Role of interventional radiology in the management of hepatocellular carcinoma: current status
Introduction
Surgery is considered the most effective chance at cure for appropriately selected patients with hepatocellular carcinoma (HCC); however, most patients are not surgical candidates at the time of presentation (1). For these patients, accurate prognostication is critically important for making treatment decisions that best balance oncologic control while maintaining their performance status. Multiple staging systems have been proposed to facilitate appropriate treatment allocation and are based on varied combinations of factors, such as, the extent of tumor burden, liver function, and performance status. The Barcelona Clinic Liver Cancer system (BCLC) (2) is currently the most widely accepted of these staging systems and is endorsed by multiple international multidisciplinary liver disease societies. The system stratifies patients into five grades (0, A, B, C, D) and appropriates treatment options accordingly. Interventional therapies, specifically radiofrequency ablation (RFA) and trans-arterial chemoembolization (TACE), are recommended by the BCLC for stage 0 and stage B patients, respectively. As technical and clinical innovation continue to outpace large randomized controlled trials, interventional therapies have expanded outside of these formal indications and can benefit carefully selected patients across multiple disease stages, especially at centers of excellence with multidisciplinary tumor boards. For the purposes of this article, interventional therapies used in the treatment of HCC are broadly classified as either ablative or embolotherapy. A brief overview of the different treatments comprising these two categories is first provided to familiarize the reader with these procedures and is then followed by a review of how these therapies fit into the evolving HCC treatment paradigm.
Overview of interventional oncology treatment modalities
Ablative techniques
Ablative therapies are minimally invasive procedures performed using a percutaneous, laparoscopic, or open surgical approach, and can be used for curative or palliative tumor treatment. As a review of interventional techniques, this article will focus on the percutaneous applications of ablation. Generally, ablative therapies can be categorized as thermal or non-thermal techniques. Other than cryoablation, most thermal techniques use different methods to heat tissues to temperatures greater than 60 °C, causing instantaneous and irreversible damage to proteins resulting in coagulative necrosis (3). Besides the entire tumor, a margin of approximately 0.5–1 cm should also be included during ablation to control any adjacent microscopic disease (4,5).
RFA is the most ubiquitous and well-studied percutaneous thermal therapy for hepatic tumors and is included in multiple societal guidelines for the treatment of HCC (6,7). In RFA, a generator creates alternating high frequency RF waves (460–480 kHz) to agitate ions and disperse heat centrifugally from a needle-shaped electrode into surrounding tissues. This technique requires the application of a grounding pad to the patient’s skin. The amount of energy delivered is based on Ohm’s law and inversely relates to tissue impedance, which has multiple practical implications. For example, at temperatures greater than 105 °C, the vaporization and carbonization of tissues occurs, increasing local impedance and limiting energy transfer to target tissues. This results in unexpected alterations to the size, shape, and homogeneity of the ablation zone, potentially inhibiting complete coverage of the tumor and margin with tumoricidal temperatures. Strategies to decrease this effect are used, such as, internally cooled probes which maintain a lower temperature around the needle tip, however, the ablation size, temperature and speed remains limited. Impedance is also the basis of heat-sink effect. Current follows the path of least resistance and flowing blood or bile in adjacent structures draws charge away from the tumor, limiting the amount of heat delivered to target tissue (8).
RFA can be performed using a monopolar system, consisting of a single grounding pad and needle-shaped electrode which is inserted centrally into the tumor. This approach is generally used to treat tumors up to ~3 cm with a single probe. Monopolar systems are also used in an overlapping fashion to treat larger tumors; however, this approach can be fraught with issues, with effective ablation relying on the accuracy of probe positioning to ensure complete tumor coverage. Some experts advocate for using multipolar systems to treat larger tumors (9), which use pairs of electrodes placed around the periphery of a tumor, delivering heat concentrically to create an ablation zone centrally between the electrodes.
The success of RFA as an effective and low morbidity tumor treatment has paved the way for a wide array of other thermal technologies to be used in HCC treatment, many of which have demonstrated potential in early studies and may offer advantages, but currently remain less well-established at this time. These modalities will be described in the following paragraphs and are listed in Table 1.
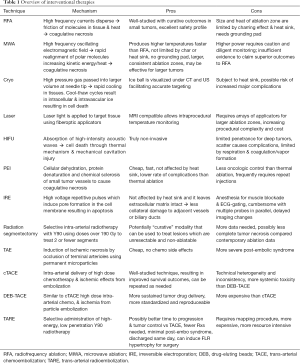
Full table
Microwave ablation (MWA) emits electromagnetic radiation from an antenna probe for tumor therapy, typically using frequencies of either 915 MHz or 2.45 GH. High energy concentration is created around the probe tip, oscillating water molecules to generate heat from friction within the tissues. The excitation of water molecules happens across a larger radius compared to RFA, thus causing a more immediate and homogenous heating. Since MWA relies less on passive tissue heat conduction, MWA zones are also less susceptible to heat sink effects. Char and vaporization is also of less consequence because of this physical property of microwaves. The penetration depth of microwaves is dependent on the frequency used, with the 915 MHz probes yielding larger ablation zones, however, lower energy density (10). It is important to recognize there are stark differences between the ablation zones created by different microwave systems, especially first-generation microwave probes which produced smaller, suboptimal ablation zones. Newer, second generation MWA systems are capable of generating hotter, larger ablation zones (can be greater than 5 cm) at a faster rate than RFA technology (11,12), leading to increasing adoption amongst interventionalists.
Cryoablation (Cryo) is a thermal ablation technique that uses the rapid expansion of gas in the distal tip of a probe to cause rapid cooling to temperatures as low as −140 °C, dependent on the gas used and probe design (13). Temperatures less than −40 °C are cytotoxic and rapid freeze/thaw cycles are used to create intracellular and intravascular ice crystals that lead to cell death. Cryoablation has not been widely used for hepatic tumor ablation because of a higher complication rate compared to RFA in older studies including hemorrhage, biliary injury, and “cryoshock”, a severe systemic reaction specific to cryotherapy that is characterized by massive cytokine release and multi-organ failure (14). That said, some authors are now reporting reasonably low complication rates with newer probes resulting in comparable outcomes to RFA (15,16).
High-intensity frequency ultrasound (HIFU) is a non-invasive technique, performed using MRI guidance, to precisely aim multiple ultrasound waves at a 3-dimensional focal point. This focal point typically measures in the range of 1 to 5 mm in diameter and 10 to 50 mm in length, depending on the source’s parameters. HIFU causes local sound pressure which is absorbed and converted to heat causing coagulative necrosis from a combination of hyperthermia and acoustic cavitation in target tissue, while sparing adjacent parenchyma and structures (17). Zhang et al. treated 39 patients with HCC tumors within 1 cm of main hepatic blood vessels with no major blood vessel injury observed in any subject (18). Morbidity from the procedure is also well tolerated in patients with cirrhosis. Complications have been in observed in about 13% of patients, mostly skin or subcutaneous tissue injuries (19). A limiting drawback of HIFU is the complex set-up required. This procedure often requires general anesthesia to render patients immobile and control their respiration, while the patient is positioned prone in a water bath to remove the air between the transducer and target area.
Laser ablation is an alternative thermal ablation technique, far less investigated than RFA or MWA, which uses thin optical fibers to transmit intense light (range, 600–1,000 nm) that is scattered and absorbed by the tissues and converted into heat to cause coagulative necrosis. The lasers have limited energy penetration, which can be altered by adjusting the frequency, and creates smaller ablation zones than other electromagnetic techniques (20). It also does not penetrate well through charred tissue. Multiple applicators can be used to develop larger ablation zones. An advantage of using optical fibers is that they can be well visualized on MRI, and the absence of metal also produces less steak artifact on CT.
Non-thermal techniques include chemical ablation and irreversible electroporation (IRE). Both techniques are most useful for treating tumors adjacent to vascular structures or central biliary ducts, since they are not limited by heat sink. Chemical ablation is the percutaneous injection of chemicals directly into a tumor, most commonly absolute ethanol, which causes cellular dehydration and protein denaturation resulting in tumor necrosis (21). Reasonable diffusion of the chemical throughout the tumor is often achieved, however, repeat injections are required to complete the ablations. The technique is inexpensive, safe and, reasonably effective but has been demonstrated to be inferior to thermal ablation for most cases and is therefore not widely practiced. IRE is a newer, non-thermal technique using multiple probes aligned in parallel to deliver short, repetitive, high voltage electrical pulses to the tissue to damage cell membranes and cause apoptosis, while largely preserving the extracellular matrix (22). Since IRE does not rely on thermal effects, its purported advantage is that it is less likely to damage adjacent structures and can be used to treat tumors adjacent to central bile ducts or vascular structures. Kingham et al. showed no increase in recurrence when treating perivascular tumors (23). IRE requires unique considerations related to its electrical interference, affecting the cardiac rhythm causing arrhythmias and also muscle contractions. These side effects can be mostly mitigated by using cardiac gating and paralytic agents. IRE has mostly been used to treat locally advanced pancreatic cancer and the HCC data is limited, with relatively worse local control compared to thermal ablation. Additionally, it is technically cumbersome requiring the parallel placement of multiple probes, with slight deviations in alignment possibly resulting in failed treatment. Currently, it is best used in small, unresectable, hepatic tumors that are also unsuitable for thermal ablation because of neighboring vital structures.
Embolotherapy techniques
Primary hepatocellular cancers almost exclusively receive their blood supply from the hepatic arterial system, while normal liver parenchyma is mostly supplied by the portal vein (24). Trans-arterial chemoembolization (TACE), a procedure formally included in the BCLC staging system for patients with stage B intermediate disease, leverages this difference in blood flow to deliver highly targeted treatment. Multiple variations of this procedure exist and are generally defined by two principles. First, that selective administration of therapeutics into the hepatic artery should improve drug tumor bioavailability and limit systemic exposure to unwanted effects (25). Second, that embolization of the tumor-specific blood supply using small particles will induce ischemic necrosis and also prevent the washout of any co-delivered therapeutic. The mechanism of cell death from TACE is somewhat unclear and is some combination of ischemic necrosis and/or chemotherapeutic cytotoxicity.
The two most common methods of TACE are conventional TACE (cTACE) or TACE using drug-eluting beads (DEB-TACE). cTACE is most commonly performed by creating an oily emulsion with ethiodized oil and chemotherapy (26). Ethiodized oil is radiopaque, allowing the operator to visualize the emulsion during therapeutic delivery, and also provides some embolic properties. This is mixed with either a single agent or combination of chemotherapeutics, most commonly doxorubicin, epirubicin, cisplatin or miriplatin. After infusion of the emulsion, additional embolic material is injected ranging from gelfoam to microspheres. In general, TACE procedures should be performed as superselective (i.e., segmental, subsegmental) as safely feasible and can be repeated either on schedule or when residual or recurrent disease is identified.
DEB-TACE has gained favor amongst some interventionalists after pharmacokinetic studies have shown cTACE may expose patients to similar systemic doses of chemotherapy as intravenous infusion (27). This is thought to be related to delays between emulsion administration and embolic injection. DEB-TACE uses hydrogel beads which can be loaded with doxorubicin using an ion-exchange mechanism, providing simultaneous embolization and drug delivery. The doxorubicin is more slowly released from the beads, resulting in decreased systemic doses compared to cTACE, despite higher doses of chemotherapy (28,29). The recommended doses range from 75 to 150 mg of doxorubicin depending on the extent of disease (30). Another perceived advantage of DEB-TACE, is the technical standardization relative to cTACE since there is less inter-operator variability and more repeatability. When performing DEB-TACE, small beads are preferred when safely possible (standard size: 100–300 um).
Trans-arterial embolization (TAE) or “Bland” embolization is performed only using embolic particles, causing terminal arterial vessel blockade and inducing ischemic necrosis (25). Historically, gelatin sponge autologous blood clot, cyanoacrylate glue, polyvinyl alcohol and microspheres were administered selectively, however, modern techniques now aim to deliver carefully calibrated 50 or 40–120 um microspheres as superselective as safely possible (31). Repeat embolization can be performed as needed for persistent viable tumor or new lesions.
Trans-arterial radioembolization (TARE) or selective internal radiation therapy (SIRT) is the trans-arterial delivery of microspheres containing yttrium-90 (Y90). Y90 is a beta-emitter with a half-life of 64.2 hrs and mean and maximal tissue penetration of 2.5 and 10 mm. The radioisotope is delivered using one of two FDA-approved methods for intra-arterial delivery, either resin microspheres bound to Y90 (SIR-sphere; Sirtex Medical) which are approved for the treatment of colorectal metastases or glass microspheres with Y90 embedded within (Therasphere; BTG Corporation) which are approved for treating HCC. Theraspheres have an average diameter of 20–30 µm and an average specific activity of 2,500 Bq/sphere, in comparison to SIR-spheres, which have an average diameter of 20–60 µm and an average specific activity of 50 Bq/sphere, so many more SIR-spheres are required to deliver the same prescribed radiation dose. This particle difference contributes to a minimal embolic effect noted with glass microspheres versus the moderate embolic effect when using resin microspheres, which can result in angiographic stasis when administered (32). Although the technical parameters of both Y90 vehicles differ, retrospective data shows comparable safety and efficacy in the treatment of HCC, while no randomized controlled trials have been performed to assess for clinical superiority between either technique. Mapping angiography is performed as a separate procedure prior to TARE to evaluate for collateral flow to enteric and other non-target organs, as well as, to calculate a lung shunt fraction. Standard treatment is usually delivered in a segmental, lobar or bilobar fashion. Radiation segmentectomy (RS) is a similar technique except higher doses of radiation are delivered in a more selective fashion, to achieve “ablative” radiation doses to a tumor. Usually 2 segments or less are targeted and a threshold dose of at least 190 Gy is used, which has been confirmed using pathologic correlation from explanted livers (33).
The role of interventional oncology in the management of HCC
The treatment modalities described above are useful primary and adjunctive therapies in the treatment and palliation of HCC. The rest of this article will provide an overview of how these therapies are utilized in the management of HCC, organized by indications across disease stages. This information is summarized in Table 2.

Full table
Curative interventional therapy for very early or early stage disease
Ablation
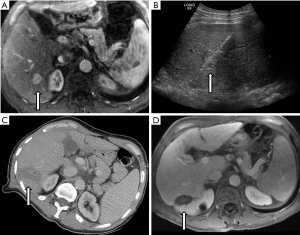
Surgery is the most widely accepted primary intervention for very early or early stage HCC; specifically, the anatomical resection of tumors in patients without significant underlying liver disease and liver transplantation for patients with abnormal liver function (6,7). RFA is recommended as a curative therapy in patients precluded from surgical intervention with 2 or 3 nodules less than 3 cm or with a solitary tumor. This recommendation is based on a large body of data showing oncologic and survival outcomes comparable to resection, at least for tumors less than 3 cm in diameter. An example case is provided in Figure 1.
Multiple, randomized prospective studies have compared radiofrequency ablation to surgical resection (SR) for very early or early HCC in patients who were surgical candidates. Chen et al. (34) prospectively randomized 180 patients with solitary HCC smaller than 5 cm to surgery or RFA. There was no significant difference in overall or disease-specific survival at 1-, 2-, 3-, or 4-yr survival with 29 months average follow-up and no difference in sub-group analysis separating tumors by size (<3 or 3.1–5 cm). SR group had longer hospital stays and more post-op complications. Lü et al. (35) randomized 105 patients with early stage HCC listed for transplantation to surgery or thermal ablation and showed no significant difference in overall or disease-free survival at 1, 2, or 3 years with shorter hospital stays in the ablation group. Feng et al. (36) randomized 168 patients with solitary HCC to surgery or RFA, but the patients were informed of their treatment group prior to consent. No significant difference was found in overall or recurrence-free survival at 1-, 2-, or 3-year. Fang et al. (37) randomized 120 patients with solitary or multiple small HCC (<3 cm) to surgery or RFA and found no significant difference in overall and disease-free survival at 1, 2, and 3 years with mean follow-up of 40 months. The incidence of post-operative complications (27% vs. 5%) and hospital stay (11.8 vs. 4.3) were significantly higher in the SR group. Ng et al. (83) randomized 218 patients with early stage HCC to either RFA or SR. RFA was performed percutaneously in 45% cases, the rest were laparoscopic or open. There was no significant difference in overall survival at 1-, 2-, 5-, and 10-yr survival with median follow-up of 93 months, however, there was a trend towards poorer disease-free survival in RFA starting 2 years post treatment in the RFA group. In subgroup analysis, no significant difference in overall survival or disease-free survival when limited to very early stage HCC or all solitary tumors. RFA group had less blood loss, shorter procedure times, shorter hospital stays and less post-operative complications. Lee et al. (84) randomly assigned 68 patients with solitary tumors 2–4 cm in size to SR or RFA. This study also showed no significant difference in 3- and 5-yr overall survival rates with median follow-up of 64 months. Intra-hepatic local recurrence was more frequent when more repeat treatments were required in the RFA group, but the rate of distant intra-hepatic and extra-hepatic recurrences remained similar.
Only one RCT has shown inferior overall survival with RFA compared to SR. Huang et al. (85) randomized 230 patients within Milan criteria to SR or RFA and concluded that overall survival and tumor recurrence rates were significantly better with resection. Their subgroup analysis by tumor size and multifocality, also showed surgery was significantly superior. The adverse events and length of hospitalization were significantly longer with resection. This study has been criticized for flawed methodology (86,87). First, the authors used a single monopolar electrode and performed sequential overlapping ablations under ultrasound guidance, which has been suggested to be suboptimal for achieving complete ablation of tumor and safety margin, especially for tumors measuring 3–5 cm (88). Also, the loss to follow-up rate for the SR group was much higher than with RFA (15.7% vs. 6.1%), possibly inflating the SR group’s survival outcomes, considering their overall survival rate is among the highest published. Additionally, multiple patients with benign disease were treated, raising questions regarding their available imaging staging accuracy.
A recent Cochrane meta-analysis by Majumdar et al. included four of these clinical trials evaluating RFA vs. SR for very early and early stage HCC including 574 surgically eligible patients with reported outcomes (38). Their analysis showed no difference in overall mortality at maximal follow-up. Cancer-related mortality was significantly lower in the surgery group; however, this outcome was only reported in the Huang trial. Serious adverse events (11.3 per 100 vs. 1.6 per 100 participants) and total adverse events were significantly higher in the surgery group. Three trials reported an additional mean 8.42 days longer hospital stay in the surgery group. There were lower intrahepatic and distant recurrence rates in the surgery group, however, without an impact on overall survival.
Mironov et al. (39) analyzed data from the population-based cancer registry SEER database, including 920 patients with T1 disease and found no significant difference in overall or tumor-free survival for up to 5 years, when comparing thermal ablation to SR for tumors <4 cm. Tumors from 4.1–5 cm benefited from surgery with improved overall and disease-specific survival. Considering the inherent selection bias within this dataset with sicker patients likely receiving thermal ablation. This study supports equivalence in oncologic control of thermal ablation to surgical resection for tumors <4 cm using a larger, “real-life” dataset.
Overall, it appears there is consensus that either surgery or thermal ablation should be performed in patients with very early or early stage HCC. The majority of data evaluating RFA and surgery as a primary treatment in surgical candidates shows no significant difference in overall mortality, however, mixed results from multiple studies suggest possibly higher intra-hepatic recurrence rates with ablation, leaving resection as the treatment of choice in low-risk patients. Further trials comparing surgery and thermal ablation are required to determine the relative benefits and harms of these treatments.
Comparison of ablative techniques
Although chemical ablation was the seminal ablative technique, thermal ablation has demonstrated superiority in RCT’s (40-43) and meta-analyses (38,89) and should be favored in the majority of ablation candidates. Shen et al. performed a meta-analysis including four RCTs with 766 patients that showed RFA has significantly better 3-year overall survival and lower recurrence rates for small HCC’s compared to chemical ablation. The perceived downside is that RFA has more complications and is more expensive. Majumdar compared fourteen clinical trials, with 2,533 participants, evaluating non-surgical interventions in patient’s ineligible for surgery. Overall survival at maximal follow-up was reported in ten trials including 1,417 participants, showing superiority of RFA compared to chemical ablation with mortality highest in percutaneous acetic acid injection (HR 1.77) and in percutaneous ethanol injection (HR 1.49) and no difference in adverse events. In the five trials reporting cancer-related mortality at maximal follow-up, RFA was significantly higher vs. PEI (16.8% vs. 8.6%).
Thermal ablation is favored in most scenarios, however, there is no consensus regarding which thermal technique to select. The body of evidence comparing thermal techniques consists mostly of heterogenous, poor-quality studies and the rapid pace of innovation limits the extrapolation of old data to new techniques. Specifically, newer microwave probes have been drastically improved compared to older microwave systems, and are gaining interest because of faster heating times, limited char/vaporization or heat sink effects, and higher, more-tumoricidal temperatures compared to RFA. For example, a retrospective cohort of 452 HCC patients with 163 HCC nodules within 5 mm of a major vascular structure, showed no difference in technical effectiveness, tumor progression rates or 1-, 3-, 5-yr cumulative survival rates when treating the peri-vascular lesions, demonstrating no difference related to heat sink effect (90). With regards to complications, a large retrospective cohort (91) demonstrated MWA has a comparable safety profile to RFA, with 2.6% of the 1,136 patients treated having major complications.
The improved heating parameters and comparable safety profile to RFA have influenced increased clinical adoption of MWA, although, there is still a paucity of data demonstrating clinical superiority to RFA. A meta-analysis by Facciorusso et al. (92) included seven comparative studies of 774 patients, evaluating RFA and MWA in mostly very early or early stage HCC. Six studies were retrospective and there was a single RCT (93). This showed an increased non-significant trend towards increased complete response with MWA (OR 1.12, P=0.67) no difference in local recurrence, and a non-significant trend towards increased overall survival with RFA (OR 0.95, P=0.85). When limiting the analysis to the three studies that enrolled larger tumor sizes, MWA significantly outperformed RFA (OR 0.46, P=0.02). There was also a non-significant trend towards increased rates of major complication with MWA, which may explain the increased survival trend with RFA. This study compared these two modalities using the highest quality evidence available, however, the included studies were mostly performed prior to significant improvements in MWA technology. The data from older studies is only applicable to the systems evaluated and cannot be extrapolated to all MWA. Newer comparative studies are needed to better evaluate the risk benefit profiles of improved MWA systems and RFA.
Cryoablation is less used because of a perceived higher complication rate and decreased efficacy compared to RFA, based on studies with first-generation cryoprobes (94). Recently, Wang et al. (16) randomized 360 HCC patient with 1 or 2 nodules <5 cm to RFA or Cryo and showed comparable results to RFA, with a trend towards better local recurrence rates for cryo. There was no difference in the rate of complete necrosis after 1 treatment (95.6% RFA vs. 98.3% cryo) and no difference in overall survival at 3- and 5-yr (66%, 38% vs. 67%, 40%). There was a mildly increased local recurrence rate at 3-yr. Interestingly, they rate of major adverse events was the same (4%), contradicting data from first generation cryoablation techniques.
Laser ablation, albeit less studied, has been shown to have a comparable safety and efficacy profile to RFA in retrospective studies, as well as in an RCT. Di Costanzo et al. (95) randomized 140 patients to receive RFA or LA in a non-inferiority trial and demonstrated no significant difference in radiologic response, time to local progression and overall survival endpoints.
There are currently no comparative studies evaluating IRE. There has been a demonstration of efficacy by pathologic evaluation at explant showing complete necrosis in 5 of 6 patients with small HCC and preservation of the bile ducts within treated areas (96). Also, a retrospective study evaluated IRE in 58 patients with 75 small HCC tumors not amenable to surgery or ablation. The 1-yr overall progression free survival was 70%, in nodules that would have otherwise not been treated. No biliary complications were noted, however, a treatment related death occurred secondary to liver failure in a patient with Child Pugh B cirrhosis and a 4.5 cm HCC tumor (97).
Embolotherapy
Although no formal role for embolotherapy exists in very early or solitary early stage HCC, it may offer durable control in patients who are not candidates for surgery or ablation. A two-center study (33) evaluated radiation segmentectomy in 102 patients with solitary HCC <5 cm, that were not resection or ablation candidates. A small cohort of 3 patients had eventual LT and pathologic evaluation of the explanted livers showed 100% of tumors had at least 90% tumor necrosis with 52% demonstrating complete necrosis. Complete necrosis was significantly associated with a treatment dose of >190 Gy. The rate and median time to progression was 26% and 33.1 months and the survival data was comparable to existing ablation literature. No major adverse events occurred, only mild and transient adverse events. This procedure can be performed on an outpatient basis and there were no readmissions in their cohort. Although well tolerated, careful patient selection is paramount, as patients with cirrhosis and poor hepatic reserve, such as elevated bilirubin and low albumin levels related to liver failure are more likely to have a major adverse event (98). Another retrospective review (45) showed excellent long-term control in 70 patients treated with RS with no progression in 72% of patients and a median time to progression of 2.4 years. These studies support RS deserving further attention as a durable and possibly curative modality available to patients deemed high-risk for surgery or ablation.
There are also several retrospective studies (99,100) evaluating superselective cTACE in patients with solitary lesions smaller than 3 cm. Yang et al. used inverse probability weighting in a retrospective study to correct for patient selection biases between patients with single HCC <3 cm who received SR, RFA, or cTACE and found no significant difference in overall survival after correction, although TACE remained an independent risk factor for recurrence. Hsu et al. reviewed 185 patients with possibly resectable, early stage HCC, and Child Pugh A cirrhosis who chose either TACE or SR. There was no significant difference in overall survival at 1-, 3-, and 5-yr, however, recurrence-free survival was significantly decreased in the TACE group. Overall survival is comparable but considering the increased risk of recurrence, when TACE is used in patients who are high-risk for surgery or ablation, it requires more vigilant surveillance and re-treatment as needed.
Combined TACE + RFA
For solitary HCC smaller than 7 cm, combined TACE + RFA has been shown to have better outcomes than RFA alone. Peng et al. (44) randomized 189 patients with solitary HCC <7 cm to TACE + RFA or RFA alone and found better overall and recurrence-free survival. Additionally, a Bayesian network meta-analysis (101) found TACE + RFA to be the most effective strategy for early stage HCC with highest ranked 1-, 3-, and 5-yr survival rates, ranking above RFA and SR. These results have led authors to compare RFA + TACE to surgical resection. A meta-analysis (102) combined four retrospective studies and showed comparable 1- and 3-yr overall survival and 1-yr progression free survival, however, 3-yr progression free survival was significantly lower for RFA + TACE.
Interventional therapy to facilitate surgical resection
Surgical resection affords patients with large tumors the best oncologic outcomes, however, the risk of postoperative liver failure excludes some patients with insufficient estimated future liver remnant (FLR). Portal vein embolization (PVE) effectively induces compensatory hypertrophy in the contralateral lobe to increase the FLR and reduce the risk of post-operative liver failure after major hepatic resections (46,103). Two trans-arterial techniques garnering interest for facilitating major hepatic resection in HCC patients with insufficient FLR are sequential TACE + PVE and Y90 radiation lobectomy.
For sequential TACE + PVE, cTACE or DEB-TACE are performed prior to PVE. Yoo et al. (47) retrospectively compared 71 patients who received TACE + PVE vs. 64 patients who received PVE alone to evaluate for differences in FLR hypertrophy and oncologic control. The combined treatment group had a significantly increased mean FLR percentage increase (7.3% vs. 5.8%), as well as, increased overall and recurrence-free survival. Ronot et al. (48) performed TACE + PVE in 54 patients of which 72% underwent right hepatectomy. Interestingly, BCLC stage did not significantly influence survival. Additionally, 8 out of 15 patients in the unresectable cohort underwent subsequent TACE, 6 of which were ipsilateral, without complication. Even though resection was not performed, prior TACE + PVE did not exclude these patients from safely receiving subsequent palliative therapy.
Radiation lobectomy may also have a pre-operative role for inducing FLR hypertrophy, considering multiple recent studies showed increased contralateral hypertrophy after TARE. For example, a series of 83 patients (104) with mixed tumors receiving lobar TARE, had a median FLR hypertrophy of 45%. Portal vein thrombosis was significantly associated in this group with FLR hypertrophy. Another non-comparative series of 13 patients (49), 10 with HCC, underwent right lobar (+ or – segment 4) TARE prior to SR and had a median percent FLR hypertrophy of 30% with 50–99% necrosis in 92% of the resected tumors. In a retrospective, comparative cohort of 52 patients with mixed hepatic tumors matched for several variables known to influence FLR hypertrophy, RE resulted in a mean FLR volume increase of 29%, significantly less than the 61.5% mean FLR volume increase in the PVE arm (50).
Future studies comparing radiation lobectomy and TACE + PVE should be performed to assess efficacy in FLR hypertrophy and oncologic control but should also assess the peri-operative benefits of FLR hypertrophy induced by TARE. Since TARE and PVE induce hypertrophy by different mechanisms, it is unclear if the same perioperative benefits from PVE can be extrapolated to TARE as there may be additional unrealized effects.
Bridging/downstaging to transplant for early and intermediate stage HCC
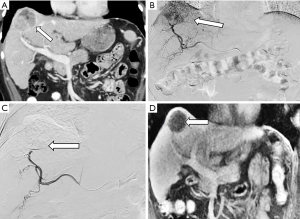
Liver transplant is the recommended curative therapy for many patients with early stage HCC. In many regions, HCC patients are given exceptional status to bolster listing priority to limit patient dropout from progression of disease (105). Therefore, it is critical to estimate post-transplant survival and recurrence rates from a patient’s pre-transplant presentation to help balance allocation for other transplant indications. From an oncologic perspective, pre-operative assessment of tumor burden, the largest tumor diameter and sum of total tumor diameters, is currently the most important consideration when determining candidacy for liver allocation (106,107). The Milan criteria (1 lesion <5 cm or 3 lesions <3 cm, with no evidence of extra-hepatic spread) is the gold standard transplant criteria for HCC (108). Those within Milan criteria have comparable survival outcomes to liver transplant patients without HCC (108,109). Expand selection criteria has been emphasized with the successful prospective validation of the UCSF criteria and additional proposed systems including the “Up-to-seven” model (110) and extended Toronto criteria (111). While awaiting organ availability, disease can progress beyond transplant criteria, rendering that person no longer a transplant candidate. Bridging therapy with interventional treatment aims to keep patients within transplant criteria. An example case is provided in Figure 2.
A multi-specialty, international consensus statement declared there is insignificant evidence to make a recommendation about bridging therapy for UNOS T1 disease (single HCC <2 cm). No existing studies compare T1 HCC patients who receive LRT to those who do not. A systematic review by Kulik et al. (112) compared a study (113) where T1 patients with cirrhosis received LRT and had a list drop-off rate of 5.3% with another study (114) including patients with T1 HCC and cirrhosis who did not receive LRT and had a dropout rate of 15.2% and 88% progressed to T2 HCC at 2.4 years.
It is currently recommended that patients with UNOS T2 disease (HCC 2–5 cm or up to 3 lesions <3 cm) should receive locoregional bridging therapy if their estimated wait time is six months or greater (115). In current practice, the difficulty of predicting the timing of organ allocation leaves most patients receiving locoregional bridging therapy.
Multiple large retrospective studies (51-53) have compared LRT + transplantation to transplantation alone showing significantly improved rates of recurrence and post-transplant survival in those receiving pre-transplant LRT. A variety and combination of ablation and embolotherapy have reported comparable safety and efficacy in the bridge to transplant setting (54-56). Additionally, it is useful to consider the effects of bridging/downstaging LRT on the listed patients who eventually dropout and never receive LT. A retrospective study (59) of 359 patients listed for LT within a 6.5-year period compared patients who received LRT to those who did not. LRT was associated with significantly improved survival in patients who were delisted (1,249.6 vs. 742.1 days,). Also, in the LT group, patients with tumor diameters >30 mm had significantly improved survival (1,949.4 vs. 1,694.8 days).
When a patient’s disease progresses and surpasses regional transplant criteria, an evaluation for downstaging therapy is recommended. Downstaging is the use of neoadjuvant therapy in patients presenting with tumor burden exceeding transplant criteria to reduce their disease to within pre-defined transplant criteria. Up to 25–40% of HCC patients listed for LT belong to a UNOS T3 sub-group and are receiving exception points and downstaging LRT (116). Multiple centers have enriched their transplantable HCC cohort by leveraging LRT to downstage and transplant these patients with comparable results. Yao et al. (60) performed an intention to treat study comparing 118 patients outside Milan criteria receiving downstaging LRT to 488 patients with T2 disease on presentation. 64 patients were successfully downstaged and underwent LT. These patients had comparable post-transplant survival and recurrence rates compared to the T2 disease control cohort. The downstaging group had increased risk of dropout with risk factors including AFP >1,000 ng/mL or Child Pugh B or C.
Response to LRT does not just provide oncologic control to improve dropout but also provides useful information regarding patients’ tumor biology. Tumor response to LRT is associated with favorable tumor histology, and a tumor’s response to LRT has become a selection tool to inform priority for transplant allocation to improve post-transplant survival and recurrence rates (61,117).
It is important that further research is performed to better evaluate the value of LRT for patients with different degrees of tumor burden and the effects on post-transplant mortality and recurrence. A meta-analysis aimed to evaluate bridging/downstaging LRT for patients with UNOS T1, T2 and T3 disease but was seriously limited given the high degree of selection bias in available studies. There was no significant data to evaluate the utility of LRT in T1 patients (112). Based on three retrospective studies (57,58,118) comparing T2 HCC patients receiving LRT or not, a non-significant reduction in dropout due to progression and all-cause dropout (RR: 0.32, 0.38) was shown in the LRT group. There was no significant change in recurrence rates or post-transplantation survival. The type of locoregional therapy performed also had no significant impact on bridging success. An analysis of studies comparing downstaged and transplanted T3 patients to T2 patients who were transplanted was also performed and showed similar post-transplantation survival outcomes for both groups.
Palliative intervention for intermediate and advanced stage HCC
Patients with intermediate stage HCC who are not candidates for liver transplantation or resection can benefit from LRT. Embolotherapy has been included in HCC management guidelines since two seminal RCTS (62,63) and multiple meta-analyses (64,65) demonstrated a survival benefit for patients receiving TACE without curative options. A modern multi-institutional study from Asia (66) demonstrated a median survival of 3.1 years for intermediate stage HCC patients treated with cTACE. Selecting the type of interventional therapy for intermediate HCC patients should be based on local expertise and preferences as there is insufficient evidence to recommend one technique over another.
For example, there is a paucity of data to support the use of chemoembolization over bland embolization. The RCT by Llovet et al. (62) was terminated when the survival benefit from TACE compared to supportive care was established. This trials smaller TAE arm also showed a trend towards improved survival but was not adequately powered at this trial endpoint. Additionally, there have been improvements in the selectivity which can be obtained with current microcatheters and in the embolic materials now delivered compared to the temporary gelfoam used in this trial. A meta-analysis of RCTs (67) did not find superiority of one technique over another and a recent RCT (68) compared TAE using modern small microspheres vs. DEB-TACE and showed no significant different in radiologic response, adverse events, or overall survival.
There is also insufficient data to support the use of DEB-TACE over cTACE or vice versa. The first RCT (69) comparing cTACE to DEB-TACE in stage B HCC patients (PRECISION V) showed no statistically significant difference between treatment arms. Subgroup analysis did show an improved radiologic response in patients with worse liver function, performance status, and recurrent or bilobar disease. No significant difference was confirmed in multiple follow-up meta-analyses (70,71). A recent retrospective study comparing DEB-TACE and cTACE in intermediate HCC patients challenged the safety of DEB-TACE purporting a higher risk of hepatobiliary complications (119). Regardless of TACE-type, patient selection is paramount. Patients with declining performance status (ECOG 2+) or decompensated cirrhosis are unlikely to benefit from TACE and are at increased risk of further hepatic decompensation.
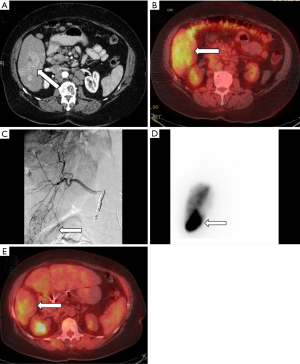
Two pilot RCTs (72,73) compared radioembolization to chemoembolization in unresectable HCC with progression-free survival as the primary endpoint. Progression-free survival was similar in both studies, however, patients received TARE only once while TACE was performed on a 6-month basis. A larger single center observational cohort (74) compared TARE to TACE in unresectable, non-ablatable early HCC and intermediate stage HCC patients. The primary endpoint was time to progression and was significantly prolonged in TARE with no difference in the number of treatments (TARE 26 months vs. TACE 6.8 months). There was also no difference in overall survival censored to transplant (TARE 18.6 months vs. TACE 17.7 months). Salem et al. (120) performed a prospective study with 56 patients which showed statistically significant improvements in quality of life factors and a trend for overall better quality of life using FACT-Hep scores. Additionally, a recent meta-analysis (121) showed statistically significant improved overall survival at 2 years but no difference at 1-, 3-, or 4-year. An example TARE case is shown in Figure 3.
For advanced stage HCC, BCLC guidelines recommend treatment with sorafenib, however, the role of embolotherapy continues to evolve. TARE has generated increasing interest after the completion of two RCTs (75,76). Both studies were designed to evaluate for superiority of TARE and failed to show significant differences in the primary outcome of overall survival, however, both showed significantly improved tumor response with TARE. Additionally, Chow et al. showed significantly improved progression free survival and time to progression in TARE over sorafenib. Vilgrain et al. showed significantly increased total and median adverse events in the sorafenib arm. Multiple additional trials are ongoing evaluating TARE head-to-head with sorafenib and also as adjuvant therapy.
TACE has also shown comparable safety and efficacy to sorafenib in patients with advanced stage HCC (77,78). For example, a small retrospective comparative study showed a non-statistically significant increased median survival in advanced stage HCC patients treated with TACE over sorafenib (79). However, RCT’s combining TACE and sorafenib for unresectable HCC have yet to demonstrate significantly improved survival outcomes (80,122).
In carefully selected patients, embolotherapy can be used to obtain favorable outcomes in select HCC patients with portal vein thrombosis or macrovascular portal vein invasion. Considering its minimally embolic effect, TARE is the favored embolotherapy in patients with portal vein thrombosis. In an initial phase II study (81) evaluating TARE including 108 patients, 30 had PVT. Patients with lobar PVT in this study had a similar number of adverse events as patients with no PVT or branch PVT. Chemoembolization has also been reported with promising results in patients with macrovascular invasion, however, no controlled studies are available. A prospective study (82) of 164 patients with segmental or sub-segmental portal vein invasion were treated with cTACE or supportive care, based on multidisciplinary tumor board recommendations and patient preferences, and showed markedly improved 1- and 2-yr survival, 30.9%, 9.2%, and 3.8%, 0%, respectively.
Conclusions
Interventional therapy plays an integral role throughout the different disease stages of HCC, beyond formal guidelines. These therapies are generally well tolerated and offer relatively low morbidity therapeutic options for palliation and cure. Ablation, embolization, and a combination of the two are also useful adjuncts to help bridge or downstage patients for surgical resection and liver transplantation. Future studies must aim to best identify which patients will benefit most from these therapies at different stages, such as minimally invasive curative ablation in very early and early stage disease, as well as, which combinations of therapy can best extend survival in patients with intermediate or advanced disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- El-Serag HB, Siegel AB, Davila JA, et al. Treatment and outcomes of treating of hepatocellular carcinoma among Medicare recipients in the United States: a population-based study. J Hepatol 2006;44:158-66. [Crossref] [PubMed]
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [Crossref] [PubMed]
- Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol 2000;174:323-31. [Crossref] [PubMed]
- Nakazawa T, Kokubu S, Shibuya A, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol 2007;188:480-8. [Crossref] [PubMed]
- Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol 2013;36:166-75. [Crossref] [PubMed]
- Bruix J, Sherman M. American Association for the Study of Liver Diseases. Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Kim SK, Rhim H, Kim YS, et al. Radiofrequency thermal ablation of hepatic tumors: pitfalls and challenges. Abdom Imaging 2005;30:727-33. [Crossref] [PubMed]
- Seror O. Ablative therapies: Advantages and disadvantages of radiofrequency, cryotherapy, microwave and electroporation methods, or how to choose the right method for an individual patient? Diagn Interv Imaging 2015;96:617-24. [Crossref] [PubMed]
- Sun Y, Cheng Z, Dong L, et al. Comparison of temperature curve and ablation zone between 915- and 2450-MHz cooled-shaft microwave antenna: results in ex vivo porcine livers. Eur J Radiol 2012;81:553-7. [Crossref] [PubMed]
- Li X, Zhang L, Fan W, et al. Comparison of microwave ablation and multipolar radiofrequency ablation, both using a pair of internally cooled interstitial applicators: results in ex vivo porcine livers. Int J Hyperthermia 2011;27:240-8. [Crossref] [PubMed]
- Fan W, Li X, Zhang L, et al. Comparison of microwave ablation and multipolar radiofrequency ablation in vivo using two internally cooled probes. AJR Am J Roentgenol 2012;198. [Crossref] [PubMed]
- Knavel EM, Brace CL. Tumor ablation: common modalities and general practices. Tech Vasc Interv Radiol 2013;16:192-200. [Crossref] [PubMed]
- Adam R, Hagopian EJ, Linhares M, et al. A comparison of percutaneous cryosurgery and percutaneous radiofrequency for unresectable hepatic malignancies. Arch Surg 2002;137:1332-9; discussion 40. [Crossref] [PubMed]
- Yang Y, Wang C, Lu Y, et al. Outcomes of ultrasound-guided percutaneous argon-helium cryoablation of hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2012;19:674-84. [Crossref] [PubMed]
- Wang C, Wang H, Yang W, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology 2015;61:1579-90. [Crossref] [PubMed]
- Mearini L. High intensity focused ultrasound, liver disease and bridging therapy. World J Gastroenterol 2013;19:7494-9. [Crossref] [PubMed]
- Zhang L, Zhu H, Jin C, et al. High-intensity focused ultrasound (HIFU): effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur Radiol 2009;19:437-45. [Crossref] [PubMed]
- Cheung TT, Chu FS, Jenkins CR, et al. Tolerance of high-intensity focused ultrasound ablation in patients with hepatocellular carcinoma. World J Surg 2012;36:2420-7. [Crossref] [PubMed]
- Pacella CM, Bizzarri G, Magnolfi F, et al. Laser thermal ablation in the treatment of small hepatocellular carcinoma: results in 74 patients. Radiology 2001;221:712-20. [Crossref] [PubMed]
- Shiina S, Tagawa K, Unuma T, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer 1991;68:1524-30. [Crossref] [PubMed]
- Lee EW, Chen C, Prieto VE, et al. Advanced hepatic ablation technique for creating complete cell death: irreversible electroporation. Radiology 2010;255:426-33. [Crossref] [PubMed]
- Kingham TP, Karkar AM, D'Angelica MI, et al. Ablation of Perivascular Hepatic Malignant Tumors with Irreversible Electroporation. J Am Coll Surg 2012;215:379-87. [Crossref] [PubMed]
- Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol 1954;30:969-77. [PubMed]
- Pleguezuelo M, Marelli L, Misseri M, et al. TACE versus TAE as therapy for hepatocellular carcinoma. Expert Rev Anticancer Ther 2008;8:1623-41. [Crossref] [PubMed]
- de Baere T, Arai Y, Lencioni R, et al. Treatment of Liver Tumors with Lipiodol TACE: Technical Recommendations from Experts Opinion. Cardiovasc Intervent Radiol 2016;39:334-43. [Crossref] [PubMed]
- Kalayci C, Johnson PJ, Raby N, et al. Intraarterial adriamycin and lipiodol for inoperable hepatocellular carcinoma: a comparison with intravenous adriamycin. J Hepatol 1990;11:349-53. [Crossref] [PubMed]
- Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol 2007;46:474-81. [Crossref] [PubMed]
- Poon RT, Tso WK, Pang RW, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol 2007;5:1100-8. [Crossref] [PubMed]
- Lencioni R, de Baere T, Burrel M, et al. Transcatheter treatment of hepatocellular carcinoma with Doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol 2012;35:980-5. [Crossref] [PubMed]
- Maluccio MA, Covey AM, Porat LB, et al. Transcatheter arterial embolization with only particles for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol 2008;19:862-9. [Crossref] [PubMed]
- Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol 2006;17:1251-78. [Crossref] [PubMed]
- Vouche M, Habib A, Ward TJ, et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology 2014;60:192-201. [Crossref] [PubMed]
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321-8. [Crossref] [PubMed]
- Lü MD, Kuang M, Liang LJ, et al. Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: a randomized clinical trial. Zhonghua Yi Xue Za Zhi 2006;86:801-5. [PubMed]
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012;57:794-802. [Crossref] [PubMed]
- Fang Y, Chen W, Liang X, et al. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J Gastroenterol Hepatol 2014;29:193-200. [Crossref] [PubMed]
- Majumdar A, Roccarina D, Thorburn D, et al. Management of people with early- or very early-stage hepatocellular carcinoma: an attempted network meta-analysis. Cochrane Database Syst Rev 2017;3. [PubMed]
- Mironov O, Jaberi A, Kachura JR. Thermal Ablation versus Surgical Resection for the Treatment of Stage T1 Hepatocellular Carcinoma in the Surveillance, Epidemiology, and End Results Database Population. J Vasc Interv Radiol 2017;28:325-33. [Crossref] [PubMed]
- Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 2003;228:235-40. [Crossref] [PubMed]
- Brunello F, Veltri A, Carucci P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand J Gastroenterol 2008;43:727-35. [Crossref] [PubMed]
- Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005;129:122-30. [Crossref] [PubMed]
- Lin SM, Lin CJ, Lin CC, et al. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 2005;54:1151-6. [Crossref] [PubMed]
- Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 2013;31:426-32. [Crossref] [PubMed]
- Lewandowski RJ, Gabr A, Abouchaleh N, et al. Radiation Segmentectomy: Potential Curative Therapy for Early Hepatocellular Carcinoma. Radiology 2018;287:1050-8. [Crossref] [PubMed]
- Ribero D, Abdalla EK, Madoff DC, et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg 2007;94:1386-94. [Crossref] [PubMed]
- Yoo H, Kim JH, Ko GY, et al. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization only before major hepatectomy for patients with hepatocellular carcinoma. Ann Surg Oncol 2011;18:1251-7. [Crossref] [PubMed]
- Ronot M, Cauchy F, Gregoli B, et al. Sequential transarterial chemoembolization and portal vein embolization before resection is a valid oncological strategy for unilobar hepatocellular carcinoma regardless of the tumor burden. HPB (Oxford) 2016;18:684-90. [Crossref] [PubMed]
- Lewandowski RJ, Donahue L, Chokechanachaisakul A, et al. (90) Y radiation lobectomy: Outcomes following surgical resection in patients with hepatic tumors and small future liver remnant volumes. J Surg Oncol 2016;114:99-105. [Crossref] [PubMed]
- Garlipp B, de Baere T, Damm R, et al. Left-liver hypertrophy after therapeutic right-liver radioembolization is substantial but less than after portal vein embolization. Hepatology 2014;59:1864-73. [Crossref] [PubMed]
- Agopian VG, Morshedi MM, McWilliams J, et al. Complete pathologic response to pretransplant locoregional therapy for hepatocellular carcinoma defines cancer cure after liver transplantation: analysis of 501 consecutively treated patients. Ann Surg 2015;262:536-45; discussion 43-5. [Crossref] [PubMed]
- Oligane HC, Xing M, Kim HS. Effect of Bridging Local-Regional Therapy on Recurrence of Hepatocellular Carcinoma and Survival after Orthotopic Liver Transplantation. Radiology 2017;282:869-79. [Crossref] [PubMed]
- Sheth RA, Patel MS, Koottappillil B, et al. Role of Locoregional Therapy and Predictors for Dropout in Patients with Hepatocellular Carcinoma Listed for Liver Transplantation. J Vasc Interv Radiol 2015;26:1761-8; quiz 8.
- Hodavance MS, Vikingstad EM, Griffin AS, et al. Effectiveness of Transarterial Embolization of Hepatocellular Carcinoma as a Bridge to Transplantation. J Vasc Interv Radiol 2016;27:39-45. [Crossref] [PubMed]
- Tohme S, Sukato D, Chen HW, et al. Yttrium-90 radioembolization as a bridge to liver transplantation: a single-institution experience. J Vasc Interv Radiol 2013;24:1632-8. [Crossref] [PubMed]
- Pauwels X, Azahaf M, Lassailly G, et al. Drug-Eluting Beads Loaded With Doxorubicin (DEBDOX) Chemoembolisation Before Liver Transplantation for Hepatocellular Carcinoma: An Imaging/Histologic Correlation Study. Cardiovasc Intervent Radiol 2015;38:685-92. [Crossref] [PubMed]
- DuBay DA, Sandroussi C, Kachura JR, et al. Radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. HPB (Oxford) 2011;13:24-32. [Crossref] [PubMed]
- Frangakis C, Geschwind JF, Kim D, et al. Chemoembolization decreases drop-off risk of hepatocellular carcinoma patients on the liver transplant list. Cardiovasc Intervent Radiol 2011;34:1254-61. [Crossref] [PubMed]
- Habibollahi P, Hunt S, Gade T, et al. The Impact of Bridging LRT on Survival in Patients Listed for Liver Transplantation. Cardiovasc Intervent Radiol 2018;41:112-9. [Crossref] [PubMed]
- Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology 2015;61:1968-77. [Crossref] [PubMed]
- Finkenstedt A, Vikoler A, Portenkirchner M, et al. Excellent post-transplant survival in patients with intermediate stage hepatocellular carcinoma responding to neoadjuvant therapy. Liver Int 2016;36:688-95. [Crossref] [PubMed]
- Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9. [Crossref] [PubMed]
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164-71. [Crossref] [PubMed]
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003;37:429-42. [Crossref] [PubMed]
- Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002;224:47-54. [Crossref] [PubMed]
- Ikeda M, Mitsunaga S, Shimizu S, et al. Current status of hepatocellular carcinoma in Japan. Chin Clin Oncol 2013;2:40. [PubMed]
- Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol 2007;30:6-25. [Crossref] [PubMed]
- Brown KT, Do RK, Gonen M, et al. Randomized Trial of Hepatic Artery Embolization for Hepatocellular Carcinoma Using Doxorubicin-Eluting Microspheres Compared With Embolization With Microspheres Alone. J Clin Oncol 2016;34:2046-53. [Crossref] [PubMed]
- Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52. [Crossref] [PubMed]
- Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: A meta-analysis. Dig Liver Dis 2016;48:571-7. [Crossref] [PubMed]
- Gao S, Yang Z, Zheng Z, et al. Doxorubicin-eluting bead versus conventional TACE for unresectable hepatocellular carcinoma: a meta-analysis. Hepatogastroenterology 2013;60:813-20. [PubMed]
- Pitton MB, Kloeckner R, Ruckes C, et al. Randomized comparison of selective internal radiotherapy (SIRT) versus drug-eluting bead transarterial chemoembolization (DEB-TACE) for the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol 2015;38:352-60. [Crossref] [PubMed]
- Kolligs FT, Bilbao JI, Jakobs T, et al. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int 2015;35:1715-21. [Crossref] [PubMed]
- Salem R, Gordon AC, Mouli S, et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology 2016;151:1155-63.e2. [Crossref] [PubMed]
- Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol 2017;18:1624-36. [Crossref] [PubMed]
- Chow PKH, Gandhi M, Tan SB, et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J Clin Oncol 2018;36:1913-21. [Crossref] [PubMed]
- Kalva SP, Pectasides M, Liu R, et al. Safety and effectiveness of chemoembolization with drug-eluting beads for advanced-stage hepatocellular carcinoma. Cardiovasc Intervent Radiol 2014;37:381-7. [Crossref] [PubMed]
- Prajapati HJ, Dhanasekaran R, El-Rayes BF, et al. Safety and efficacy of doxorubicin drug-eluting bead transarterial chemoembolization in patients with advanced hepatocellular carcinoma. J Vasc Interv Radiol 2013;24:307-15. [Crossref] [PubMed]
- Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology 2012;263:590-9. [Crossref] [PubMed]
- Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer 2011;47:2117-27. [Crossref] [PubMed]
- Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 2008;47:71-81. [Crossref] [PubMed]
- Luo J, Guo RP, Lai EC, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol 2011;18:413-20. [Crossref] [PubMed]
- Ng KKC, Chok KSH, Chan ACY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg 2017;104:1775-84. [Crossref] [PubMed]
- Lee HW, Lee JM, Yoon JH, et al. A prospective randomized study comparing radiofrequency ablation and hepatic resection for hepatocellular carcinoma. Ann Surg Treat Res 2018;94:74-82. [Crossref] [PubMed]
- Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010;252:903-12. [Crossref] [PubMed]
- Cho YK. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2011;254:838-9; author reply 9. [Crossref] [PubMed]
- Seror O, N'Kontchou G, Ganne N, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2011;254:837-author reply 837-8. [Crossref] [PubMed]
- Seror O, N'Kontchou G, Ibraheem M, et al. Large (>or=5.0-cm) HCCs: multipolar RF ablation with three internally cooled bipolar electrodes--initial experience in 26 patients. Radiology 2008;248:288-96. [Crossref] [PubMed]
- Shen A, Zhang H, Tang C, et al. Systematic review of radiofrequency ablation versus percutaneous ethanol injection for small hepatocellular carcinoma up to 3 cm. J Gastroenterol Hepatol 2013;28:793-800. [Crossref] [PubMed]
- Huang S, Yu J, Liang P, et al. Percutaneous microwave ablation for hepatocellular carcinoma adjacent to large vessels: a long-term follow-up. Eur J Radiol 2014;83:552-8. [Crossref] [PubMed]
- Liang P, Wang Y, Yu X, et al. Malignant liver tumors: treatment with percutaneous microwave ablation--complications among cohort of 1136 patients. Radiology 2009;251:933-40. [Crossref] [PubMed]
- Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int J Hyperthermia 2016;32:339-44. [Crossref] [PubMed]
- Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology 2002;223:331-7. [Crossref] [PubMed]
- Huang YZ, Zhou SC, Zhou H, et al. Radiofrequency ablation versus cryosurgery ablation for hepatocellular carcinoma: a meta-analysis. Hepatogastroenterology 2013;60:1131-5. [PubMed]
- Di Costanzo GG, Tortora R, D'Adamo G, et al. Radiofrequency ablation versus laser ablation for the treatment of small hepatocellular carcinoma in cirrhosis: a randomized trial. J Gastroenterol Hepatol 2015;30:559-65. [Crossref] [PubMed]
- Cheng RG, Bhattacharya R, Yeh MM, et al. Irreversible Electroporation Can Effectively Ablate Hepatocellular Carcinoma to Complete Pathologic Necrosis. J Vasc Interv Radiol 2015;26:1184-8. [Crossref] [PubMed]
- Sutter O, Calvo J, N'Kontchou G, et al. Safety and Efficacy of Irreversible Electroporation for the Treatment of Hepatocellular Carcinoma Not Amenable to Thermal Ablation Techniques: A Retrospective Single-Center Case Series. Radiology 2017;284:877-86. [Crossref] [PubMed]
- Salem R, Thurston KG. Radioembolization with yttrium-90 microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies: part 3: comprehensive literature review and future direction. J Vasc Interv Radiol 2006;17:1571-93. [Crossref] [PubMed]
- Yang HJ, Lee JH, Lee DH, et al. Small single-nodule hepatocellular carcinoma: comparison of transarterial chemoembolization, radiofrequency ablation, and hepatic resection by using inverse probability weighting. Radiology 2014;271:909-18. [Crossref] [PubMed]
- Hsu KF, Chu CH, Chan DC, et al. Superselective transarterial chemoembolization vs hepatic resection for resectable early-stage hepatocellular carcinoma in patients with Child-Pugh class a liver function. Eur J Radiol 2012;81:466-71. [Crossref] [PubMed]
- Lan T, Chang L, Rahmathullah MN, et al. Comparative Efficacy of Interventional Therapies for Early-stage Hepatocellular Carcinoma: A PRISMA-compliant Systematic Review and Network Meta-analysis. Medicine (Baltimore) 2016;95. [Crossref] [PubMed]
- Guo W, He X, Li Z, et al. Combination of Transarterial Chemoembolization (TACE) and Radiofrequency Ablation (RFA) vs. Surgical Resection (SR) on Survival Outcome of Early Hepatocellular Carcinoma: A Meta-Analysis. Hepatogastroenterology 2015;62:710-4. [PubMed]
- Kishi Y, Abdalla EK, Chun YS, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg 2009;250:540-8. [PubMed]
- Vouche M, Lewandowski RJ, Atassi R, et al. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol 2013;59:1029-36. [Crossref] [PubMed]
- OPaTN. Policies. 2018. Available online: https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf
- Toso C, Asthana S, Bigam DL, et al. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology 2009;49:832-8. [Crossref] [PubMed]
- Germani G, Gurusamy K, Garcovich M, et al. Which matters most: number of tumors, size of the largest tumor, or total tumor volume? Liver Transpl 2011;17 Suppl 2:S58-66. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Mazzaferro V, Bhoori S, Sposito C, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl 2011;17 Suppl 2:S44-57. [Crossref] [PubMed]
- Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35-43. [Crossref] [PubMed]
- Sapisochin G, Goldaracena N, Laurence JM, et al. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: A prospective validation study. Hepatology 2016;64:2077-88. [Crossref] [PubMed]
- Kulik L, Heimbach JK, Zaiem F, et al. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: A systematic review and meta-analysis. Hepatology 2018;67:381-400. [Crossref] [PubMed]
- Huo TI, Huang YH, Su CW, et al. Validation of the HCC-MELD for dropout probability in patients with small hepatocellular carcinoma undergoing locoregional therapy. Clin Transplant 2008;22:469-75. [Crossref] [PubMed]
- Mehta N, Sarkar M, Dodge JL, et al. Intention to treat outcome of T1 hepatocellular carcinoma with the "wait and not ablate" approach until meeting T2 criteria for liver transplant listing. Liver Transpl 2016;22:178-87. [Crossref] [PubMed]
- Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012;13:e11-22. [Crossref] [PubMed]
- Mazzaferro V. Squaring the circle of selection and allocation in liver transplantation for HCC: An adaptive approach. Hepatology 2016;63:1707-17. [Crossref] [PubMed]
- Tsuchiya K, Asahina Y, Tamaki N, et al. Risk factors for exceeding the Milan criteria after successful radiofrequency ablation in patients with early-stage hepatocellular carcinoma. Liver Transpl 2014;20:291-7. [Crossref] [PubMed]
- Andorno E, Bottino G, Morelli N, et al. Preliminary results of liver transplantation for hepatocellular carcinoma among allocation organ policy strategies, neoadjuvant treatments, and intention-to-treat analysis. Transplant Proc 2008;40:1972-3. [Crossref] [PubMed]
- Monier A, Guiu B, Duran R, et al. Liver and biliary damages following transarterial chemoembolization of hepatocellular carcinoma: comparison between drug-eluting beads and lipiodol emulsion. Eur Radiol 2017;27:1431-9. [Crossref] [PubMed]
- Salem R, Gilbertsen M, Butt Z, et al. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol 2013;11:1358-65.e1. [Crossref] [PubMed]
- Lobo L, Yakoub D, Picado O, et al. Unresectable Hepatocellular Carcinoma: Radioembolization Versus Chemoembolization: A Systematic Review and Meta-analysis. Cardiovasc Intervent Radiol 2016;39:1580-8. [Crossref] [PubMed]
- Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol 2016;64:1090-8. [Crossref] [PubMed]


