). Further studies are needed to clarify the significance of immune checkpoint blockade in an adjuvant setting stratified by PD-L1 expression, MSI status, and EBV status.
There were some limitations in this study. This was a retrospective study with a small number of cases from a single institution. Although there may be a lack of correction for multiple comparisons in statistics, it still remains difficult to select a proper method suitable for the various experimental properties. The CPS was classified into three categories without assessing the individual patient scores. However, to the best of our knowledge, this is the largest cohort study to provide information on the PD-L1 CPS evaluated by the 22C3 and 28-8 assays.
In conclusion, our study demonstrated that the PD-L1 CPS in gastric cancer patients was highly concordant between the 22C3 and 28-8 pharmDx assays using various CPS cutoffs. This study suggests the potential interchangeability of these two assays to determine PD-L1 expression levels in gastric cancer patients.
Acknowledgments
The authors thank all the patients who participated in this study and their families.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://dx.doi.org/10.21037/jgo-21-505
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jgo-21-505
Peer Review File: Available at https://dx.doi.org/10.21037/jgo-21-505
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jgo-21-505). YN reports grants from Ono Pharma and Bristol-Myers Squibb, personal fees from Astrazeneca, Eli Lilly, Yakult Honsha, Daiichi Sankyo, and Taiho; TM reports grants from MSD, Daiichi Sankyo, Ono Pharma, and Novartis, personal fees from Takeda, Chugai, Merck Bio Pharma, Taiho, Bayer, Lilly Japan, Yakult Honsha, Sanofi, Daiichi Sankyo, Ono, and Bristol myers squibb; HT reports grants from Dainippon Sumitomo Pharma, Array BioPharma, MSD Oncology, Ono Pharmaceutical, Daiichi Sankyo, Sysmex, and Novartis, personal fees from Bayer, Sanofi, Takeda, Chugai Pharma, Taiho Pharmaceutical, Lilly Japan, Merck Serono, Yakult Honsha, Medical & Biological Laboratories Co., Ltd., Bristol-Myers Squibb Japan, MSD K.K, Novartis, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Nippon Kayaku, Ono Pharmaceutical, and Takeda; SI reports grants from AstraZeneca, Ono Pharmaceutical, and Merck Sharp & Dohme; YY reports grants from ArcherDx nad Chugai-pharma, and personal fees from MSD, Chugai-pharma, AstraZeneca, Pfizer, Roche/Ventana, Agilent/Dako, Thermo Fisher Science, ArcherDx, Novartis, Elli-Lily, Amgen, and Sysmex; and KM reports grants from Solasia Pharma, Merck Serono, Daiichi Sankyo, Parexel International, Pfizer, MSD, Amgen, and ONO Pharmaceutical, consulting fees from AstraZeneca, ONO Pharmaceutical, and Amgen, and personal fees from ONO Pharmaceutical, Chugai, Takeda, Taiho, Sanofi, Bristol-Myers Squibb, Eli Lilly, and Bayer. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by Institutional Review Board (IRB) at Aichi Cancer Center Hospital (IRB No. 2020-1-496). Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Pyrhönen S, Kuitunen T, Nyandoto P, et al. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 1995;71:587-91. [Crossref] [PubMed]
- Murad AM, Santiago FF, Petroianu A, et al. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 1993;72:37-41. [Crossref] [PubMed]
- Glimelius B, Ekström K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 1997;8:163-8. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Pedoeem A, Azoulay-Alfaguter I, Strazza M, et al. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol 2014;153:145-52. [Crossref] [PubMed]
- Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461-71. [Crossref] [PubMed]
- Fuchs CS, Doi T, Jang RW, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol 2018;4:e180013. Erratum in: JAMA Oncol 2019 Apr 1;5(4):579. [Crossref] [PubMed]
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27-40. [Crossref] [PubMed]
- Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol 2020;6:1571-80. [Crossref] [PubMed]
- Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;392:123-33. [Crossref] [PubMed]
- Rimm DL, Han G, Taube JM, et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol 2017;3:1051-8. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 2018;24:1449-58. [Crossref] [PubMed]
- Kulangara K, Zhang N, Corigliano E, et al. Clinical Utility of the Combined Positive Score for Programmed Death Ligand-1 Expression and the Approval of Pembrolizumab for Treatment of Gastric Cancer. Arch Pathol Lab Med 2019;143:330-7. [Crossref] [PubMed]
- NCCN clinical practice guidelines in Oncology GC, Ver3. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed Jul 17, 2021).
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22:276-82. [Crossref] [PubMed]
- Morsch R, Rose M, Maurer A, et al. Therapeutic implications of PD-L1 expression in bladder cancer with squamous differentiation. BMC Cancer 2020;20:230. [Crossref] [PubMed]
- Huang X, Ding Q, Guo H, et al. Comparison of three FDA-approved diagnostic immunohistochemistry assays of PD-L1 in triple-negative breast carcinoma. Hum Pathol 2021;108:42-50. [Crossref] [PubMed]
- Zajac M, Scott M, Ratcliffe M, et al. Concordance among four commercially available, validated programmed cell death ligand-1 assays in urothelial carcinoma. Diagn Pathol 2019;14:99. [Crossref] [PubMed]
- Krigsfeld GS, Prince EA, Pratt J, et al. Analysis of real-world PD-L1 IHC 28-8 and 22C3 pharmDx assay utilisation, turnaround times and analytical concordance across multiple tumour types. J Clin Pathol 2020;73:656-64. [Crossref] [PubMed]
- Ahn S, Kim KM. PD-L1 expression in gastric cancer: interchangeability of 22C3 and 28-8 pharmDx assays for responses to immunotherapy. Mod Pathol 2021;34:1719-27. [Crossref] [PubMed]
- Kawazoe A, Shitara K, Kuboki Y, et al. Clinicopathological features of 22C3 PD-L1 expression with mismatch repair, Epstein-Barr virus status, and cancer genome alterations in metastatic gastric cancer. Gastric Cancer 2019;22:69-76. [Crossref] [PubMed]
- Kawazoe A, Kuwata T, Kuboki Y, et al. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer 2017;20:407-15. [Crossref] [PubMed]
- Kim JW, Nam KH, Ahn SH, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer 2016;19:42-52. [Crossref] [PubMed]
- Eto S, Yoshikawa K, Nishi M, et al. Programmed cell death protein 1 expression is an independent prognostic factor in gastric cancer after curative resection. Gastric Cancer 2016;19:466-71. [Crossref] [PubMed]
- Pietrantonio F, Miceli R, Raimondi A, et al. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability As a Biomarker in Gastric Cancer. J Clin Oncol 2019;37:3392-400. [Crossref] [PubMed]


