
Management of altered mental status and delirium in cancer patients
Introduction
Patients with cancer are well known for the numerous complications associated with their disease and/or with the rigorous treatment modalities they receive (1). Two frequently misdiagnosed and potentially fatal neuropsychiatric complications are altered mental status (AMS) and delirium (2,3). The diagnosis and treatment of AMS and delirium have long puzzled clinicians (4,5), especially in patients with oncological diseases. Patients with cancer very frequently present with AMS and/or delirium especially in the end of life stage (2). Consequently, the evaluation of these conditions in terms of diagnosis and management becomes more challenging, particularly in the era where patient’s quality of life is a medical priority (6).
Definitions
- AMS: a broad term that encompasses all manifestations of brain dysfunction including confusion, clouding of consciousness, disorientation, inattention, altered behavior, or drowsiness (7,8).
- Delirium: a more specific term commonly used to describe an acute state of confusion resulting from organic brain dysfunction. Delirium is characterized by a sudden onset of fluctuating level of consciousness. Despite being a common presentation, delirium has been described vaguely throughout the years. With increased awareness of researchers, there has risen a pressing urge to construct various assessment and diagnostic tools that aid physicians in managing delirious patients (9-13). A general physician can easily miss a case of delirium, and this undoubtedly has profound drastic consequences on patient outcomes (13,14).
Epidemiology
AMS and delirium are of the most prevalent neurological presentations of patients with cancer (15,16). These conditions are commonly found in hospitalized patients, even in the absence of cancer. Siddiqi et al. conducted a systematic review of 46 cohorts and found that the prevalence of delirium in newly admitted patients is 20% and the incidence per admission ranges from 3% to 29% (17). Nonetheless, delirium is even more prevalent among patients with advanced cancer. It comes just after adjustment disorders in neuropsychiatric diagnoses among cancer patients (18). Delirium is more commonly observed in the older cancer patient age groups and is equally prevalent in both genders (2,19,20). Studies show that 22‒44% of patients with cancer experience delirium and that the incidence rises to 87% in the final days of life (21-24). Han et al. found that delirium was missed 76% of the time in the emergency department, suggesting that the incidence might even be higher (13). Additionally, evidence shows that hospitalized cancer patients with no signs of delirium on admission are highly prone to developing delirium during their admission. In a prospective cohort study of patients with terminal cancer, Gagnon et al. found that 20% presented with delirium on admission, in comparison to 31% of patients who were free of delirium on admission but became so during their admission (25). Similarly, Lawlor et al. studied a cohort of 113 patients with advanced cancer and reported delirium in 42% of admitted patients. Of the remaining, 45% developed delirium after admission (19).
Etiology
Structural and non-structural causes
AMS and delirium can occur as a result of structural and/or nonstructural causes (3). Nonstructural causes of delirium are more common among patients with cancer and are typically related to drug intoxication and withdrawal or other metabolic imbalances (3,26). Doriath et al. found that toxic or metabolic encephalopathy was the main cause of delirium in 57% of confused cancer patients, with structural brain lesions coming in second at 36% (27).
Evidence suggests that delirium is a multifactorial condition. Doriath et al. reported that 61% of patients with toxic or metabolic encephalopathy had more than one contributing factor. The most common contributing factors were electrolyte abnormalities, renal insufficiency, liver dysfunction, anti-cancer drugs, infections and use of opiates (27). Tuma and DeAngelis studied 140 confused patients with non-central nervous system cancer, 50% of whom had disseminated systemic metastasis. The authors reported that only 33% of patients had a single cause for AMS, whereas multiple causes were identified for 67% of patients. Among the AMS cases, 54% were opioid-related and 64% were caused by metabolic abnormalities such as hypoxia, hyponatremia and renal failure. Infections and recent surgeries were also found to be common causes of delirium (2).
On another hand, structural causes of AMS are related to pathological processes within the brain, such as ischemic or hemorrhagic stroke, hydrocephalus, intracranial tumors, and mass effect producing lesions (28). Tuma and DeAngelis reported structural lesions as a cause of AMS in 36% of cases, with 26% being caused by metastatic lesions to the brain (2). Doriath et al. obtained similar findings, with AMS being associated with structural causes in 34% of cases, almost all of which were caused by metastatic disease (27).
Predisposing and precipitating factors
It is believed that delirium is caused by a precipitating factor in a patient predisposed to having the problem. This concept was first introduced by Inouye in 2006 (29). Predisposing factors for delirium include advanced cancer, visual impairment, advanced disease, baseline cognitive impairment and dementia (29). Precipitating factors, on the other hand, are those that trigger delirium such as toxins, metabolic disturbances, brain insults, dehydration, physical restraints, urinary catheters and multidrug regimens (6,29). Laurila et al. found that both predisposing factors and precipitating factors are directly correlated to the patient’s susceptibility to delirium. The authors reported an average of 3 precipitating factors and an average of 5 predisposing factors per patient, which supports a multi-factorial etiology of delirium (30). This is further supported by Magny et al., who studied a sample of 208 geriatric patients presenting with dementia from the community and reported that 80% of patients had a predisposing cognitive or neurologic disorder. Additionally, they noted that infections, followed by drugs and dehydration, were the most common precipitating factors (31). In a similar study conducted on cancer patients, Sagawa et al. noted that the most frequent precipitating factor is the use of opioids, with inflammation and electrolyte abnormalities also being common causes (28).
Other overlooked but increasingly important factors are loneliness and unfamiliar environments, which also add to the psychological stress afflicting the cancer patient (32). Given the complex nature of delirium and its multi-factorial etiologies, it is difficult to contain delirium under one heading. Instead, delirium would be better described as a spectrum of disorders with numerous and often compounding causative factors. As a result, cancer patients form a subgroup that is highly prone to such a dire neuropsychiatric complication.
Pathophysiology
Multiple theories have been proposed to explain the pathogenesis of delirium. Most of these theories, reviewed by Maldonado, center on the roles of neurotransmitters, inflammatory cytokines, and blood-brain barrier integrity in the development of delirium. The neurotransmitter hypothesis builds upon the role of neurotransmitter changes in the development and treatment of delirium and AMS (33). This theory was originally devised following observations of delirium in patients using neurotransmitter-altering medications or substances such as opioids (34). This theory suggests that delirium can be the result of a decrease in acetylcholine levels and an increase in dopamine levels, with alterations in glutamate and gamma-aminobutyric acid (GABA) (33). Knowing that the cholinergic system is involved in regulating selective attention and memory, any disruption in cholinergic transmission is postulated to be a potential cause of delirium (35,36). This theory is supported by multiple studies showing that delirium can be induced by anti-cholinergic drugs and reversed by administration of cholinergic agonists (33). Opioid use is known to be associated with an anticholinergic effect (37) and is also well known to play a role in producing delirium (26). Patients with cancer are at a high risk, as they rely on highly potent opioids for pain control, such as morphine (26). There is also evidence suggesting that low levels of acetylcholine are found in delirious patients not using anticholinergic medication. This suggests that endogenous anticholinergic substances may be produced during acute illness (38,39).
On another note, the presence of inflammatory factors/cytokines resulting from malignancies may also increase the propensity of cancer patients to develop delirium. This is explained by the neuro-inflammatory hypothesis, which implies that peripheral inflammation results in the activation of CNS parenchymal cells, which in turn produce inflammatory cytokines that alter the normal functioning of neuronal synapses (40). This hypothesis could explain the “sickness behavior” seen in patients with inflammation or infections. In fact, most of these patients develop non-specific symptoms such as fever, fatigue, malaise and anorexia as well as behavioral symptoms such as depressed mood, anhedonia and cognitive changes. This could also be a potential explanation for the cognitive and behavioral changes seen in delirium (33).
The association between delirium and peripheral inflammation is also addressed by the Oxidative Stress Hypothesis. Pathologic processes, such as hypoxia, infections and malignancies, can produce oxidative stress and also compromise the body’s redox systems which play a role in neutralizing this condition (41). This theory suggests that this state of oxidative stress is capable of causing cerebral damage and is therefore a potential cause for the delirium in patients with peripheral pathologies (33,42).
Presentation
Delirium can affect cancer patients of all ages and can have myriad presentations; however, it is believed to be more common in the elderly cancer patients. Additionally, symptoms tend to fluctuate during the episode and are usually worse during the nighttime (11,22,43). The diversity of symptoms and the fluctuation of symptom severity are challenging that physicians face in diagnosing this condition particularly in the hypoactive subtype (44). In fact, delirium does not have pathognomonic features that can be readily seen; a physician must therefore maintain a high index of suspicion, take a detailed history and use proper assessment tools to detect these conditions (45).
There are three well-recognized clinical subtypes of delirium, separated according to the patient’s psychomotor activity and level of arousal. These subtypes include the hyperactive subtype, hypoactive subtype and mixed subtype (46). The hyperactive subtype displays features similar to those seen in psychosis or mania: restlessness, hallucinations, delusions and hypervigilance (47). Centeno et al. describes patients that fall under this subtype as disinhibited, uncomfortable or agitated. The patient may fidget around the room, attempt to pull out lines, or repeat movements, names, etc. (18). Contrarily, delirium in patients with the hypoactive subtype usually manifests with withdrawal, psychomotor retardation or lack of movement, lack of orientation, paucity of speech and unresponsiveness. This type of delirium may mimic a depressed mood (47). The hypoactive subtype is more likely to be missed, given the absence of positive symptoms (48). Patients with mixed subtype delirium usually present with features of both. Patients more commonly present with the hypoactive or mixed symptom subtypes (48,49).
AMS and delirium can cause significant stress on patients who are already enduring difficult times in their struggle with cancer. Patients have recalled their experience in great detail and have described it as being severely stressful at times (22,23). This also applies to the patients’ families, who are significantly affected by the delirium. Families of delirious patients suffer from considerable stress, struggle to understand the etiology of delirium, and fear its recurrence (50,51).
Diagnosis
Effectively diagnosing delirium is a recurring discussion among researchers, especially that many delirium cases go undetected (13). The Diagnostic and Statistical Manual of Mental Disorders (DSM) is the gold standard for diagnosing delirium (52). One study at The University of Texas MD Anderson Cancer Center showed that 41% of delirium cases remained undetected by clinicians, even after they were trained on how to assess and diagnose it (24). Similar results were reported in the emergency department of another tertiary academic medical center, where 76% of delirium cases were missed (13).
These results are unfortunate, as studies show that delirium has more favorable outcomes when detected early (53). However, early detection can be troublesome, as some patients may appear cognitively normal with good orientation. The fluctuating pattern of the symptoms also makes the diagnosis difficult (43). This further solidifies the need for a thorough understanding of proper detection and management strategies for this psychiatric disorder, both in the general population and in the cancer patient population.
The fifth edition of the DSM (52) states that for a diagnosis of delirium to be made, five criteria must be met:
- Alteration in attention and awareness;
- Alteration in attention and awareness develops acutely (usually over a few hours to a few days), constitutes an acute change from baseline attention and awareness, with severity fluctuation throughout the day;
- Alteration in cognition, which may include memory deficit, language deficit, disorientation, or changes in perception;
- Criteria 1 and 3 cannot be better explained by an established neurocognitive disorder and are not occurring in the context of a severely reduced level of arousal (coma);
- Evidence from the history, physical examination, or laboratory findings that the observed changes are direct physiological consequences of another medical condition, substance intoxication, withdrawal from a substance, and/or exposure to a toxin.
Patient history
As a first step, the clinician should look for clues within the patient’s presentation, such as new-onset agitation that peaks at night or variations in sleep patterns and memory. A relatively acute onset of symptoms with a fluctuating pattern is helpful in distinguishing delirium from more chronic conditions such as dementia (11). Also, it is important to take into consideration the clinical clues given by family members or caretakers because hints as such can provide invaluable insight that the patient him/herself may not display or report. The patient’s family may also provide information about their baseline cognition and attention (18). The history should also include a thorough medication history, as multiple drugs are well known to cause delirium and/or AMS. Of particular importance are any recently introduced or discontinued drugs that might have been associated with the onset of delirium (27,54). This can include steroids, opioids, anti-depressants or benzodiazepines (54-56). Moreover, the physician should assess for signs of alcohol intake/withdrawal, symptoms of infection, and any new stressors or environmental changes (11). The presence of focal nonspecific neurological signs such as tremors and asterixis may accompany AMS and the presence of focal deficits such as unilateral weakness may suggest cerebrovascular or inflammatory events. In patients with such deficits, neuro-imaging is compulsory to rule out any acute event in the brain (11).
It is important to remember that the diagnosis of delirium in cancer patients is even more complex. Changes in mental status may result from multiple intertwining factors such as the disease itself, the medical therapy, and any comorbidities or baseline cognitive disorders (44). These patients are also under a tremendous amount of stress, and cancer diagnoses are commonly associated with a number of mental health issues, such as adjustment disorders, depression, and major depressive episodes (57,58). The physician must therefore identify and differentiate hypoactive delirium from a normal stress response or depressed mood, and hyperactive delirium from dementia or psychosis (5). Moreover, it is important to maintain suspicion to some of the less common causes of AMS that are more specific to the cancer patient population. This includes new brain lesions, paraneoplastic syndromes (usually associated with ovarian teratoma and small cell lung carcinoma), hepatic encephalopathy, renal failure, and vitamin deficiencies. Additionally, cerebrovascular events causing mental status changes may occur as a result of the increased coagulability associated with malignancies (26,44).
Delirium is considered a medical emergency and prompts investigation to rule out infections and other serious causes. The treating team should therefore ensure airway integrity, obtain blood studies, and monitor vitals while looking for underlying causes (11). However, it is also important for the care provider to balance the need for medical interventions with the patient’s comfort. For example, a delirious patient who requires a scan may find it to be a disorienting and traumatic experience despite the routine nature of the procedure. This requires the treating team to be more tactful in their approach in order to minimize the patient’s suffering (44).
Assessment and diagnostic tools
Multiple tools have been developed in order to facilitate the detection of delirium. Wong et al. showed that among such tools, the Confusion Assessment Method (CAM) had the most evidence supporting its use as a bedside assessment questionnaire (59). This questionnaire has been validated for use in the general population in different settings. The Confusion Assessment Method for Intensive Care Unit (CAM-ICU) is a version of the CAM that was developed for use in the ICU and can be performed in patients who are unable to communicate verbally (60). Other tools used for delirium detection include the Delirium Observation Screening Scale, the Nursing Delirium Screening Scale, and the Mini Mental Status Examination (MMSE). However, Bush et al. have noted that while the CAM and most of the other tools have been validated for the general patient population, no validation studies have been done on the cancer patient population. Additionally, the commonly used MMSE was shown be the least favorable, although it still provides a decent assessment of baseline cognition (43,59). On another note, instruments such as the Memorial Delirium Assessment Scale and the Delirium Severity Scale provide a good assessment of delirium severity and have been validated for use in the cancer patient population and in terminally ill patients respectively (18), while the Nursing Delirium Symptom Score has been shown to be accurate in bedside screening of delirium (61).
Treatment
The treatment of AMS and delirium includes both pharmacological and nonpharmacological interventions. Nonpharmacological interventions require collaborative effort from the physicians, nurses, and family/caregivers (11). They focus on preserving the patient’s safety and integrity, while minimizing any unnecessary stress. The essential need for patient and staff safety must always be kept in mind. The patient should be made comfortable and unnecessary and potentially harmful devices (lines, catheters, etc.) should be removed or fixated to prevent harm to the patient/caregiver or staff. Bed exercises and ambulation are encouraged. These should be tailored to the patient’s capacity. On the other hand, the use of physical restraints should be avoided as they risk exacerbating the symptoms and cause significant distress (43). Patient needs, such as bathroom access, should be addressed and promptly tended to. Although the patient with AMS or delirium needs constant monitoring, unnecessary procedures (such as excessive blood tests or pressure monitoring) should be avoided and any potentially aggravating stimuli (such as excessive light, noise, or commotion) should be minimized or removed (62). In case of any vision or hearing impairment, eyeglasses or hearing devices should be provided as needed (43). The patient must be kept calm, with every effort made to keep the patient familiar with his or her surroundings; this is done by ensuring that a familiar face or setting is near at hand (11). Family members/caregivers should be counseled as to what delirium is and its fluctuating course. The physician needs to be mindful of the fact that this is a stressful time for the family members as much as the patient (23). Educating the caregiver/family member on the efforts they can make to decrease agitation and support the patient as medical treatment is provided will help the patient during recovery and decrease stress in an already-difficult situation (11,22).
Before pharmacological therapy is initiated, an underlying cause of delirium or multiple contributors to the delirium must be sought out and addressed. This includes discontinuing offending medications (opioids in particular), treating infections and rehydrating the patient to support the kidney in flushing out metabolites. As for the use of medications in delirium management in cancer patients, evidence is relatively scarce. Most studies are underpowered, and there is a great need for large randomized clinical trials that compare the use of different medications with non-pharmacological interventions and evaluate the effectiveness of each drug. Table 1 presents the most typical pharmacological interventions used for delirium as well as the evidence on their uses in cancer patients. The European Society for Medical Oncology (ESMO) Guidelines Committee on delirium management in cancer patients recognize Olanzapine, Quetiapine and Aripiprazole as drugs that may be helpful in treating delirium. However, the Committee did not recommend the use of Haloperidol and Risperidone, which were proven by Agar et al. to be non-beneficial when compared with non-pharmacological management (43,63). Further evidence is needed in order to identify the pharmacological interventions that may help improve symptoms and outcomes in cancer patients suffering from delirium.
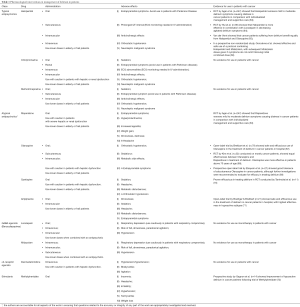
Full table
Prognosis
There is an abundance of evidence suggesting that delirium is a predictor of long-term morbidity or mortality in the general population. In fact, a meta-analysis of 16 studies conducted by Zhang et al. reported increased risks of complications, longer hospital stays, prolonged mechanical ventilation, and increased mortality rates in patients presenting with delirium (73). Delirium has also been suggested as a risk factor for future development of dementia (74). For patients with cancer, the development of AMS is a particularly challenging complication. A study of patients with terminal cancer by Fang et al. compared in-hospital mortality rates among terminal cancer patients admitted to the hospital with or without delirium. The results showed a 77.6% mortality rate among in terminal cancer patients presenting with no delirium compared with a mortality rate of 50.9% in patients admitted without delirium (75). Additionally, patients with cancer presenting with delirium also exhibit shorter survival time in comparison with cancer-free patients with delirium (24).
Mortality risk may vary depending on the delirium subtype, as well as the potential etiological factors involved. The hypoactive subtype of delirium is associated with a higher mortality rate and a shorter mean survival time compared to the mixed and the hyperactive subtypes (75,76). In addition, patients with delirium caused by structural brain lesions display a poorer prognosis in comparison with patients with toxic or metabolic encephalopathies or infections. Moreover, shorter survival was noted in patients with more metabolic abnormalities (27).
On another hand, studies show that at least 50% of delirium cases among cancer patients are reversible, as there is usually no organic change to the brain (19). Patients who do not recover fully continue to show signs of cognitive impairment and functional decline (77). Reversibility may depend on the precipitating and predisposing factors, and can improve once these factors are addressed (78). For example, delirium resulting from medication use, electrolyte abnormalities or dehydration is usually reversible (18,43), unlike delirium caused by a hypoxic event or organ dysfunction (19).
Conclusions
With the increasing prevalence of malignancy in the community (79), it is important for physicians and nurses to become more cautious about some of the complications that are highly prevalent to cancer patients. Delirium is a complication that is commonly missed in this population and is associated with considerable distress for both the patients and their caregivers. The onset of delirium is associated with poor prognosis in cancer patients and its treatment can be challenging due to its multifactorial etiology. Physicians and nurses involved in the care of oncology patients should therefore receive adequate training in the use of diagnostic tools for delirium and maintain a low threshold for its detection. Additionally, further studies are needed to validate the available diagnostic tools in the cancer patient subgroup.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin 2016;66:337-50. [Crossref] [PubMed]
- Tuma R, DeAngelis LM. Altered mental status in patients with cancer. Arch Neurol 2000;57:1727-31. [Crossref] [PubMed]
- Xiao HY, Wang YX, Xu TD, et al. Evaluation and treatment of altered mental status patients in the emergency department: Life in the fast lane. World J Emerg Med 2012;3:270-7. [Crossref] [PubMed]
- Han JH, Schnelle JF, Ely EW. The relationship between a chief complaint of "altered mental status" and delirium in older emergency department patients. Acad Emerg Med 2014;21:937-40. [Crossref] [PubMed]
- Hustey FM, Meldon SW. The prevalence and documentation of impaired mental status in elderly emergency department patients. Ann Emerg Med 2002;39:248-53. [Crossref] [PubMed]
- Elsayem AF, Bruera E, Valentine AD, et al. Delirium frequency among advanced cancer patients presenting to an emergency department: A prospective, randomized, observational study. Cancer 2016;122:2918-24. [Crossref] [PubMed]
- Morandi A, Pandharipande P, Trabucchi M, et al. Understanding international differences in terminology for delirium and other types of acute brain dysfunction in critically ill patients. Intensive Care Med 2008;34:1907-15. [Crossref] [PubMed]
- Tindall SC, Walker HK, Hall WD, et al. Clinical Methods: The History, Physical, and Laboratory Examinations. Boston: Butterworth-Heinemann, 1990.
- Wilber ST, Ondrejka JE. Altered Mental Status and Delirium. Emerg Med Clin North Am 2016;34:649-65. [Crossref] [PubMed]
- Lorenzl S, Fusgen I, Noachtar S. Acute confusional States in the elderly--diagnosis and treatment. Dtsch Arztebl Int 2012;109:391-9. [PubMed]
- Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol 2009;5:210-20. [Crossref] [PubMed]
- Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis and treatment. Crit Care Clin 2008;24:657-722. vii. [Crossref] [PubMed]
- Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med 2009;16:193-200. [Crossref] [PubMed]
- de la Cruz M, Fan J, Yennu S, et al. The frequency of missed delirium in patients referred to palliative care in a comprehensive cancer center. Support Care Cancer 2015;23:2427-33. [Crossref] [PubMed]
- Clouston PD, DeAngelis LM, Posner JB. The spectrum of neurological disease in patients with systemic cancer. Ann Neurol 1992;31:268-73. [Crossref] [PubMed]
- Pereira J, Hanson J, Bruera E. The frequency and clinical course of cognitive impairment in patients with terminal cancer. Cancer 1997;79:835-42. [Crossref] [PubMed]
- Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing 2006;35:350-64. [Crossref] [PubMed]
- Centeno C, Sanz A, Bruera E. Delirium in advanced cancer patients. Palliat Med 2004;18:184-94. [Crossref] [PubMed]
- Lawlor PG, Gagnon B, Mancini IL, et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer: a prospective study. Arch Intern Med 2000;160:786-94. [Crossref] [PubMed]
- Weinrich S, Sarna L. Delirium in the older person with cancer. Cancer 1994;74:2079-91. [Crossref] [PubMed]
- Reid VL, McDonald R, Nwosu AC, et al. A systematically structured review of biomarkers of dying in cancer patients in the last months of life; An exploration of the biology of dying. PLoS One 2017;12:e0175123. [Crossref] [PubMed]
- Bruera E, Bush SH, Willey J, et al. Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer 2009;115:2004-12. [Crossref] [PubMed]
- Breitbart W, Gibson C, Tremblay A. The delirium experience: delirium recall and delirium-related distress in hospitalized patients with cancer, their spouses/caregivers, and their nurses. Psychosomatics 2002;43:183-94. [Crossref] [PubMed]
- Elsayem AF, Bruera E, Valentine A, et al. Advance directives, hospitalization, and survival among advanced cancer patients with delirium presenting to the emergency department: a prospective study. Oncologist 2017;22:1368-73. [Crossref] [PubMed]
- Gagnon P, Allard P, Masse B, et al. Delirium in terminal cancer: a prospective study using daily screening, early diagnosis, and continuous monitoring. J Pain Symptom Manage 2000;19:412-26. [Crossref] [PubMed]
- Caraceni A. Drug-associated delirium in cancer patients. EJC Suppl 2013;11:233-40. [Crossref] [PubMed]
- Doriath V, Paesmans M, Catteau G, et al. Acute confusion in patients with systemic cancer. J Neurooncol 2007;83:285-9. [Crossref] [PubMed]
- Sagawa R, Akechi T, Okuyama T, et al. Etiologies of delirium and their relationship to reversibility and motor subtype in cancer patients. Jpn J Clin Oncol 2009;39:175-82. [Crossref] [PubMed]
- Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord 1999;10:393-400. [Crossref] [PubMed]
- Laurila JV, Laakkonen ML, Tilvis RS, et al. Predisposing and precipitating factors for delirium in a frail geriatric population. J Psychosom Res 2008;65:249-54. [Crossref] [PubMed]
- Magny E, Le Petitcorps H, Pociumban M, et al. Predisposing and precipitating factors for delirium in community-dwelling older adults admitted to hospital with this condition: A prospective case series. PLoS One 2018;13:e0193034. [Crossref] [PubMed]
- Colussi AM, Mazzer L, Candotto D, et al. The elderly cancer patient: a nursing perspective. Crit Rev Oncol Hematol 2001;39:235-45. [Crossref] [PubMed]
- Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry 2013;21:1190-222. [Crossref] [PubMed]
- Hshieh TT, Fong TG, Marcantonio ER, et al. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci 2008;63:764-72. [Crossref] [PubMed]
- Perry E, Walker M, Grace J, et al. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci 1999;22:273-80. [Crossref] [PubMed]
- Chudasama Y, Dalley JW, Nathwani F, et al. Cholinergic modulation of visual attention and working memory: dissociable effects of basal forebrain 192-IgG-saporin lesions and intraprefrontal infusions of scopolamine. Learn Mem 2004;11:78-86. [Crossref] [PubMed]
- Rada P, Barson JR, Leibowitz SF, et al. Opioids in the hypothalamus control dopamine and acetylcholine levels in the nucleus accumbens. Brain Res 2010;1312:1-9. [Crossref] [PubMed]
- Flacker JM, Wei JY. Endogenous anticholinergic substances may exist during acute illness in elderly medical patients. J Gerontol A Biol Sci Med Sci 2001;56:M353-5. [Crossref] [PubMed]
- Mulsant BH, Pollock BG, Kirshner M, et al. Serum anticholinergic activity in a community-based sample of older adults: relationship with cognitive performance. Arch Gen Psychiatry 2003;60:198-203. [Crossref] [PubMed]
- Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin 2008;24:789-856. ix. [Crossref] [PubMed]
- Aliev G, Obrenovich ME, Smith MA, et al. Hypoperfusion, Mitochondria Failure, Oxidative Stress, and Alzheimer Disease. J Biomed Biotechnol 2003;2003:162-3. [Crossref] [PubMed]
- Engel GL, Romano J. Delirium, a syndrome of cerebral insufficiency. 1959. J Neuropsychiatry Clin Neurosci 2004;16:526-38. [Crossref] [PubMed]
- Bush SH, Lawlor PG, Ryan K, et al. Delirium in adult cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2018;29:iv143-65. [Crossref] [PubMed]
- Edelstein A, Alici Y. Diagnosing and Managing Delirium in Cancer Patients. Oncology (Williston Park) 2017;31:686-92. iii. [PubMed]
- Bush SH, Marchington KL, Agar M, et al. Quality of clinical practice guidelines in delirium: a systematic appraisal. BMJ Open 2017;7:e013809. [Crossref] [PubMed]
- Raju K, Coombe-Jones M. An overview of delirium for the community and hospital clinician. Progress in Neurology and Psychiatry 2015;19:23-7. [Crossref]
- Johnson MH. Assessing confused patients. Journal of neurology, neurosurgery, and psychiatry 2001;71 Suppl 1:i7-i12. [PubMed]
- Spiller JA, Keen JC. Hypoactive delirium: assessing the extent of the problem for inpatient specialist palliative care. Palliat Med 2006;20:17-23. [Crossref] [PubMed]
- Alici-Evcimen Y, Breitbart W. An update on the use of antipsychotics in the treatment of delirium. Palliat Support Care 2008;6:177-82. [Crossref] [PubMed]
- Carbone MK, Gugliucci MR. Delirium and the Family Caregiver: The Need for Evidence-based Education Interventions. Gerontologist 2015;55:345-52. [Crossref] [PubMed]
- Namba M, Morita T, Imura C, et al. Terminal delirium: families' experience. Palliat Med 2007;21:587-94. [Crossref] [PubMed]
- The DSM-5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med 2014;12:141. [Crossref] [PubMed]
- Heymann A, Radtke F, Schiemann A, et al. Delayed treatment of delirium increases mortality rate in intensive care unit patients. J Int Med Res 2010;38:1584-95. [Crossref] [PubMed]
- Bush SH, Bruera E. The assessment and management of delirium in cancer patients. Oncologist 2009;14:1039-49. [Crossref] [PubMed]
- Michaud L, Burnand B, Stiefel F. Taking care of the terminally ill cancer patient: delirium as a symptom of terminal disease. Ann Oncol 2004;15 Suppl 4:iv199-203. [Crossref] [PubMed]
- White C, McCann MA, Jackson N. First do no harm.. Terminal restlessness or drug-induced delirium. J Palliat Med 2007;10:345-51. [Crossref] [PubMed]
- Gil Moncayo FL, Costa Requena G, Perez FJ, et al. Psychological adjustment and prevalence of psychiatric disorders in cancer patients. Med Clin (Barc) 2008;130:90-2. [Crossref] [PubMed]
- Pitman A, Suleman S, Hyde N, et al. Depression and anxiety in patients with cancer. Bmj 2018;361:k1415. [Crossref] [PubMed]
- Wong CL, Holroyd-Leduc J, Simel DL, et al. Does this patient have delirium?: value of bedside instruments. Jama 2010;304:779-86. [Crossref] [PubMed]
- Grover S, Avasthi A. Clinical Practice Guidelines for Management of Delirium in Elderly. Indian journal of psychiatry 2018;60:S329-40. [PubMed]
- Gaudreau JD, Gagnon P, Harel F, et al. Fast, systematic, and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. J Pain Symptom Manage 2005;29:368-75. [Crossref] [PubMed]
- Braillon A. Delirium in patients with advanced cancer: Screening to cure or caring to prevent? Cancer 2017;123:704. [Crossref] [PubMed]
- Agar MR, Lawlor PG, Quinn S, et al. Efficacy of Oral Risperidone, Haloperidol, or Placebo for Symptoms of Delirium Among Patients in Palliative Care: A Randomized Clinical Trial. JAMA Intern Med 2017;177:34-42. [Crossref] [PubMed]
- Hui D, Frisbee-Hume S, Wilson A, et al. Effect of Lorazepam With Haloperidol vs Haloperidol Alone on Agitated Delirium in Patients With Advanced Cancer Receiving Palliative Care: A Randomized Clinical Trial. JAMA 2017;318:1047-56. [Crossref] [PubMed]
- van der vorst M, Neefjes E, Manon S, et al. Efficacy and side effect profile of olanzapine versus haloperidol for symptoms of delirium in hospitalized patients with advanced cancer: A multicenter, investigator-blinded, randomized, controlled trial (RCT). J Clin Oncol 2017;35:abstr 231.
- Goncalves F, Almeida A, Pereira S. A Protocol for the Control of Agitation in Palliative Care. Am J Hosp Palliat Care 2016;33:948-51. [Crossref] [PubMed]
- Breitbart W, Tremblay A, Gibson C. An open trial of olanzapine for the treatment of delirium in hospitalized cancer patients. Psychosomatics 2002;43:175-82. [Crossref] [PubMed]
- Kim SW, Yoo JA, Lee SY, et al. Risperidone versus olanzapine for the treatment of delirium. Hum Psychopharmacol 2010;25:298-302. [Crossref] [PubMed]
- Elsayem A, Bush SH, Munsell MF, et al. Subcutaneous olanzapine for hyperactive or mixed delirium in patients with advanced cancer: a preliminary study. J Pain Symptom Manage 2010;40:774-82. [Crossref] [PubMed]
- Tanimukai H, Tsujimoto H, Matsuda Y, et al. Novel Therapeutic Strategies for Delirium in Patients With Cancer: A Preliminary Study. Am J Hosp Palliat Care 2016;33:456-62. [Crossref] [PubMed]
- Boettger S, Breitbart W. An open trial of aripiprazole for the treatment of delirium in hospitalized cancer patients. Palliat Support Care 2011;9:351-7. [Crossref] [PubMed]
- Gagnon B, Low G, Schreier G. Methylphenidate hydrochloride improves cognitive function in patients with advanced cancer and hypoactive delirium: a prospective clinical study. J Psychiatry Neurosci 2005;30:100-7. [PubMed]
- Zhang Z, Pan L, Ni H. Impact of delirium on clinical outcome in critically ill patients: a meta-analysis. Gen Hosp Psychiatry 2013;35:105-11. [Crossref] [PubMed]
- Davis DH, Muniz Terrera G, Keage H, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain 2012;135:2809-16. [Crossref] [PubMed]
- Fang CK, Chen HW, Liu SI, et al. Prevalence, detection and treatment of delirium in terminal cancer inpatients: a prospective survey. Jpn J Clin Oncol 2008;38:56-63. [Crossref] [PubMed]
- Kim SY, Kim SW, Kim JM, et al. Differential Associations Between Delirium and Mortality According to Delirium Subtype and Age: A Prospective Cohort Study. Psychosom Med 2015;77:903-10. [Crossref] [PubMed]
- Buurman BM, Hoogerduijn JG, de Haan RJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One 2011;6:e26951. [Crossref] [PubMed]
- Kang JH, Shin SH, Bruera E. Comprehensive approaches to managing delirium in patients with advanced cancer. Cancer Treat Rev 2013;39:105-12. [Crossref] [PubMed]
- Fitzmaurice C, Dicker D, Pain A, et al. The Global Burden of Cancer 2013. JAMA Oncol 2015;1:505-27. [Crossref] [PubMed]


