Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments
Introduction
As advances in the treatment of breast cancer continue to progress, health care providers and patients are increasingly focused on post-treatment quality of life. Accordingly, an in-depth understanding of breast cancer-related lymphedema (BCRL) and its treatments is necessary for all clinical providers (1). BCRL, a much-feared sequela of breast cancer treatment, results from disruption to the lymphatic system that prevents adequate drainage from lymphatic vessels causing protein-rich lymph fluid to accumulate in the interstitial space (2,3). This excess fluid can cause abnormal swelling in the breast, trunk or upper extremity on the side of treatment. Depending on the extent of edema, symptoms of BCRL can include arm tightness, heaviness/fullness, pain, and impaired limb function (3-5). Furthermore, as BCRL progresses, adipose deposition and fibrosis can result (6). BCRL negatively affects a patient’s quality of life, causing elevated rates of depression and anxiety in addition to physical impairment compared to patients without BCRL (7-9). It is imperative to not only understand the risk factors influencing BCRL but also to use this knowledge to inform preventative measures and diagnostic approaches. The purpose of this review is to outline evidence-based risk factors, precautionary measures, and treatment modalities in order to establish an integrative knowledge base from which clinicians and researchers can draw to understand, diagnose, prevent, and treat BCRL.
Incidence of BCRL
In a recent meta-analysis, the overall estimated incidence of chronic arm edema after breast cancer was found to be 21.4%, indicating that BCRL is a widespread problem affecting 1 in every 5 patients following breast cancer treatment (10). Due to the lack of diagnostic criteria for BCRL, the reported incidence varies from less than 5% to more than 50% (10-12). The likelihood of any one individual developing lymphedema depends largely on that patient’s individual risk factors. Literature examining these risk factors generally investigates the risk associated with either treatment-related or non-treatment-related risk factors. There are likely other non-modifiable risk factors including genetics and anatomy, which are less researched and not well-understood.
Treatment-related risk factors for BCRL
The main treatment-related risk factors for BCRL literature include axillary lymph node dissection (ALND) and regional lymph node radiation (RLNR). There is strong evidence that both ALND (10,11,13-17), and RLNR (10,11,17-20) are independent risk factors for BCRL. Additionally, emerging evidence indicates lack of breast reconstruction (21-23) as another treatment-related risk factor. Conversely, discord exists in the literature regarding risk posed by taxane-based chemotherapy (Table 1).

Full table
Type of axillary surgery
Axillary surgery type largely determines an individual’s risk for developing lymphedema. Both ALND and the less invasive sentinel lymph node biopsy (SLNB) put patients at life-long risk for developing lymphedema due to the removal of either many axillary, in the case of ALND, or few sentinel, in the case of SLNB, lymph nodes (10,11,13-18,32). However, a recent meta-analysis of BCRL incidence in patients with unilateral breast cancer estimated that patients who receive ALND have a lymphedema incidence four times higher than those who receive SLNB [19.9% (95% CI: 13.5–28.2) and 5.6% respectively] (10). Thus, SLNB is an effective option for staging the axilla while minimizing the risk of lymphedema in patients with clinically node negative breast cancer (33), including a contralateral SLNB for those patients undergoing contralateral prophylactic mastectomy in conjunction with therapeutic mastectomy (24). These results are supported by Kilbreath and colleagues, who prospectively screened for lymphedema and found similar incidence rates when they stratified their data by number of nodes removed. For patients who have had more than five or more nodes removed, the incidence rate was 18.2%; for patients with less than five nodes removed, the incidence rate was 3.3% (18). This suggests that BCRL risk associated with axillary surgery may depend on the number of nodes removed, a metric that is generally accepted as an approximation for overall surgical damage to the lymphatic system (32). Indeed, Kim and colleagues showed that BCRL incidence rates in patients with 10 or more axillary lymph nodes removed were significantly greater than in patients with less than 10 dissected lymph nodes (27% vs. 6% respectively; P<0.001), and McLaughlin and colleagues found a significant difference in the number of axillary lymph nodes removed for patients who did not develop BCRL compared to those that did (19 vs. 22 respectively; P<0.0001) (16,32). Together, these data remind clinicians and researchers that the extent of axillary surgery may be an important prognostic factor for BCRL development, one which may be modified with the advancing surgical techniques outlined below. Moreover, De Groef and colleagues cautioned against the assumption that SLNB does not substantially affect arm morbidity irrespective of BCRL. In their prospective study, 50% of patients who underwent SLNB reported pain and 49% of patients experienced impaired shoulder function 1 year after surgery (25). This, and the fact that SLNB itself poses a risk for LE development, must be considered during the development of new treatments and protocols for patients undergoing treatment for breast cancer.
RLNR
Radiotherapy to the regional nodes, or RLNR, has been shown to be a significant risk factor for lymphedema development (11,17-20,26). Warren and colleagues demonstrated that RLNR, either supraclavicular with or without posterior axillary boost, significantly increased LE risk compared to breast/chest wall RT alone (HR: 1.70; 95% CI: 1.07–2.70) (20). A new meta-analysis by Shaitelman and colleagues calculates the pooled incidence for patients undergoing breast/chest wall radiation alone as 7.4% (95% CI: 5.1–10.0), but the pooled incidence for various combinations of RLNR varies from 10.8% to 15.5% (17). When stratified by type of axillary surgery, patients undergoing ALND and RLNR, had 18.2% pooled incidence (95% CI: 12.4–23.9), which represented a significant increase in lymphedema risk compared to ALND patients who received breast/chest wall radiation only (OR: 2.74; 95% CI: 1.38–5.44) (17). Furthermore, while patients with positive SLNB who receive adjuvant RLNR in lieu of ALND have lower rates of clinically diagnosed lymphedema 5 years after surgery (23% vs. 11%, P<0.0001), the risk of RLNR in and of itself should not be underestimated (34). Thus, patients undergoing RLNR, even without ALND, should be considered a high-risk group for developing lymphedema, and all patients undergoing ALND and/or RLNR should be prospectively screened.
Lack of breast reconstruction
The effect of reconstruction on risk of BCRL has become an emerging area of interest in the literature (21-23,28). Recently, in a large prospective cohort study, Miller and colleagues investigated immediate implant reconstruction and immediate autologous reconstruction compared to mastectomy without reconstruction (21). Mastectomy itself has been occasionally cited as a risk factor for BCRL (10,11,18,19,35), and several studies have shown that delayed autologous reconstruction reduced the severity of BCRL (36-38). They found that immediate reconstruction significantly reduced the risk of lymphedema (HR: 0.432; P<0.0001). Specifically, immediate implant reconstruction offered a greater reduction in risk compared to immediate autologous reconstruction (HR: 0.500; P=0.0322) (21). These results are similar to those of an earlier study by Avraham and colleagues who found that those undergoing tissue expander breast reconstruction had significantly lower rates of BCRL (5% vs. 18%; P<0.0004) (39). Moreover, a retrospective study by Card and colleagues that found patients who did not undergo reconstruction were more likely to develop BCRL compared to patients who had reconstruction (adjusted OR: 0.37; 95% CI: 0.02–0.63) (23). In a previous study, the same authors acknowledged that immediate reconstruction decreased risk of BCRL, but they found no difference in BCRL incidence based on type of immediate reconstruction (22). In contrast to these studies, Basta and colleagues did not find reconstruction or lack thereof as a significant factor influencing LE risk, but this study’s retrospective nature and lack of objective measurement-based diagnostic criteria limit the scope of its findings (27).
Adjuvant and neoadjuvant chemotherapy
Whereas ALND and RLNR are known risk factors for BCRL and lack of reconstruction likely increases the risk of BCRL as well, less conclusive evidence exists to suggest chemotherapy as a risk factor. Some studies indicate adjuvant chemotherapy as a potential risk factor for BCRL (10,18,32,40-42) whereas other studies do not (19,31). In particular, taxane-based chemotherapy is of interest in BCRL literature because of taxane-induced fluid retention in patients during treatment (43-45). In a recent prospective cohort study by Kilbreath and colleagues, arm swelling at 6 and 12 months was associated with adjuvant taxane therapy, and swelling at both time points were independent risk factors for LE development (18). Zhu and colleagues’ recent retrospective analysis lends support to Kilbreath et al.’s findings. They found that docetaxel-based chemotherapy significantly increased the cumulative incidence of BCRL compared to non-docetaxel based chemotherapy (19.91% vs. 32.09%; P=0.011) and was an independent risk factor for BCRL (HR: 1.73; P=0.017) (42). Conversely, Swaroop and colleagues did not find taxane-based chemotherapy as a risk factor for BCRL. They did, however, find docetaxel treatment, but not paclitaxel treatment, to be a risk factor for mild swelling compared to no chemotherapy and non-taxane based chemotherapy (31). Thus, while it is clear taxanes, specifically docetaxel, cause edema, there is not a clear consensus in the literature that taxane-based chemotherapy is a risk factor for BCRL.
The effect of neoadjuvant chemotherapy on BCRL risk is unclear. Neoadjuvant chemotherapy is utilized in breast cancer treatment to decrease the size of the primary tumor and any affected lymph nodes, allowing for less extensive surgery. It has been suggested that neoadjuvant chemotherapy could, in theory, decrease BCRL incidence by reducing the number of positive lymph nodes (29,30). Specht and colleagues found that there was an increased risk of BCRL in patients with residual lymph node disease after neoadjuvant chemotherapy (30). More studies, using objective and standardized BCRL measurement techniques and definitions, are needed to define the role of neoadjuvant and adjuvant chemotherapy in BCRL risk.
Non-treatment-related risk factors for BCRL
Studies have demonstrated several non-treatment-related risk factors for BCRL, including body mass index (BMI) at time of diagnosis, subclinical edema, and cellulitis on the side of treatment (Table 2). Efforts aimed at addressing these risk factors may represent a prudent avenue for BCRL prevention.
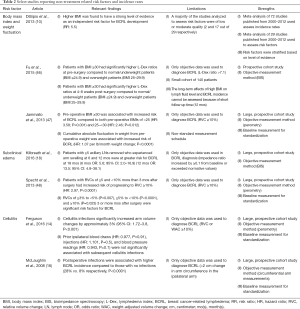
Full table
BMI
High BMI at time of breast cancer diagnosis is a well-established risk factor for developing BCRL (14,16,20,22,40,46,49-55). In a prospective cohort screening for BCRL using perometry, Jammallo and colleagues found a BMI greater than or equal to 30 kg/m2 was an independent risk factor for BCRL (47). This result was similar to Ridner and colleagues’ smaller prospective study using perometry in which they found that patients with a BMI of 30 kg/m2 or above were 3.6 times more likely to develop lymphedema (95% CI: 1.42–9.04; P=0.007) (55). Similarly, Fu and colleagues investigated BMI’s influence on lymph fluid by using bioimpedance spectroscopy (BIS) to screen for lymphedema in a small cohort of 140 women. They found that obese women (BMI ≥30) were more likely to have an L-Dex score of greater than 7.1, which was their operative definition for lymphedema, compared to both the normal/underweight group (18.5–24.9, <18.5 respectively) and the overweight group (25.0–29.9) before surgery and throughout the following year (46). This result supports the previous arm volume studies which indicate BMI ≥30 at time of diagnosis as a modifier of BCRL risk.
In addition to high BMI at diagnosis, there is some supporting evidence suggesting weight fluctuations during and after treatment may be a risk factor for BCRL (15,35,47). Jammallo et al. demonstrated that post-operative weight fluctuations greater than 10 pounds per month, either lost or gained, increased BCRL risk (47). Thus, more research regarding weight fluctuation is needed before optimal weight loss and/or management programs can be implemented clinically to modify a patient’s risk for BCRL.
Subclinical edema
Subclinical edema has been shown to be a risk factor for BCRL (18,20,48). Specht and colleagues first studied the relationship between subclinical swelling and progression to lymphedema—defined as >10% relative volume change—to assess at what level of swelling would intervention be warranted. They prospectively screened 1,173 patients treated for breast cancer with perometry, and they found small increases in arm volume (≥3% but <5%) as well as larger increases in arm volume (≥3% but <10%) within 3 months of surgery increased BCRL risk. After the third postoperative month, only larger increases in arm volume (≥5% but <10%) were correlated with increased BCRL incidence (48). Additionally, in their large cohort screened prospectively with BIS, Kilbreath and colleagues found that, for women with five or more lymph nodes removed, axillary swelling at 6 and 12 months postoperatively are independent risk factors for BCRL at 18 months. Interestingly, arm swelling at 6 and 12 months were associated with arm swelling in the first month of surgery (18). Together, these studies demonstrate the importance of screening for early postoperative arm volume increases to inform long-term BCRL risk.
Cellulitis
Cellulitis is a well-established BCRL risk factor in the literature (13,14,16,35,49,51,56,57). In their recent large prospective cohort study, Ferguson and colleagues demonstrated that cellulitis infections significantly increased BCRL risk (P<0.001). They also showed that ipsilateral ‘risk events’, such as blood draws and injections, did not correlate with cellulitis infections, and they further suggest that these routine medical procedures may not expose the axilla to substantial infection risk when done in a sterile environment (14). Nonetheless, patients who have undergone treatment for breast cancer should be wary of the risks of postoperative infections. In a cohort of patients receiving both unilateral and bilateral breast conserving therapy, Indelicato and colleagues found that patients with BCRL were more likely to develop delayed breast cellulitis (56). Thus, cellulitis and BCRL may represent a feedback loop in which cellulitis increases BCRL risk and BCRL increases risk of further infections (13,15). More research is needed to fully delineate the relationship between cellulitis and BCRL to help mitigate risk of both.
Prevention
Precautionary guidelines
The National Lymphedema Network (NLN) outlines precautionary lifestyle recommendations for those at risk of lymphedema, which they define as anyone who has had lymph nodes removed or radiation therapy during treatment for cancer (58). It should be clarified that, for patients treated for breast cancer, RLNR is the specific type of radiotherapy that increases a patient’s risk for BCRL. These precautionary guidelines range from minimally burdensome (e.g., good skin care) to potentially demanding (e.g., potential use of a compression garment during air travel, avoidance of blood pressure cuffs and other forms of limb constriction, and reduction of trauma to the at-risk limb by avoiding venipuncture) lifestyle alterations. Yet, implementation of these guidelines may not significantly reduce a patient’s risk of developing BCRL (13,14,18,59). Ferguson et al. found that, in their cohort, blood pressure readings, blood draws, and air travel did not significantly increase BCRL risk in patients who underwent unilateral breast cancer surgery (14). In patients who underwent bilateral breast cancer surgery, a recent prospective cohort study by Asdourian and colleagues demonstrated similar results, namely that lifestyle risk factors were not associated with an increased weight adjusted volume change (60). Doubt regarding precautionary guidelines is further enhanced by Kilbreath et al.’s recent study, which found that air travel, arm trauma, medical procedures, and arm use did not increase risk of BCRL in women with five or more lymph nodes removed (18). Indeed, evidence for many of these clinically-guided, precautionary guidelines lack high level scientific evidence; however, as Asdourian et al. highlighted, there is a need for more rigorous research regarding precautionary behaviors prior to implementing practice changes (13). This recommendation is echoed in the 2016 International Society of Lymphedema Consensus document. They state, “The recent promulgation of lists of risk factors for secondary lymphedema has become a highlighted issue due to publications of ‘do’s and don’ts’. These are largely anecdotal and not sufficiently investigated. While some precautions rest on solid physiological principles, others are less supported.” It goes on to state that “standard use of some of these ‘don’ts’ for risk reduction of lymphedema may not be appropriate and possibly subjects patients to therapies which are unsupported until a point in the future when evaluation and prognostication evidence has demonstrated more clearly specific risks and the corresponding preventative measures” (61).
Screening programs and early intervention
Historically, BCRL has been treated using an impairment-based model which relies on both the patient and the provider to detect visible limb swelling and to accurately diagnose/treat the swelling as lymphedema. However, as the BCRL field has progressed, the consensus has shifted, instead recommending a preventative, prospective screening approach (47,48,52-58). With the advent of better, more precise diagnostic technology, such as perometry and BIS, patients can be easily screened for BCRL both during and after treatment (12,62-67). Massachusetts General Hospital has successfully established one such prospective BCRL screening program in its multidisciplinary breast cancer clinic, using perometry as a standard objective tool, together with subjective patient reported outcome measures (PROM) (62). Patients with elevated measurements beyond baseline are referred to a certified lymphedema therapist for clinical evaluation and consult. Elements of any successful prospective screening program should include a validated, reliable, objective measurement tool, a standardized measurement protocol, a preoperative baseline measurement, a longitudinal series of follow up measurements that account for natural arm asymmetry and weight changes, and PROM. Such screening should also include clinical examination by a certified lymphedema therapist at the discretion of the team (62,67-73).
Using this model, BCRL can be diagnosed in earlier stages, allowing for earlier intervention (61,74). Stout and colleagues successfully screened 196 patients for BCRL, and patients were given compression garments if there was a >3% increase in arm volume compared to the preoperative measurement and contralateral arm volume changes. After 4 weeks, the patients who received the early intervention had significant volume reduction that was maintained at the four month follow up visit (68). Similarly, Soran and colleagues prospectively screened 186 patients with either BIS or circumferential arm measurements, treating those diagnosed with subclinical lymphedema with physical therapy, compression garments, and education. Of the 33% of patients in the BIS group who were diagnosed with subclinical lymphedema, only 4.4% developed clinical lymphedema compared to 36.4% in the control group, demonstrating the effectiveness of prospective screening and early intervention in reducing BCRL (75). While these studies demonstrate potential efficacy for early intervention, they are limited by their small sample size. Large, randomized trials are needed to fully evaluate the benefits of early intervention for subclinical edema. Nonetheless, the International Society for Lymphology upholds that prospective screening models and early intervention allow for greater treatment success and potential cost savings (61).
Improving treatment techniques
Improving treatment techniques to minimize lymphatic disruption is another avenue of research in BCRL prevention (33). One of the main ways to prevent lymphatic disruption is to perform less invasive nodal surgeries. The concept of removing only the sentinel lymph node(s) (SLN)—or the lymph nodes that first receive drainage from the tumor—was a concept proposed by Cabanas in the 1970s in the treatment of penile cancer (76). By the 1990s, melanoma researchers and breast cancer researchers began using tracers like blue dye and/or radioactive colloids to map the drainage of ducts into the sentinel lymph nodes (77-80), and Krag and colleagues demonstrated that biopsy of the sentinel lymph nodes could accurately predict axillary-node metastasis in patients treated for breast cancer (80). As research further demonstrated the efficacy of using SLNs to indicate nodal metastasis without decreasing survival rates (81,82), modern surgical trends shifted towards using the more conservative SLNB as opposed to ALND to stage the axilla in patients treated for breast cancer. As suspected, the advent of SLNB as an alternative to ALND in clinically node negative patients with breast cancer has drastically reduced the risk of BCRL (83) and arm morbidities (83-86).
For patients who present with node-positive breast cancer, there are fewer options for modifying treatment to reduce BCRL risk. Current clinical practice guidelines recommend that most patients with lymph node metastases undergo ALND (87,88); however, there is compelling evidence that neoadjuvant chemotherapy treatment may downstage the axillary lymph nodes and allow for less extensive surgery (89-91). Both the SENTINA study and the ACOSOG Z1071 clinical trials showed a less than 10% false-negative rate when three or more sentinel lymph nodes were biopsied, demonstrating that at least three negative SLNs reliably indicates absence of further nodal metastases (89,90). In a recent prospective study by Mamtani and colleagues, they avoided ALND for 40% of their patients who presented with nodal metastases by downstaging with neoadjuvant chemotherapy (91). These patients all had three or more negative SLNs and had no contraindications to SLNB. Furthermore, in Mamtani et al.’s study, only 14% of patients had fewer than three identified SLNs, which is a much lower proportion than the SENTINA and ACOSOG Z1071 studies. This study provides further evidence that ALND may not be indicated in patients who have been successfully downstaged with neoadjuvant chemotherapy and with three or more negative SLNs. Large trials demonstrating positive long-term regional recurrence outcomes are needed to validate this novel approach. In addition, results from the AMAROS (34) and the Z0011 (92,93) trials suggest that, for patients with positive SLNB, ALND may not be indicated if it replaced with adjuvant therapy. Compared to ALND, the AMAROS trial demonstrated that RLNR offered comparable locoregional control with significantly less arm morbidity, including significantly less lymphedema comparatively (34).
Moreover, new surgical techniques are being developed to minimize axillary lymphatic disruption in both SLNB and ALND procedures. The first procedure, axillary reverse mapping (ARM), has shown promise in recent years as a new method to map out and preserve axillary lymphatics during surgery and potentially reduce post-surgical lymphedema incidence in patients (94-98). It is based on the hypothesis that, because the axilla and breast have mostly separate drainage pathways, upper extremity lymphedema can be prevented by avoiding the removal of tracer-identified lymph nodes and lymphatics that only drain the axilla. In 2007, the first published reports of ARM demonstrated that blue dye injected into the ipsilateral arm could be used to visualize these axillary lymphatics and lymph nodes, thereby differentiating them from the SLNs that drain the breast (99,100). Since then, other methods of visualizing ARM nodes have developed, including using radioactive colloids and lymphoscintigraphy (95,101,102), using the fluorescent dye IndoCyanine Green with a fluorescence imaging system (103-106), and using combinations of tracers (106-109).
In studies looking at BCRL after ARM procedures, the reduced incidence rates provide evidence for the efficacy of ARM’s ability to reduce BCRL (94-96,98,100,102,106,109-116). One recent study by Tummel and colleagues demonstrated promising results. They measured arm volume using water displacement at baseline and every 6 months, and they characterized objective lymphedema as any increase ≥20% in volume of the affected side over the contralateral side, whilst considering baseline measurements and changes in contralateral arm volume. Six hundred and fifty-four ARM procedures were performed with either an SLNB or an ALND, and, except during the beginning of the study, benign-appearing ARM nodes and lymphatics were preserved during ALND (67.3% of ALND patients with ARM lymphatics identified). In a subset of patients for which ARM lymphatics were identified and preserved, objective BCRL rates at a median of 26 months after surgery were 1.2% for SLNB patients and 6.9% for ALND patients (110). These represent a marked decrease to typical BCRL incidence rates for SLNB and ALND. Yet, it should be noted that their definition of ≥20% increase in arm volume was conservative, and the same cohort of patients might have had a higher incidence of BCRL if other BCRL definitions were used. This highlights an important limitation in BCRL assessment in ARM studies. Because these studies use varying definitions of BCRL and inconsistent methods of measuring edema across studies, it is difficult to compare BCRL incidence rates after sparing ARM nodes, thereby limiting our ability to assess ARM as a surgical tool for BCRL reduction (94,98). The limitations of current ARM studies highlight the need for standard definitions of BCRL that use quantitative measurements, ones which take into account preoperative baseline arm volume and changes in the contralateral arm and/or weight (69-72). Future studies of ARM must include this type of rigorous assessment of BCRL in order to fully understand the potential benefit it could have (98). Nevertheless, the potential of ARM to reduce post-surgical axillary morbidity should not be underestimated.
However, ARM lymph nodes and lymphatics cannot, and potentially should not, always be spared. There have been incidences of the SLN coinciding with the ARM node in some patients (95,96,106,110-113,117-120), and studies have reported ARM/SLN crossover rates anywhere between 0–28% (96,100,101,103,104,106,110-116,118-122). This is not surprising because research has shown that interconnections do exist between the two, relatively independent drainage pathways (123). Any connections between the two pathways potentially allow metastatic breast cancer cells to invade axillary lymphatics, making the pathological status of ARM nodes an important area of investigation to determine oncologic safety. In the case of crossover between the SLN and ARM nodes, the crossover nodes are removed during SLNB, and upon examination, some studies identified metastases in these concordant nodes (96,111,120). Moreover, the rates of metastases in non-crossover ARM nodes are important to consider when assessing oncologic safety. In trials examining ARM during ALND, studies have reported metastatic involvement of ARM nodes anywhere from 0% to 43% (99-101,103-105,107,108,112,114-116,118,121,122,124-128). Because of these varying rates of ARM nodal involvement, it is unclear whether this procedure can be safely used in clinically node positive patients undergoing primary ALND. However, there is evidence indicating that ARM can successfully be implemented into SLNB procedures for clinically node negative patients and that non-crossover ARM nodes can be spared in these patients with positive SLNB (95,96,129). Yet, larger, randomized clinical trials comparing the efficacy and safety of ARM in conjunction with axillary surgery to SLNB and ALND alone are needed before clinical guidelines can implement ARM for lymphedema reduction in patient populations (94).
Boccardo and colleagues, recognizing that the preservation of ARM lymphatics might not always be feasible or possible due to complications such as extensive axillary disease or failure to find blue lymphatics, established the Lymphatic Microsurgical Preventative Healing Approach (LYMPHA) technique (130-132). LYMPHA involves constructing lymphatic-venous anastomoses (LVAs) between the arm lymphatics and a collateral branch of the axillary vein during axillary surgery. LVAs have been successfully used in the surgical treatment of primary and secondary lymphedema for many years (133,134), but the preventative approach of creating these anastomoses between ARM lymphatics and the axillary vein collateral during axillary surgery represents a creative method of preserving lymphatic function when ARM lymph node removal is necessitated (130). After 4 years, only 3 out of 79 patients demonstrated clinical LE, which corresponds to an incidence rate of 4.05% (132). Feldman and colleagues had similar results in their LYMPHA trial: only 3 out of the 24 patients who had a successful LYMPHA procedure with ALND developed LE after 26 months compared to 4 out of 8 patients (50%) who had unsuccessful LYMPHA procedures due to lack of adequate axillary vein or extensive lymphatic/axillary disease (135). Interestingly, Tummel and colleagues constructed LVAs when blue ARM lymphatics had to be transected during their ARM studies during SLNB and ALND. In the subset of patients who had blue lymphatics transected, the BCRL incidence rate was significantly lower when the lymphatics were reanastomosed (18.7% vs. 0%; P=0.009). However, these results are limited because there was a low incidence of ARM lymphatic transection as well as transected ARM reanastomosis (110). LYMPHA in conjunction with ARM procedures may prove to be an effective adjustment to SLNB and ALND, particularly for those in which ARM lymphatics and nodes must be transected or removed. The technique could be implemented with relatively little burden to hospitals in which surgeons trained in microsurgical techniques work, particularly if performed at the time of mastectomy with immediate reconstruction (136). However, as noted by Ahn and Port (59,137), access to surgeons with microsurgical skills is not ubiquitous, limiting the feasibility of this technique as a widespread preventative procedure in the immediate future. Nevertheless, to fully determine the efficacy of ARM in conjunction with LYMPHA as a preventative approach to reducing BCRL, a large, multicenter, prospective cohort study that uses standardized, objective definitions of BCRL along with clinical assessments is needed.
Treatments
Non-invasive treatments
First-line intervention to treat BCRL involves two distinct phases: reduction therapy and maintenance therapy. Reductive therapy typically involves complete decongestive therapy (CDT) administered by a certified lymphedema therapist, whose goal is to decrease symptoms and limb volume. CDT is individualized for each patient, but it typically includes manual lymphatic drainage (MLD), compression bandaging, exercise, skin care, and patient education. There is no consensus on optimal treatment parameters, including frequency, for CDT. Once minimized limb volume is achieved with CDT, typically after several weeks, maintenance therapy begins. This may include self- or caregiver-administered MLD, compression garments, exercise, and skin care.
Research regarding non-invasive treatment for BCRL has focused on CDT in its entirety, but there has been less well-established research regarding the efficacy of each individual CDT component. Compression bandaging as a first line intervention for subclinical BCRL has been used increasingly to prevent swelling progression (68) and it has been shown to reduce arm volume successfully with or without the addition of MLD for BCRL (138,139). However, MLD is an important tool for volume reduction. One recent meta-analysis concluded that MLD is not only safe and well-tolerated, but it may also be most beneficial to patients with mild to moderate BCRL in addition to compression bandaging (140). Current ISL consensus highlights the need for more research regarding MLD as a monotherapy (61).
Furthermore, exercise is an important aspect of CDT as well as a helpful tool in the long-term management of BCRL. An exercise program including both aerobic and resistance exercises, initially supervised to ensure proper technique and progression, does not incite or exacerbate BRCL (141-146). Moreover, there is no limitation on the maximum amount of weight that can be lifted as long as weight-lifting exercises are supervised and progressive (141). Patients, both with and without BCRL, are encouraged to progress toward meeting the American College of Sports Medicine guidelines for patients who have undergone treatment for breast cancer. These recommendations include 150 min/week of moderate activity (e.g., walking) or 75 min/week of vigorous activity (e.g., running), as well as 6–8 resistance exercises for the major muscle groups of the upper and lower extremities (147).
Other non-invasive, technological treatments for BCRL exist. Intermittent pneumatic compression (IPC) pumps have been used to treat BCRL, but there is unclear evidence demonstrating their efficacy. In their meta-analysis, Shao and colleagues found that the addition of IPC pumps to routine management of BCRL did not significantly improve treatment outcomes (148). However, others have shown IPC to be an effective addition to CDT (149) or an alternative to MLD and compression bandaging when combined with self-lymphatic drainage (150). Rogan and colleagues, in their meta-analysis of different treatment modalities, concluded that while IPC pumps may be useful in the reductive phase of treatment, their utility is limited because they can only stimulate drainage in unaffected lymphatic collectors (146). Additionally, low level laser therapy (LLLT) has become a recent modality of interest to treat BCRL (61,151,152). One small randomized pilot trial demonstrated that 20 minutes of LLLT combined with compression bandaging is just as effective at reducing volume compared to 40 minutes of MLD, potentially offering a time saving treatment that would reduce burdensome treatment (152). A meta-analysis of nine studies examining LLLT as a BCRL treatment modality concluded that LLLT alone or in conjunction with other treatments decreases pain and swelling in patients (151).
Surgical treatments
While non-invasive treatment remains the standard of care for BCRL, surgical management is another avenue to treat persistent lymphedema, particularly for patients who do not respond to non-invasive treatments. There are two main surgical strategies: ablative procedures and physiologic procedures (153-157).
Ablative procedures, also known as debulking procedures, reduce limb volume by surgically removing edematous tissue. Typically, liposuction or suction-assisted protein lipectomy (SAPL) are used as volume reduction treatments because they are less invasive than older debulking procedures and do not require skin grafting. Liposuction/SAPL procedures are best suited for individuals with solid, non-pitting edema whose volume excess is largely due to fat deposits instead of fluid accumulation (61,153,155,158-162). In studies looking at patients with upper and lower extremity lymphedema, liposuction/SAPL has shown significant volume reductions (61,158,162-166). However, because these procedures do not address the underlying physiologic causes of lymphedema, namely the inadequate drainage of lymphatic fluid from the extremity, compression garments must be worn continuously to maintain the decreased volume (158,163,164,167,168).
Conversely, physiologic procedures treat the etiology of BCRL by reestablishing and/or redirecting axillary lymphatic flow. Re-approximation or rerouting of lymphatic drainage pathways can be achieved by establishing unobstructed connections with distal healthy tissue or proximal venous tissues. Because these procedures work to resolve fluid accumulation in the extremity, physiologic procedures are indicated for patients with pitting edema who have not progressed to fibrotic, solid edema (153,158).
Procedures utilizing distal tissues generally involve lymphatic grafts or vascularized flaps containing lymphatic soft tissue. The lymphaticolymphatic bypass procedure is an example of the former, during which healthy lymphatic vessels are harvested from the lower extremity and anastomosed to the affected arm’s axillary lymphatics at one end and to healthy supraclavicular lymphatics on the other (169). In reports, these rerouted lymphatic pathways achieved long term patency and improved lymphatic transport, and they have proven effective in reducing upper extremity volume (169-172). However, harvesting the lymphatic graft can potentially cause lymphedema in the donor extremity. In lieu of using lymphatic grafts, autologous venous grafts from donor extremities can be used to bypass the blockage in a similar way to lymphatic grafts without the potential disruption to the donor lymphatic pathways (173,174). The other major surgical treatment involving introduction of distal tissues to the axilla is a vascularized lymph node transfer (VLNT). A VLNT involves harvesting a lymph node flap with its corresponding vascular supply from a donor site and introducing it into the affected extremity (154,175-180). Blood supply is achieved by anastomosing the lymph node flap’s blood vessels and the native axillary blood vessels. The donor lymph node flaps can be taken from various areas, but most surgeons typically use lateral groin lymph nodes to treat upper-extremity lymphedema (155,176,178,179). Studies have also shown successful results when harvesting lymph node flaps for VLNT in conjunction with abdominal flaps, which surgeons then use for simultaneous breast reconstruction (181,182). Furthermore, while this procedure does put the donor site at risk for developing lymphedema (183,184), the mapping of lymphatic drainage patterns allows for the selective removal of lymph nodes not primarily responsible for draining the donor extremity (158,185).
Surgical treatments for BCRL also include physiologic procedures, such as LVA, which utilize proximal tissues instead of grafts or flaps to reestablish lymphatic drainage. In fact, LYMPHA, mentioned above, is a prophylactic LVA performed at the time of axillary surgery between transected lymphatics and the collateral branch of the axillary vein (130). During palliative LVAs, multiple lymphatic vessels are anastomosed to venules, thereby allowing lymphatic drainage into the venous system (133,134,186-190). Recently, Poumellec and colleagues successfully used a stepped LVA approach, during which three total anastomoses were created at the wrist, forearm, and elbow, to treat upper extremity lymphedema. Of the 31 patients treated, 93.5% showed a decrease in arm circumference with a mean reduction of 24.7%, and of the 3 patients with late-stage BCRL, 1 showed no circumference decrease whereas the other 2 patients had recurrences (186). In their prospective study, Chang and colleagues performed LVAs in 89 women with upper extremity lymphedema, and symptom improvement was reported by 96% of patients. One year after surgery, the mean volume reduction was 42% overall, and patients with less progressive lymphedema (stages 1 and 2) had significantly greater volume reductions compared to patients with stage 3 or 4 lymphedema (187). As with all of the previously mentioned procedures, large, randomized clinical trials are needed to fully evaluate the palliative benefits of LVAs alone and compared to other treatment techniques.
Conclusions
BCRL remains a potentially life-altering sequela of breast cancer treatment that affects approximately one in five patients (10). Well-established risk factors include ALND, RLNR, high BMI at time of diagnosis, edema 3–5% within 3 months of surgery, edema 5–10% at any time after surgery, and cellulitis infections. Nevertheless, research has precipitated significant advances in BCRL screening and treatment. Most notably, establishment of risk factors, evolving evidence around precautionary guidelines, and adapting surgical treatments to reduce lymphatic disruption are powerful areas of evolving BCRL research and care. Techniques such as ARM and LYMPHA have shown promising reductions in post-operative BCRL incidence. Moreover, BCRL treatment offers many therapeutic modalities, including conservative and surgical methods that can be tailored to suit the individual patient. For BCRL research to continue to advance, clinical researchers must utilize objective, standardized measurements that are comparable to other studies in addition to PROM/symptom monitoring and clinical examination. Doing so would allow researchers to better compare individual study results using different preventative/treatment techniques, thereby allowing providers to make informed decisions regarding patient treatment.
One theme that becomes apparent while reviewing the literature is the need for a multidisciplinary approach to diagnose, treat, and prevent BCRL. Success necessitates communication and coordination with a patient’s medical, surgical, and radiation oncologists and nurse practitioners as well as with their physical therapists. Fundamental to BCRL screening are pre-operative measurements to determine a patient’s natural baseline asymmetry. To successfully diagnose BCRL early, health care providers on the patient’s treatment team must make a concerted effort to ensure these measurements are obtained preoperatively. Screening must be longitudinal throughout and beyond treatment for breast cancer, incorporating objective measures, subjective data and clinical examination. Such a screening program allows for early detection and treatment of swelling before it can progress. Ideally, preoperative imaging studies could allow surgeons to identify patients with pre-existing lymphatic disruption and develop individualized surgical plans to minimize BCRL risk. After surgery and treatment, it is imperative to monitor any upper extremity edema, a responsibility that extends beyond the clinical staff. Patients themselves are important components of any treatment team, and clinical providers must educate patients about BCRL and their individual risk for developing it. Quality, comprehensive, individualized patient education should allow a patient to be vigilant, not fearful, in monitoring her at-risk limb. It is a mutual goal of the entire team to maximize every patient’s quality of life beyond treatment for breast cancer. A multidisciplinary team-based approach to understanding, screening for, preventing, diagnosing, and treating BCRL is strongly recommended to provide best care for patients who have been treated for breast cancer and who are at risk of BCRL.
Acknowledgements
Funding: The project was supported by Award Number R01CA139118 (AG Taghian) and Award Number P50CA08393 (AG Taghian) from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. This program is supported by the Adele McKinnon Research Fund for Breast Cancer-Related Lymphedema.
Footnote
Conflicts of Interest: AG Taghian has been loaned equipment from ImpediMed for use in an investigator initiated clinical trial. ImpediMed has had no involvement in the conception or reporting of our research activities. AG Taghian has been a consultant for VisionRT (image-guidance radiation oncology). The other authors have no conflicts of interest to declare.
References
- Erickson VS, Pearson ML, Ganz PA, et al. Arm edema in breast cancer patients. J Natl Cancer Inst 2001;93:96-111. [Crossref] [PubMed]
- Hespe GE, Nitti MD, Mehrara BJ. Pathophysiology of lymphedema. In: Greene A, Slavin S, Brorson H. editors. Lymphedema Presentation, Diagnosis, and Treatment. Cham: Springer, 2015.
- Chowdhry M, Rozen WM, Griffiths M. Lymphatic mapping and preoperative imaging in the management of post-mastectomy lymphoedema. Gland Surg 2016;5:187-96. [PubMed]
- Fu MR, Rosedale M. Breast cancer survivors’ experiences of lymphedema-related symptoms. J Pain Symptom Manage 2009;38:849-59. [Crossref] [PubMed]
- Armer JM, Radina ME, Porock D, et al. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res 2003;52:370-9. [Crossref] [PubMed]
- Hespe GE, Nores GG, Huang JJ, et al. Pathophysiology of lymphedema—Is there a chance for medication treatment? J Surg Oncol 2017;115:96-8. [Crossref] [PubMed]
- Chachaj A, Malyszczak K, Pyszel K, et al. Physical and psychological impairments of women with upper limb lymphedema following breast cancer treatment. Psychooncology 2010;19:299-305. [Crossref] [PubMed]
- Vassard D, Olsen MH, Zinckernagel L, et al. Psychological consequences of lymphoedema associated with breast cancer: A prospective cohort study. Eur J Cancer 2010;46:3211-8. [Crossref] [PubMed]
- Khan F, Amatya B, Pallant JF, et al. Factors associated with long-term functional outcomes and psychological sequelae in women after breast cancer. Breast 2012;21:314-20. [Crossref] [PubMed]
- DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol 2013;14:500-15. [Crossref] [PubMed]
- Tsai RJ, Dennis LK, Lynch CF, et al. The risk of developing arm lymphedema among breast cancer survivors: A meta-analysis of treatment factors. Ann Surg Oncol 2009;16:1959-72. [Crossref] [PubMed]
- Shah C, Vicini FA. Breast cancer-related arm lymphedema: Incidence rates, diagnostic techniques, optimal management and risk reduction strategies. Int J Radiat Oncol Biol Phys 2011;81:907-14. [Crossref] [PubMed]
- Asdourian MS, Skolny MN, Brunelle C, et al. Precautions for breast cancer-related lymphoedema: Risk from air travel, ipsilateral arm blood pressure measurements, skin puncture, extreme temperatures, and cellulitis. Lancet Oncol 2016;17:e392-405. [Crossref] [PubMed]
- Ferguson CM, Swaroop MN, Horick N, et al. Impact of ipsilateral blood draws, injections, blood pressure measurements, and air travel on the risk of lymphedema for patients treated for breast cancer. J Clin Oncol 2016;34:691-8. [Crossref] [PubMed]
- Sayegh HE, Asdourian MS, Swaroop MN, et al. Diagnostic methods, risk factors, prevention, and management of breast cancer-related lymphedema: Past, present, and future directions. Curr Breast Cancer Rep 2017;9:111-21. [Crossref] [PubMed]
- McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Objective measurements. J Clin Oncol 2008;26:5213-9. [Crossref] [PubMed]
- Shaitelman SF, Chiang YJ, Griffin KD, et al. Radiation therapy targets and the risk of breast cancer-related lymphedema: A systematic review and network meta-analysis. Breast Cancer Res Treat 2017;162:201-15. [Crossref] [PubMed]
- Kilbreath SL, Refshauge KM, Beith JM, et al. Risk factors for lymphoedema in women with breast cancer: A large prospective cohort. Breast 2016;28:29-36. [Crossref] [PubMed]
- Gärtner R, Jensen MB, Kronborg L, et al. Self-reported arm-lymphedema and functional impairment after breast cancer treatment -- a nationwide study of prevalence and associated factors. Breast 2010;19:506-15. [Crossref] [PubMed]
- Warren LEG, Miller CL, Horick N, et al. The impact of radiation therapy on the risk of lymphedema after treatment for breast cancer: A prospective cohort study. Int J Radiat Oncol Biol Phys 2014;88:565-71. [Crossref] [PubMed]
- Miller CL, Colwell AS, Horick N, et al. Immediate implant reconstruction is associated with a reduced risk of lymphedema compared to mastectomy alone: A prospective cohort study. Ann Surg 2016;263:399-405. [Crossref] [PubMed]
- Crosby MA, Card A, Liu J, et al. Immediate breast reconstruction and lymphedema incidence. Plast Reconstr Surg 2012;129:789e-95e. [Crossref] [PubMed]
- Card A, Crosby M, Liu J, et al. Reduced incidence of breast cancer-related lymphedema following mastectomy and breast reconstruction versus mastectomy alone. Plast Reconstr Surg 2012;130:1169-78. [Crossref] [PubMed]
- Miller CL, Specht MC, Skolny MN, et al. Sentinel lymph node biopsy at the time of mastectomy does not increase the risk of lymphedema: Implications for prophylactic surgery. Breast Cancer Res Treat 2012;135:781-9. [Crossref] [PubMed]
- De Groef A, Van Kampen M, Tieto E, et al. Arm lymphoedema and upper limb impairments in sentinel node-negative breast cancer patients: A one year follow-up study. Breast 2016;29:102-8. [Crossref] [PubMed]
- Miller CL, Specht MC, Skolny MN, et al. Risk of lymphedema after mastectomy: Potential benefit of applying ACOSOG Z0011 protocol to mastectomy patients. Breast Cancer Res Treat 2014;144:71-7. [Crossref] [PubMed]
- Basta MN, Fischer JP, Kanchwala SK, et al. A propensity-matched analysis of the influence of breast reconstruction on subsequent development of lymphedema. Plast Reconstr Surg 2015;136:134e-43e. [Crossref] [PubMed]
- Lee KT, Mun GH, Lim SY, et al. The impact of immediate breast reconstruction on post-mastectomy lymphedema in patients undergoing modified radical mastectomy. Breast 2013;22:53-7. [Crossref] [PubMed]
- Kim M, Park IH, Lee KS, et al. Breast cancer-related lymphedema after neoadjuvant chemotherapy. Cancer Res Treat 2015;47:416-23. [Crossref] [PubMed]
- Specht MC, Miller CL, Skolny MN, et al. Residual lymph node disease after neoadjuvant chemotherapy predicts an increased risk of lymphedema in node-positive breast cancer patients. Ann Surg Oncol 2013;20:2835-41. [Crossref] [PubMed]
- Swaroop MN, Ferguson CM, Horick NK, et al. Impact of adjuvant taxane-based chemotherapy on development of breast cancer-related lymphedema: Results from a large prospective cohort. Breast Cancer Res Treat 2015;151:393-403. [PubMed]
- Kim M, Kim SW, Lee SU, et al. A model to estimate the risk of breast cancer-related lymphedema: Combinations of treatment-related factors of the number of dissected axillary nodes, adjuvant chemotherapy, and radiation therapy. Int J Radiat Oncol Biol Phys 2013;86:498-503. [Crossref] [PubMed]
- Lopez Penha TR, van Roozendaal LM, Smidt ML, et al. The changing role of axillary treatment in breast cancer: Who will remain at risk for developing arm morbidity in the future? Breast 2015;24:543-7. [Crossref] [PubMed]
- Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 2014;15:1303-10. [Crossref] [PubMed]
- Vignes S, Arrault M, Dupuy A. Factors associated with increased breast cancer-related lymphedema volume. Acta Oncol 2007;46:1138-42. [Crossref] [PubMed]
- Kambayashi J, Ohshiro T, Mori T. Appraisal of myocutaneous flapping for treatment of postmastectomy lymphedema. Case report. Acta Chir Scand 1990;156:175-7. [PubMed]
- Blanchard M, Arrault M, Vignes S. Positive impact of delayed breast reconstruction on breast-cancer treatment-related arm lymphoedema. J Plast Reconstr Aesthet Surg 2012;65:1060-3. [Crossref] [PubMed]
- Chang DW, Kim S. Breast reconstruction and lymphedema reply. Plast Reconstr Surg 2010;125:19-23. [Crossref] [PubMed]
- Avraham T, Daluvoy SV, Riedel ER, et al. Tissue expander breast reconstruction is not associated with an increased risk of lymphedema. Ann Surg Oncol 2010;17:2926-32. [Crossref] [PubMed]
- Ahmed RL, Schmitz KH, Prizment AE, et al. Risk factors for lymphedema in breast cancer survivors, the Iowa Women’s Health Study. Breast Cancer Res Treat 2011;130:981-91. [Crossref] [PubMed]
- Jung SY, Shin KH, Kim M, et al. Treatment factors affecting breast cancer-related lymphedema after systemic chemotherapy and radiotherapy in stage II/III breast cancer patients. Breast Cancer Res Treat 2014;148:91-8. [Crossref] [PubMed]
- Zhu W, Li D, Li X, et al. Association between adjuvant docetaxel-based chemotherapy and breast cancer-related lymphedema. Anticancer Drugs 2017;28:350-5. [Crossref] [PubMed]
- Brønstad A, Berg A, Reed RK. Effects of the taxanes paclitaxel and docetaxel on edema formation and interstitial fluid pressure. Am J Physiol Heart Circ Physiol 2004;287:H963-8. [Crossref] [PubMed]
- Qin YY, Li H, Guo XJ, et al. Adjuvant chemotherapy, with or without taxanes, in early or operable breast cancer: A meta-analysis of 19 randomized trials with 30698 patients. PLoS One 2011;6. [Crossref] [PubMed]
- Ohsumi S, Shimozuma K, Ohashi Y, et al. Subjective and objective assessment of edema during adjuvant chemotherapy for breast cancer using taxane-containing regimens in a randomized controlled trial: The national surgical adjuvant study of breast cancer 02. Oncology 2012;82:131-8. [Crossref] [PubMed]
- Fu MR, Axelrod D, Guth A, et al. Patterns of obesity and lymph fluid level during the first year of breast cancer treatment: A prospective study. J Pers Med 2015;5:326-40. [Crossref] [PubMed]
- Jammallo LS, Miller CL, Singer M, et al. Impact of body mass index and weight fluctuation on lymphedema risk in patients treated for breast cancer. Breast Cancer Res Treat 2013;142:59-67. [Crossref] [PubMed]
- Specht MC, Miller CL, Russell TA, et al. Defining a threshold for intervention in breast cancer-related lymphedema: What level of arm volume increase predicts progression? Breast Cancer Res Treat 2013;140:485-94. [Crossref] [PubMed]
- Bevilacqua JLB, Kattan MW, Changhong Y, et al. Nomograms for predicting the risk of arm lymphedema after axillary dissection in breast cancer. Ann Surg Oncol 2012;19:2580-9. [Crossref] [PubMed]
- Bar Ad V, Cheville A, Solin LJ, et al. Time course of mild arm lymphedema after breast conservation treatment for early-stage breast cancer. Int J Radiat Oncol Biol Phys 2010;76:85-90. [Crossref] [PubMed]
- Petrek JA, Senie RT, Peters M, et al. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001;92:1368-77. [Crossref] [PubMed]
- Park JH, Lee WH, Chung HS. Incidence and risk factors of breast cancer lymphoedema. J Clin Nurs 2008;17:1450-9. [Crossref] [PubMed]
- Norman SA, Localio AR, Kallan MJ, et al. Risk factors for lymphedema after breast cancer treatment. Cancer Epidemiol Biomarkers Prev 2010;19:2734-46. [Crossref] [PubMed]
- Helyer LK, Varnic M, Le LW, et al. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J 2010;16:48-54. [Crossref] [PubMed]
- Ridner SH, Dietrich MS, Stewart BR, et al. Body mass index and breast cancer treatment-related lymphedema. Support Care Cancer 2011;19:853-7. [Crossref] [PubMed]
- Indelicato DJ, Grobmyer SR, Newlin H, et al. Delayed breast cellulitis: An evolving complication of breast conservation. Int J Radiat Oncol Biol Phys 2006;66:1339-46. [Crossref] [PubMed]
- Shih YC, Xu Y, Cormier JN, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: A 2-year follow-up study. J Clin Oncol 2009;27:2007-14. [Crossref] [PubMed]
- National Lymphedema Network. Lymphedema Risk Reduction Practices [Internet]. 2012. Available online: https://www.lymphnet.org/resources/position-paper-lymphedema-risk-reduction-practices
- Ahn S, Port ER. Lymphedema precautions: Time to abandon old practices? J Clin Oncol 2016;34:655-8. [Crossref] [PubMed]
- Asdourian MS, Swaroop MN, Sayegh HE, et al. Association between precautionary behaviors and breast cancer-related lymphedema in patients undergoing bilateral surgery. J Clin Oncol 2017;35:3934-41. [Crossref] [PubMed]
- International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 consensus document of the International Society of Lymphology. Lymphology 2013;46:1-11. [PubMed]
- Brunelle C, Skolny M, Ferguson C, et al. Establishing and sustaining a prospective screening program for breast cancer-related lymphedema at the Massachusetts General Hospital: Lessons Learned. J Pers Med 2015;5:153-64. [Crossref] [PubMed]
- Soran A, Menekse E, Girgis M, et al. Breast cancer-related lymphedema after axillary lymph node dissection: Does early postoperative prediction model work? Support Care Cancer 2016;24:1413-9. [Crossref] [PubMed]
- Stout NL, Pfalzer LA, Springer B, et al. Breast cancer-related lymphedema: Comparing direct costs of a prospective surveillance model and a traditional model of care. Phys Ther 2012;92:152-63. [Crossref] [PubMed]
- Vicini F, Shah C, Lyden M, et al. Bioelectrical impedance for detecting and monitoring patients for the development of upper limb lymphedema in the clinic. Clin Breast Cancer 2012;12:133-7. [Crossref] [PubMed]
- Erdogan Iyigun Z, Selamoglu D, Alco G, et al. Bioelectrical impedance for detecting and monitoring lymphedema in patients with breast cancer. Preliminary results of the florence nightingale breast study group. Lymphat Res Biol 2015;13:40-5. [Crossref] [PubMed]
- Bundred NJ, Stockton C, Keeley V, et al. Comparison of multi-frequency bioimpedance with perometry for the early detection and intervention of lymphoedema after axillary node clearance for breast cancer. Breast Cancer Res Treat 2015;151:121-9. [Crossref] [PubMed]
- Stout Gergich NL, Pfalzer LA, McGarvey C, et al. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer 2008;112:2809-19. [Crossref] [PubMed]
- Sun F, Skolny MN, Swaroop MN, et al. The need for preoperative baseline arm measurement to accurately quantify breast cancer-related lymphedema. Breast Cancer Res Treat 2016;157:229-40. [Crossref] [PubMed]
- Ancukiewicz M, Russell TA, Otoole J, et al. Standardized method for quantification of developing lymphedema in patients treated for breast cancer. Int J Radiat Oncol Biol Phys 2011;79:1436-43. [Crossref] [PubMed]
- Ancukiewicz M, Miller CL, Skolny MN, et al. Comparison of relative versus absolute arm size change as criteria for quantifying breast cancer-related lymphedema: The flaws in current studies and need for universal methodology. Breast Cancer Res Treat 2012;135:145-52. [Crossref] [PubMed]
- Miller CL, Specht MC, Horick N, et al. A novel, validated method to quantify breast cancer-related lymphedema (BCRL) following bilateral breast surgery. Lymphology 2013;46:64-74. [PubMed]
- O’Toole J, Jammallo LS, Miller CL, et al. Screening for breast cancer-related lymphedema: The need for standardization. Oncologist 2013;18:350-2. [Crossref] [PubMed]
- Shah C, Arthur DW, Wazer D, et al. The impact of early detection and intervention of breast cancer-related lymphedema: A systematic review. Cancer Med 2016;5:1154-62. [Crossref] [PubMed]
- Soran A, Ozmen T, McGuire KP, et al. The importance of detection of subclinical lymphedema for the prevention of breast cancer-related clinical lymphedema after axillary lymph node dissection: A prospective observational study. Lymphat Res Biol 2014;12:289-94. [Crossref] [PubMed]
- Cabanas RM. An approach for the treatment of penile carcinoma. Cancer 1977;39:456-66. [Crossref] [PubMed]
- Krag DN, Weaver DL, Alex JC, et al. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol 1993;2:335-9; discussion 340. [Crossref] [PubMed]
- Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 1992;127:392-9. [Crossref] [PubMed]
- Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet 1997;349:1864-7. [Crossref] [PubMed]
- Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer -- A multicenter validation study. N Engl J Med 1998;339:941-6. [Crossref] [PubMed]
- Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: Results from the NSABP B-32 randomised phase III trial. Lancet Oncol 2007;8:881-8. [Crossref] [PubMed]
- Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: Overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 2010;11:927-33. [Crossref] [PubMed]
- Langer I, Guller U, Berclaz G, et al. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery. Ann Surg 2007;245:452-61. [Crossref] [PubMed]
- Land SR, Kopec JA, Julian TB, et al. Patient-reported outcomes in sentinel node-negative adjuvant breast cancer patients receiving sentinel-node biopsy or axillary dissection: National Surgical Adjuvant Breast and Bowel Project phase III protocol B-32. J Clin Oncol 2010;28:3929-36. [Crossref] [PubMed]
- Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol 2006;13:491-500. [Crossref] [PubMed]
- Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC trial. J Natl Cancer Inst 2006;98:599-609. [Crossref] [PubMed]
- Lyman GH, Somerfield MR, Bosserman LD, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2017;35:561-4. [Crossref] [PubMed]
- Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v8-30. [Crossref] [PubMed]
- Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013;310:1455-61. [Crossref] [PubMed]
- Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. Lancet Oncol 2013;14:609-18. [Crossref] [PubMed]
- Mamtani A, Barrio AV, King TA, et al. How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? Results of a prospective study. Ann Surg Oncol 2016;23:3467-74. [Crossref] [PubMed]
- Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305:569-75. [Crossref] [PubMed]
- Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg 2010;252:426-32; discussion 432-3. [PubMed]
- Parks RM, Cheung KL. Axillary reverse mapping in N0 patients requiring sentinel lymph node biopsy - A systematic review of the literature and necessity of a randomised study. Breast 2017;33:57-70. [Crossref] [PubMed]
- Noguchi M, Miura S, Morioka E, et al. Is axillary reverse mapping feasible in breast cancer patients? Eur J Surg Oncol 2015;41:442-9. [Crossref] [PubMed]
- Ahmed M, Rubio IT, Kovacs T, et al. Systematic review of axillary reverse mapping in breast cancer. Br J Surg 2016;103:170-8. [Crossref] [PubMed]
- Beek MA, Gobardhan PD, Schoenmaeckers EJ, et al. Axillary reverse mapping in axillary surgery for breast cancer: An update of the current status. Breast Cancer Res Treat 2016;158:421-32. [Crossref] [PubMed]
- Gebruers N, Tjalma WA. Clinical feasibility of axillary reverse mapping and its influence on breast cancer related lymphedema: A systematic review. Eur J Obstet Gynecol Reprod Biol 2016;200:117-22. [Crossref] [PubMed]
- Nos C, Lesieur B, Clough K, et al. Blue dye injection in the arm in order to conserve the lymphatic drainage of the arm in breast cancer patients requiring an axillary dissection. Ann Surg Oncol 2007;14:2490-6. [Crossref] [PubMed]
- Thompson M, Korourian S, Henry-Tillman R, et al. Axillary reverse mapping (ARM): A new concept to identify and enhance lymphatic preservation. Ann Surg Oncol 2007;14:1890-5. [Crossref] [PubMed]
- Britton TB, Solanki CK, Pinder SE, et al. Lymphatic drainage pathways of the breast and the upper limb. Nucl Med Commun 2009;30:427-30. [Crossref] [PubMed]
- Gennaro M, MacCauro M, Sigari C, et al. Selective axillary dissection after axillary reverse mapping to prevent breast-cancer-related lymphoedema. Eur J Surg Oncol 2013;39:1341-5. [Crossref] [PubMed]
- Noguchi M, Yokoi M, Nakano Y. Axillary reverse mapping with indocyanine fluorescence imaging in patients with breast cancer. J Surg Oncol 2010;101:217-21. [PubMed]
- Noguchi M, Noguchi M, Nakano Y, et al. Axillary reverse mapping using a fluorescence imaging system in breast cancer. J Surg Oncol 2012;105:229-34. [Crossref] [PubMed]
- Ikeda K, Ogawa Y, Kajino C, et al. The influence of axillary reverse mapping related factors on lymphedema in breast cancer patients. Eur J Surg Oncol 2014;40:818-23. [Crossref] [PubMed]
- Sakurai T, Endo M, Shimizu K, et al. Axillary reverse mapping using fluorescence imaging is useful for identifying the risk group of postoperative lymphedema in breast cancer patients undergoing sentinel node biopsies. J Surg Oncol 2014;109:612-5. [Crossref] [PubMed]
- Nos C, Kaufmann G, Clough KB, et al. Combined axillary reverse mapping (ARM) technique for breast cancer patients requiring axillary dissection. Ann Surg Oncol 2008;15:2550-5. [Crossref] [PubMed]
- Tausch C, Baege A, Dietrich D, et al. Can axillary reverse mapping avoid lymphedema in node positive breast cancer patients? Eur J Surg Oncol 2013;39:880-6. [Crossref] [PubMed]
- Yue T, Zhuang D, Zhou P, et al. A prospective study to assess the feasibility of axillary reverse mapping and evaluate its effect on preventing lymphedema in breast cancer patients. Clin Breast Cancer 2015;15:301-6. [Crossref] [PubMed]
- Tummel E, Ochoa D, Korourian S, et al. Does axillary reverse mapping prevent lymphedema after lymphadenectomy? Ann Surg 2017;265:987-92. [Crossref] [PubMed]
- Ochoa D, Korourian S, Boneti C, et al. Axillary reverse mapping: Five-year experience. Surgery 2014;156:1261-8. [Crossref] [PubMed]
- Connor C, McGinness M, Mammen J, et al. Axillary reverse mapping: A prospective study in women with clinically node negative and node positive breast cancer. Ann Surg Oncol 2013;20:3303-7. [Crossref] [PubMed]
- Boneti C, Korourian S, Diaz Z, et al. Scientific Impact Award: Axillary reverse mapping (ARM) to identify and protect lymphatics draining the arm during axillary lymphadenectomy. Am J Surg 2009;198:482-7. [Crossref] [PubMed]
- Casabona F, Bogliolo S, Valenzano Menada M, et al. Feasibility of axillary reverse mapping during sentinel lymph node biopsy in breast cancer patients. Ann Surg Oncol 2009;16:2459-63. [Crossref] [PubMed]
- Han JW, Seo YJ, Choi JE, et al. The efficacy of arm node preserving surgery using axillary reverse mapping for preventing lymphedema in patients with breast cancer. J Breast Cancer 2012;15:91-7. [Crossref] [PubMed]
- Boneti C, Korourian S, Bland K, et al. Axillary reverse mapping: Mapping and preserving arm lymphatics may be important in preventing lymphedema during sentinel lymph node biopsy. J Am Coll Surg 2008;206:1038-42. [Crossref] [PubMed]
- Noguchi M, Noguchi M, Ohno Y, et al. Feasibility study of axillary reverse mapping for patients with clinically node-negative breast cancer. Eur J Surg Oncol 2016;42:650-6. [Crossref] [PubMed]
- Deng H, Chen L, Jia W, et al. Safety study of axillary reverse mapping in the surgical treatment for breast cancer patients. J Cancer Res Clin Oncol 2011;137:1869-74. [Crossref] [PubMed]
- Ding X. ARM in breast cancer with enlarged lymph node: A Chinese single center experience. Breast 2015;24:S139. [Crossref]
- Kuusk U, Seyednejad N, McKevitt EC, et al. Axillary reverse mapping in breast cancer: A Canadian experience. J Surg Oncol 2014;110:791-5. [Crossref] [PubMed]
- Kang S, Choi J, Jeon Y, et al. Preservation of lymphatic drainage from arm in breast cancer surgery: Is it safe? Cancer Res 2009;69:201. [Crossref]
- Rubio IT, Cebrecos I, Peg V, et al. Extensive nodal involvement increases the positivity of blue nodes in the axillary reverse mapping procedure in patients with breast cancer. J Surg Oncol 2012;106:89-93. [Crossref] [PubMed]
- Pavlista D, Eliska O. Analysis of direct oil contrast lymphography of upper limb lymphatics traversing the axilla - A lesson from the past - Contribution to the concept of axillary reverse mapping. Eur J Surg Oncol 2012;38:390-4. [Crossref] [PubMed]
- Bedrosian I, Babiera GV., Mittendorf EA, et al. A phase I study to assess the feasibility and oncologic safety of axillary reverse mapping in breast cancer patients. Cancer 2010;116:2543-8. [PubMed]
- Gobardhan PD, Wijsman JH, Van Dalen T, et al. ARM: Axillary reverse mapping - The need for selection of patients. Eur J Surg Oncol 2012;38:657-61. [Crossref] [PubMed]
- Ikeda K, Ogawa Y, Komatsu H, et al. Evaluation of the metastatic status of lymph nodes identified using axillary reverse mapping in breast cancer patients. World J Surg Oncol 2012;10:233. [Crossref] [PubMed]
- Ponzone R, Cont NT, Maggiorotto F, et al. Extensive nodal disease may impair axillary reverse mapping in patients with breast cancer. J Clin Oncol 2009;27:5547-51. [Crossref] [PubMed]
- Schunemann E, Dória MT, Silvestre JBCH, et al. Prospective study evaluating oncological safety of axillary reverse mapping. Ann Surg Oncol 2014;21:2197-202. [Crossref] [PubMed]
- Luiten EJ, Beek MA, Rubio IT. Clinical utility of axillary reverse mapping (ARM) in an era of changing perceptions concerning axillary surgery. Eur J Surg Oncol 2016;42:585-7. [Crossref] [PubMed]
- Boccardo F, Casabona F, De Cian F, et al. Lymphedema microsurgical preventive healing approach: A new technique for primary prevention of arm lymphedema after mastectomy. Ann Surg Oncol 2009;16:703-8. [Crossref] [PubMed]
- Boccardo FM, Casabona F, Friedman D, et al. Surgical prevention of arm lymphedema after breast cancer treatment. Ann Surg Oncol 2011;18:2500-5. [Crossref] [PubMed]
- Boccardo F, Casabona F, De Cian F, et al. Lymphatic microsurgical preventing healing approach (LYMPHA) for primary surgical prevention of breast cancer-related lymphedema: Over 4 years follow-up. Microsurgery 2014;34:421-4. [Crossref] [PubMed]
- Campisi C, Boccardo F. Microsurgical techniques for lymphedema treatment: Derivative lymphatic-venous microsurgery. World J Surg 2004;28:609-13. [Crossref] [PubMed]
- Tourani SS, Taylor GI, Ashton MW. Long-tem patency of lymphovenous anastomoses. Plast Reconstr Surg 2016;138:492-8. [Crossref] [PubMed]
- Feldman S, Bansil H, Ascherman J, et al. Single institution experience with lymphatic microsurgical preventive healing approach (LYMPHA) for the primary prevention of lymphedema. Ann Surg Oncol 2015;22:3296-301. [Crossref] [PubMed]
- Gomberawalla A, Feldman S. LYMPHA: New innovation, not old practice. J Clin Oncol 2016;34:3108-9. [Crossref] [PubMed]
- Ahn S, Port ER. Reply to A. Gomberawalla et al and J. Nudelman. J Clin Oncol 2016;34:3110-1. [Crossref] [PubMed]
- McNeely ML, Magee DJ, Lees AW, et al. The addition of manual lymph drainage to compression therapy for breast cancer related lymphedema: A randomized controlled trial. Breast Cancer Res Treat 2004;86:95-106. [Crossref] [PubMed]
- Dayes IS, Whelan TJ, Julian JA, et al. Randomized trial of decongestive lymphatic therapy for the treatment of lymphedema in women with breast cancer. J Clin Oncol 2013;31:3758-63. [Crossref] [PubMed]
- Ezzo J, Manheimer E, McNeely ML, et al. Manual lymphatic drainage for lymphedema following breast cancer treatment. Cochrane Database Syst Rev 2015. [PubMed]
- Schmitz KH, Troxel AB, Cheville A, et al. Physical Activity and Lymphedema (the PAL trial): assessing the safety of progressive strength training in breast cancer survivors. Contemp Clin Trials 2009;30:233-45. [Crossref] [PubMed]
- Sagen A, Karesen R, Risberg MA. Physical activity for the affected limb and arm lymphedema after breast cancer surgery. A prospective, randomized controlled trial with two years follow-up. Acta Oncol 2009;48:1102-10. [Crossref] [PubMed]
- Schmitz KH, Ahmed RL, Troxel AB, et al. Weight lifting for women at risk for breast cancer-related lymphedema: a randomized trial. JAMA 2010;304:2699-705. [Crossref] [PubMed]
- Ahmed RL, Thomas W, Yee D, et al. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. J Clin Oncol 2006;24:2765-72. [Crossref] [PubMed]
- Cormie P, Galvão DA, Spry N, et al. Neither heavy nor light load resistance exercise acutely exacerbates lymphedema in breast cancer survivors. Integr Cancer Ther 2013;12:423. [Crossref] [PubMed]
- Rogan S, Taeymans J, Luginbuehl H, et al. Therapy modalities to reduce lymphoedema in female breast cancer patients: A systematic review and meta-analysis. Breast Cancer Res Treat 2016;159:1-14. [Crossref] [PubMed]
- Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 2010;42:1409-26. [Crossref] [PubMed]
- Shao Y, Qi K, Zhou QH, et al. Intermittent pneumatic compression pump for breast cancer-related lymphedema: A systematic review and meta-analysis of randomized controlled trials. Oncol Res Treat 2014;37:170-4. [Crossref] [PubMed]
- Szolnoky G, Lakatos B, Keskeny T, et al. Intermittent pneumatic compression acts syngergistically with manual lymphatic drainage in complex decongestive physiotherapy for breast cancer treatment-related lymphedema. Lymphology 2009;42:188-94. [PubMed]
- Gurdal SO, Kostanoglu A, Cavdar I, et al. Comparison of intermittent pneumatic compression with manual lymphatic drainage for treatment of breast cancer-related lymphedema. Lymphat Res Biol 2012;10:129-35. [Crossref] [PubMed]
- Smoot B, Chiavola-Larson L, Lee J, et al. Effect of low-level laser therapy on pain and swelling in women with breast cancer-related lymphedema: A systematic review and meta-analysis. J Cancer Surviv 2015;9:287-304. [Crossref] [PubMed]
- Ridner SH, Poage-Hooper E, Kanar C, et al. A pilot randomized trial evaluating low-level laser therapy as an alternative treatment to manual lymphatic drainage for breast cancer-related lymphedema. Oncol Nurs Forum 2013;40:383-93. [Crossref] [PubMed]
- Garza R, Skoracki R, Hock K, et al. A comprehensive overview on the surgical management of secondary lymphedema of the upper and lower extremities related to prior oncologic therapies. BMC Cancer 2017;17:468. [Crossref] [PubMed]
- Suami H, Chang DW. Overview of surgical treatments for breast cancer–related lymphedema. Plast Reconstr Surg 2010;126:1853-63. [Crossref] [PubMed]
- Allen RJ Jr, Cheng MH. Lymphedema surgery: Patient selection and an overview of surgical techniques. J Surg Oncol 2016;113:923-31. [Crossref] [PubMed]
- Kayıran O, De La Cruz C, Tane K, et al. Lymphedema: From diagnosis to treatment. Turk J Surg 2017;33:51-7. [Crossref] [PubMed]
- Granzow JW, Soderberg JM, Kaji AH, et al. Review of current surgical treatments for lymphedema. Ann Surg Oncol 2014;21:1195-201. [Crossref] [PubMed]
- Granzow JW, Soderberg JM, Kaji AH, et al. An effective system of surgical treatment of lymphedema. Ann Surg Oncol 2014;21:1189-94. [Crossref] [PubMed]
- Brorson H, Ohlin K, Olsson G, et al. Adipose tissue dominates chronic arm lymphedema following breast cancer: An analysis using volume rendered CT images. Lymphat Res Biol 2006;4:199-210. [Crossref] [PubMed]
- Brorson H, Ohlin K, Olsson G, et al. Breast cancer-related chronic arm lymphedema is associated with excess adipose and muscle tissue. Lymphat Res Biol 2009;7:3-10. [Crossref] [PubMed]
- Cuzzone DA, Weitman ES, Albano NJ, et al. IL-6 regulates adipose deposition and homeostasis in lymphedema. Am J Physiol Heart Circ Physiol 2014;306:H1426-34. [Crossref] [PubMed]
- Brorson H. Liposuction in lymphedema treatment. J Reconstr Microsurg 2016;32:56-65. [PubMed]
- Greene AK, Maclellan RA. Operative treatment of lymphedema using suction-assisted lipectomy. Ann Plast Surg 2016;77:337-40. [Crossref] [PubMed]
- Boyages J, Kastanias K, Koelmeyer LA, et al. Liposuction for advanced lymphedema: A multidisciplinary approach for complete reduction of arm and leg swelling. Ann Surg Oncol 2015;22 Suppl 3:S1263-70. [Crossref] [PubMed]
- Schaverien MV, Munro KJ, Baker PA, et al. Liposuction for chronic lymphoedema of the upper limb: 5 Years of experience. J Plast Reconstr Aesthet Surg 2012;65:935-42. [Crossref] [PubMed]
- Damstra RJ, Voesten HG, Klinkert P, et al. Circumferential suction-assisted lipectomy for lymphoedema after surgery for breast cancer. Br J Surg 2009;96:859-64. [Crossref] [PubMed]
- Brorson H, Svensson H. Liposuction combined with controlled compression therapy reduces arm lymphedema more effectively than controlled compression therapy alone. Plast Reconstr Surg 1998;102:1058-67. [Crossref] [PubMed]
- Granzow JW, Soderberg JM, Kaji AH, et al. Review of Current Surgical Treatments for Lymphedema. Ann Surg Oncol 2014;21:1195-201. [Crossref] [PubMed]
- Baumeister RG, Siuda S, Bohmert H, et al. A microsurgical method for reconstruction of interrupted lymphatic pathways: autologous lymph-vessel transplantation for treatment of lymphedemas. Scand J Plast Reconstr Surg 1986;20:141-6. [Crossref] [PubMed]
- Baumeister RG, Siuda S. Treatment of lymphedemas by microsurgical lymphatic grafting: what is proved? Plast Reconstr Surg 1990;85:64-74; discussion 75-6. [Crossref] [PubMed]
- Baumeister RG, Mayo W, Notohamiprodjo M, et al. Microsurgical Lymphatic Vessel Transplantation. J Reconstr Microsurg 2016;32:34-41. [PubMed]
- Weiss M, Baumeister RG, Hahn K. Post-therapeutic lymphedema: scintigraphy before and after autologous lymph vessel transplantation: 8 years of long-term follow-up. Clin Nucl Med 2002;27:788-92. [Crossref] [PubMed]
- Campisi C. Use of autologous interposition vein graft in management of lymphedema: preliminary experimental and clinical observations. Lymphology 1991;24:71-6. [PubMed]
- Campisi C, Boccardo F, Zilli A, et al. The use of vein grafts in the treatment of peripheral lymphedemas: Long-term results. Microsurgery 2001;21:143-7. [Crossref] [PubMed]
- Chen HC, O’Brien BM, Rogers IW, et al. Lymph node transfer for the treatment of obstructive lymphoedema in the canine model. Br J Plast Surg 1990;43:578-86. [Crossref] [PubMed]
- Cheng MH, Chen SC, Henry SL, et al. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: Flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg 2013;131:1286-98. [Crossref] [PubMed]
- Cheng MH, Huang JJ, Wu CW, et al. The mechanism of vascularized lymph node transfer for lymphedema. Plast Reconstr Surg 2014;133:192e-8e. [Crossref] [PubMed]
- Becker C, Assouad J, Riquet M, et al. Postmastectomy lymphedema: Long-term results following microsurgical lymph node transplantation. Ann Surg 2006;243:313-5. [Crossref] [PubMed]
- Lin CH, Ali R, Chen SC, et al. Vascularized groin lymph node transfer using the wrist as a recipient site for management of postmastectomy upper extremity lymphedema. Plast Reconstr Surg 2009;123:1265-75. [Crossref] [PubMed]
- Becker C, Hidden G, Godart S, et al. Free lymphatic transplant. Eur J Lymphol Rel Prob 1991;6:25-77.
- Saaristo AM, Niemi TS, Viitanen TP, et al. Microvascular breast reconstruction and lymph node transfer for postmastectomy lymphedema patients. Ann Surg 2012;255:468-73. [Crossref] [PubMed]
- Nguyen AT, Chang EI, Suami H, et al. An algorithmic approach to simultaneous vascularized lymph node transfer with microvascular breast reconstruction. Ann Surg Oncol 2015;22:2919-24. [Crossref] [PubMed]
- Vignes S, Blanchard M, Yannoutsos A, et al. Complications of autologous lymph-node transplantation for limb lymphoedema. Eur J Vasc Endovasc Surg 2013;45:516-20. [Crossref] [PubMed]
- Viitanen TP, Mäki MT, Seppänen MP, et al. Donor-site lymphatic function after microvascular lymph node transfer. Plast Reconstr Surg 2012;130:1246-53. [Crossref] [PubMed]
- Dayan JH, Dayan E, Smith ML. Reverse lymphatic mapping: A new technique for maximizing safety in vascularized lymph node transfer. Plast Reconstr Surg 2015;135:277-85. [Crossref] [PubMed]
- Poumellec MA, Foissac R, Cegarra-Escolano M, et al. Surgical treatment of secondary lymphedema of the upper limb by stepped microsurgical lymphaticovenous anastomoses. Breast Cancer Res Treat 2017;162:219-24. [Crossref] [PubMed]
- Chang DW, Suami H, Skoracki R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg 2013;132:1305-14. [Crossref] [PubMed]
- Campisi C, Davini D, Bellini C, et al. Lymphatic microsurgery for the treatment of lymphedema. Microsurgery 2006;26:65-9. [Crossref] [PubMed]
- Campisi C, Eretta C, Pertile D, et al. Microsurgery for treatment of peripheral lymphedema: Long-term outcome and future perspectives. Microsurgery 2007;27:333-8. [Crossref] [PubMed]
- O’Brien BM, Sykes P, Threlfall GN, et al. Microlymphaticovenous anastomoses for obstructive lymphedema. Plast Reconstr Surg 1977;60:197-211. [Crossref] [PubMed]


