
Benzodiazepines and/or neuroleptics for the treatment of delirium in palliative care?—a critical appraisal of recent randomized controlled trials
Introduction
The recently revised definition of delirium according to the fifth edition of the Diagnostic and Statistical Manual (DSM) of the American Psychiatric Association (1) acknowledges that the diagnosis of delirium is an umbrella construct. While in DSM versions prior to DSM-5 alterations of consciousness (content or level of consciousness) and level of arousal were core features, both terms were omitted in DSM-5 (2). The DSM-5 definition now relies on disturbance of cognition such as memory deficit, disorientation, language, visuospatial ability, or perception (1). In addition, disturbance in attention (i.e., reduced ability to direct, focus, sustain, and shift attention) and awareness (reduced orientation to the environment) are mandatory features (3-5). However, while the DSM-5 definition is used widely in clinical and research settings, it does not represent a consensus definition in all fields of medicine. For example, in contrast to dementia (6-12), delirium develops over a short period (hours and days), fluctuates in severity during the course of day and is often associated with an impaired day/night pattern. In contrast to the International Classifications of Diseases 10 (13) altered psychomotor activity is not part of the DSM 5 definition, but delirium can present as hyper- or hypoactive delirium (14). Both states, but especially the hypoactive form are often unrecognized by physician and this deficit has not improved over time resulting in at least $164 billion in annual health care expenditures alone in the US (15). Delirium is a frequent and life-threatening condition, associated with unfavorable short- and long-term prognosis (16,17), and diverse and multifactorial etiologies (18). In palliative care, it is especially prevalent in patients near the end of life and most often results in profound distress for patients and caregivers (17,19). A major pharmacologic strategy in this situation is palliative sedation (20). In hospitals, nursing homes and home care, delirium is a serious problem, leading to substantial health care costs and associated high levels of mortality (21-23). Delirium is a complex and challenging situation for health care professionals because it is often irreversible and in case of agitated and aggressive patients sometimes even a risk for those involved in caring for the patient (formal and informal caregivers) (24,25). To overcome these situations, benzodiazepines and neuroleptics are widely used to treat delirium in various clinical settings, including palliative care (25,26). Outside the field of palliative care, neuroleptics and benzodiazepines have been examined in trials for example in a postoperative, intensive-care or geriatric setting (15,27-29). Here, benzodiazepines were found to provoke, aggravate or mask delirium (15,27-29), reduce survival and sometimes even lead to long-term cognitive impairment (15,27-30). Thus, many clinicians avoid them (29,30) and guidelines and meta-analyses do not recommend their use (26,29,31,32), with delirium due to withdrawal being an important exception (29,30). Neuroleptics are extensively used to treat delirium or “distressing behavioral and psychological symptoms” (33) in various settings (34), as for example in the elderly, even though no substance has yet been approved for this indication and neuroleptics have shown to increase mortality (34-36). Therefore, guidelines recommend neuroleptics to be reserved for severe psychotic symptoms and for the hyperactive form of delirium with careful consideration of patient safety (36). Nevertheless, many clinicians continue to administer conventional (e.g., haloperidol) or atypical neuroleptics (e.g., risperidone) willingly to treat delirium in palliative care (37,38) or even as prophylaxis for delirium (39).
The aim of this publication is to obtain and discuss novel evidence from randomized, placebo-controlled trials (RCTs) published in 2017 on the use of benzodiazepines or neuroleptics in the treatment of delirium in palliative care and to provide basic recommendations for the pharmacotherapy in this setting.
Methods
A focused review for RCTs assessing psychopharmacotherapy with benzodiazepines or neuroleptics of delirium in patients treated in a palliative care or hospice setting was performed. No language restrictions were being applied and no specific definition for “palliative care” or “hospice” was demanded. Instead, the studies were deemed eligible if the authors of the publications reported of patients under palliative-, end-of-life-, comfort-, supportive- or hospice care, regardless of the definition of “palliative care” or “hospice” they relied on. To obtain and discuss only novel and widely accessible key-publications, only RCTs published in 2017 in PubMed listed medical journals were being included. A narrative summary and no meta-analysis of the findings of the RCTs were intended. No a-priori registration of the review protocol (e.g., in PROSPERO) or risk of bias (RoB) analysis was done due to the narrow and focused scope of this review.
The search included the following queries connected via the Boolean operator “AND”:
Query 1-Condition: delirium [tw1];
Query 2-Setting: palliative care [tw] OR hospice [tw] OR palliative medicine [tw] OR end-of-life [tw];
Query 3-Studydesign: randomi*2 [tw] OR trial [MeSH] OR randomized controlled trial [MeSH].
1, the builder tw (“text word”) searches title, abstract, MeSH headings and subheadings (single words and phrases) and other terms field such as author-supplied keywords; 2, the asterisk (“*”) is the PubMed truncation symbol.
Two researchers (Jan Gaertner and Walter Prikoszovich) independently screened the search findings and excluded hits according to title and abstract. Potential disagreement was resolved by the consultation of another author (Steffen Eychmueller).
Results
On December 17th 2017, the search yielded 42 publications. After exclusion of 40 hits, two remained for analysis (Table S1).
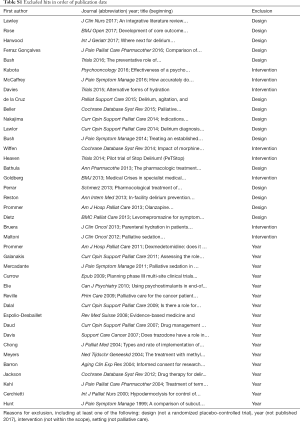
Full table
Study 1: Agar et al. (40)
Methods: the study was performed to compare the efficacy of risperidone or haloperidol versus placebo in relieving distress among patients with delirium who were receiving palliative care at 11 Australian inpatient hospice- or hospital palliative care facilities. A double blind, parallel-arm, dose-titrated randomized clinical trial was conducted. Patients with a Nursing Delirium Screening Scale (NuDESC) sum score of at least 1 were included. Patients received titrated doses of oral risperidone, haloperidol, or placebo solution for 72 hours. There was also an option to continue the blinded study medication for an additional 48 h if a partial response occurred or to allow a dose taper with symptom resolution. The researchers doses were age-adjusted, with patients 65 years or younger receiving a 0.5 mg loading dose (either risperidone or haloperidol) administered with the first dose of 0.5 mg, then 0.5 mg maintenance doses every 12 hours. Doses could be titrated by 0.25 mg on day 1 and by 0.5 mg thereafter to a maximum dose of 4 mg/d. Patients older than 65 years received 50% of the loading, initial, and maximum doses. Doses were increased if the sum of NuDESC scores for items 2, 3 and 4 (behavioral, communication and perceptual items) was 1 or more at the most recent assessment, conducted every 8 hours. Dose reduction to the prior dose was performed in the case of adverse effects, resolution of delirium [Memorial Delirium Assessment Scale (MDAS) score <7 for 48 hours], or resolution of symptoms (all NuDESC item scores <1 for 48 hours). All patients received treatment of potentially reversible causes of delirium, if these were identifiable and treatment seemed indicated. In addition, non-pharmacologic measure such as adequate hydration, vision and hearing aids, presence of family, and reorientation were provided as appropriate and available. Subcutaneous midazolam (2.5 mg, every 2 hours) was administered if patients scored 2 on the NuDESC item for “inappropriate behavior” or “illusions and hallucinations”. For serious extrapyramidal adverse effects, benztropine (1 to 2 mg) was available intravenously. The primary outcome of the study was the average of the last 2 delirium symptom scores on day 3, compared to the baseline score.
Results: the intention-to-treat (ITT) analysis included 247 patients. Of these, 218 (88%) were cancer patients and 85% were women. Mean age was 74.9 [standard deviation (SD): 9.8]. Study participants had mild to moderate delirium (median baseline MDAS scores ranged from 13.7 to 15.1), and were predominantly male (65.6%) with a mean age of 74.9 years. The majority had a cancer diagnosis (88.3%) and baseline Australia-modified Karnofsky Performance Status (AKPS) scores ranged from 30% to 50%. The risperidone group included 82 patients, 81 received haloperidol, and 84 placebos. At the end of the study, delirium symptom scores of patients in the risperidone arm were significantly higher compared to the placebo group [average: 0.48 units; 95% confidence interval (CI), 0.09−0.86; P=0.02] in the primary ITT analysis. The findings for patients receiving haloperidol were also significantly worse compared to placebo: Here, delirium symptom scores were 0.24 Units higher (95% CI, 0.06−0.42; P=0.009) compared to the control group. Concerning adverse events, patients in both intervention groups experienced more extrapyramidal effects (risperidone: 0.73; 95% CI, 0.09−1.37; P=0.03; haloperidol: 0.79; 95% CI, 0.17−1.41; P=0.01). Overall survival was significantly higher for patients receiving placebo compared to those receiving haloperidol [hazard ratio (HR), 1.73; 95% CI, 1.20−2.50; P=0.003]. Compared to patients’ risperidone, placebo patients’ overall survival did not differ significantly (HR, 1.29; 95% CI, 0.91−1.84; P=0.14).
Authors’ conclusions: treatment of underlying causes of delirium and non-pharmacologic management of delirium is more effective in treating distressing symptoms of delirium than risperidone and haloperidol.
Study 2: Hui et al. (41)
Methods: the study was conducted to compare the effect of lorazepam versus placebo on agitation in patients with delirium who were also receiving neuroleptics (haloperidol). The double-blind, parallel-group RCT recruited patients with advanced cancer that were treated on an acute palliative care ward of an US comprehensive cancer center who had at least 2 days of delirium with documented episodes of agitation before enrollment. All patients started on a standardized open-label regimen with haloperidol (2 mg) every 4 hours intravenously and another 2 mg every hour as needed for agitation. The Richmond Agitation and Sedation Scale [RASS; range, −5 (“unarousable”) to 4 (“very agitated or combative”)] score was obtained two hourlies until it was at least 1 or more (RASS score 1: “Restless-Anxious or apprehensive but movements not aggressive or vigorous”). The study protocol was amended because the initial version required a score of at least 2 (RASS score 2: “Agitated-Frequent non-purposeful movements”) but this led to recruitment problems. Therefore, the threshold was lowered. If RASS score was at least 1 (used as the definition for “epi”), the patients received study medication (either lorazepam 3 mg or placebo infused intravenously over 1.5 minutes). In addition, patients in both groups received 2 mg of haloperidol intravenously. Other drugs could be administered and scheduled haloperidol could be withheld if this was indicated according to the judgment of the attending physician and bedside nurse. As the primary outcome, the change in RASS score from baseline to 8 hours after study drug administration was obtained.
Results: a total of 90 patients were enrolled [mean age 62 years; 42 women (47%)]. Of the 90 patients, 58 (64%) received the study medication and 52 (90%) completed the trial. Patients receiving lorazepam (plus haloperidol) for agitation, had significantly lower RASS scores after 8 hours (−4.1) when compared to the control group (placebo plus haloperidol: −2.3; mean difference (MD), −1.9; 95% CI, −2.8 to −0.9; P<0.001). In the intervention group, significantly less rescue doses of haloperidol were needed (median 2.0 mg) compared to the control arm (median 4.0 mg; MD −1.0 mg; 95% CI, −2.0 to 0; P=0.009). Also, both the blinded informal caregivers and nurses perceived significantly more patients in the active arm to be comfortable (caregivers: 84% vs. 37%; MD, 47%; 95% CI, 14% to 73%, P=0 .007; nurses: 77% vs. 30%; MD, 47%; 95% CI, 17% to 71%, P=0.005). Hypokinesia was the most common side effect [intervention group: n=3 patients (19%), control group: n=4 patients (27%)]. Results for survival did not differ between the two groups.
Authors’ conclusion: addition of lorazepam to haloperidol for the treatment of agitation in delirium was more effective than haloperidol alone.
Discussion
Strengths, weaknesses and particularities of the studies
Agar et al. (40)
Strengths: the current paper is one of the first and most elaborate interventional studies assessing pharmacotherapy delirium of mild to moderate severity in palliative care. Therefore, evidence from this trial will be a cornerstone for evidence-based guideline development for the treatment of delirium in palliative care. Randomization, blinding and ITT analysis is impeccable and the choice of the primary outcome is meaningful, relevant, chance-sensitive and pragmatic, though the timing (72 h after first dose of the study regimen) should be noted (see below paragraph “particularities”). The presentation of methods and results does not leave much room for improvement or open question. It should be recognized that in the protocol, abstract, introduction and discussion section, the authors emphasize that the indication for neuroleptics are certain (i.e., behavioral) symptoms in delirious patients, but not delirium itself. This is well considered, because it is a known and sometimes fatal misunderstanding of many clinicians, that neuroleptics were indicated for “disorientation”, as “sedatives” (42) or even as prophylaxis for delirium (39). Neuroleptics do not only lack approval for these indications, their use is known to result in increased morbidity and mortality (42,43).
The study was performed as an investigator-initiated-trial, and the study group did not rely on funding from manufacturers. Therefore, the authors` were able to conduct the study with an inactive comparator and assess well-known, conventional drugs, which are used in palliative care and other fields of medicine for a long time. Because the study was performed as a multi-center trial in specialist palliative care and hospice institutions, the authors were able to include palliative care patients with a variety of advanced diseases and different ages, rather than limiting to patients with cancer or elderly persons. The latter is often the case when trials are performed in cancer centers with palliative care departments or nursing homes. From the ethical point of view, it is worthwhile mentioning, that rescue medication for distressing symptoms was readily available for patients in the control- and interventions arms and that the administration of study medication could be stopped anytime, if this was considered inadequate by the blinded bedside nurse and attending physician. Another strength of the study is the emphasis the authors have put on non-pharmacologic and individual measures to treat delirium and even more important the precipitating causes of delirium. Before pharmacotherapy is initiated for delirium, treatment, meticulous assessment and elimination of potentially reversible precipitants or aggravators of delirium is of utmost importance (i.e., polypharmacy, infections, electrolyte abnormalities, absence of hearing and vision aids, pain and anxiety, absence of trusted caregivers (“significant others”). Agar and colleagues have put significant effort in providing such support for all patients in the study (control and intervention group) and to describe these measures in detail in the publication. Also, it must be noted that prevention of delirium is of upmost importance and that complex multi-modal non-pharmacological interventions are highly effective (44,45). Before pharmacotherapy and also non-pharmacologic (“ecobiopsychosocial”) (42,46) measures for treating delirium are undertaken, the routine screening for and identification of patients at risk for delirium and subsequent ecobiopsychosocial measures as prophylaxis of delirium are of utmost importance (Table 1). Such measures include provision of hearing- and vision aids, but also calendars, clocks and instructions for patients and informal caregivers (47). For example, in tertiary care hospitals, this can be readily implemented by specialist nurse-led delirium services (47).

Full table
Weaknesses: in the baseline-table of the publication, it is evident that most patients that were included did not suffer from severe, distressing psychotic symptoms. Yet, it is known from various guidelines, that the use of neuroleptics should be restricted to those patients who are severely affected from these symptoms (47,48). On the one hand, this selection bias (wrong indication) (49) might have led to the findings that showed superiority of placebo over risperidone and haloperidol. On the other hand, it illustrates a common, problematic practice in the pharmacologic treatment of delirium in palliative care and other field of medicine (33). This is, that in contradiction to common recommendations (34), neuroleptics are often given for “inadequate behavior”, “disorientation” or “psychosocial distress”, especially in the elderly and patients with dementia (33,34) or even as prophylaxis of delirium (39). As it has been mentioned earlier in this article, this is not only questionable because of the known negative consequences concerning survival of the patients, but also because of the impact on quality of life of the patients. Patients are prone to experience a relevant increase in distressing extrapyramidal effects; moreover, they may seem “calmer” if judged by caregiver or health-care-professional, whilst experiencing profound distress, as it has been reported by patients recovering from hypoactive delirium who recalled the experience (42,47). Further research is required in the management of palliative care patients with severe delirium or with reduced performance status.
Particularities: in the study, the same dose for risperidone and haloperidol is used, so doses are not equipotent (regarding chlorpromazine equivalents). This resulted in very low doses especially for haloperidol and here, especially for patients 65 years or older, because an age-adapted dosing scheme was used. Specifically, patients 65 years or older received a 0.25 mg loading dose along with the first dose of 0.25 mg, then 0.5 mg maintenance doses every 12 hours. Doses could be titrated by 0.25 mg on day 1 and by 0.5 mg thereafter to a maximum dose of 2 mg/d. On the one hand, it is reasonable to use low doses of neuroleptics, because of safety issues (42,48) and other authors have reported that on average 2 mg haloperidol per day were an effective dose (50). Yet, this dose is surprisingly small and little more than the dose often recommended if haloperidol is used as an antiemetic in palliative care. Therefore, the findings of Agar and colleagues that patients’ survival was significantly lower in the haloperidol group are absolutely notable. It is an alarming finding and emphasizes that safety issues concerning haloperidol in palliative care must be assessed vigorously in the future. But it is also possible that differences between the three study arms at baseline may account for this adverse outcome, because patients in the haloperidol group received higher opioid doses than patients in the other arms {oral morphine equivalent dose [median (interquartile range) (mg/d)]}: haloperidol 33.0 (0−153.5); risperidone: 6.9 (0−88.2); placebo 15.0 (0−86.4). It cannot be ruled out that for example the potentiation of anticholinergic effects of opioids and neuroleptics (drug-drug interaction) may have led to increased mortality in the haloperidol group. It is also remarkable that in this study there is a high number of extrapyramidal symptoms were reported even in these very small doses. Such symptoms are usually not present in doses lower than 3 mg. Dose adjustments were rather infrequent and 12-hour intervals might be too long to attain an effect. Furthermore, it has to be mentioned that the dropout rates for the risperidone group (31 of 82 patients) were about twice the rates of haloperidol (18 of 81 patients) and placebo (15 of 84 patients).
The duration of the study was rather short. Already 72 hours after the administration of the study medication, the primary outcome was obtained. Though it is recommended, that neuroleptics should be tapered as soon as possible in delirium (47,48), 72 hours seems rather short, especially because it is known that delirium itself is often not reversible (14,16,19,24). Finally, although Agar and colleagues mentioned that missing scores were imputed using multiple imputations, it is necessary to emphasize that missing data can reduce the power and efficiency of a study and, unfortunately, can also lead to biased results (51). Another particularity of this study was that compared to the relatively small doses of neuroleptics, relatively high doses of midazolam (2.5 mg intravenous) were allowed as rescue medication.
Hui et al. (41)
Strengths: Hui and colleagues managed to conduct one of the first interventional studies on the pharmacologic treatment of episodes of agitation in delirious palliative care patients. Though a standardized RoB analysis, for example with the Cochrane Risk of Bias Tool (52) or the Jadad score (53) was not undertaken in the focused review presented here, the quality of the evidence is most likely to exceed that of previous trial. Main reasons for this is the randomized, double blind design and the performance as an investigator-initiated-trial without funding from manufacturers. Hui and coworkers chose to assess an absolutely relevant intervention, because benzodiazepines are widely used in delirious patient in palliative care and other fields of medicine, despite ambiguous or contradictory evidence and the known harmful potential of these drugs (28,54). The primary outcome (RASS) is well validated, easy to comprehend, chance-sensitive and absolutely relevant for informal caregivers, health-care-professionals and patient safety. Though the authors do not provide information about the minimally clinically relevant difference (MCID) of this outcome, the power calculation for the trial appears reasonable, because a 1.0 difference on a 10-point scale has been identified as a sufficient benchmark in other patient-, proxy- or professional-rated symptom scales in palliative care (55). The authors conclude that lorazepam (in addition to haloperidol, when compared to haloperidol alone) was superior in treating agitation in delirious patients. They did not conclude that that the interventions successfully treated delirium itself. This is important, because one must distinguish between treating agitation in delirium from treating delirium itself, which very often, this source of major misunderstanding and malpractice (54). The presentation of methods and results is impeccable and does not leave many open questions.
Weaknesses: it should be noted, that the primary outcome of this trial might be unfavorable. More precisely, a superior effect of the intervention, as measured by the primary outcome (RASS), may on the one hand sufficiently measure the reduction of agitation (or successful sedation), but convey incorrect conclusions. This means that because delirious patients who are more sedated or hypoactive may as well experience pronounced distress (54,56). The mean RASS score among patients treated with lorazepam was −2 to −3 (minimally responsive to verbal stimulus) compared with 0 to −1 (awake and alert, or drowsy) in the control arm. This means that less patients in the interventions group were able to communicate, what may have been very relevant for patients and the significant others, since the vast majority of patients died. Furthermore, it might be possible, that the combination of haloperidol and lorazepam did not treat delirium, but rather masked hyperactive delirium symptoms by sedating the patients and, more likely, converting those patients to hypoactive delirium, which might explain, why patients in the haloperidol and lorazepam group had greater severity of delirium than the haloperidol and placebo group, despite fewer episodes of hyperactivity (57). The impact of lorazepam on delirium itself can be found by studying the tables in the manuscript. A secondary outcome, the Memorial Delirium Assessment Scale (MDAS) (58) was obtained and it is obvious, that the intervention did not result in a reduction of delirium severity. Taking together ongoing delirium with reduced psychomotor activity, it can be argued that lorazepam simply shifted patients from hyperactive (or mixed) delirium to the hypoactive form, which is just as distressing for the patients (54,56). A closer look at the full text of the article reveals that 8 hours after administration of the study medication, sedation was profound: One of two patients scored RASS −3 to −5 [RASS −3: moderate sedation: any movement (but no eye contact) to voice; RASS −5: unarousable: no response to voice or physical stimulation]. Already 30 minutes after being given the study medication, 1 of 3 patients was RASS −3 to −5. In the control group (haloperidol only) this was the case for only 3% (after 30 minutes) and 8% (after 8 hours) of the patients respectively. Interestingly, the mean RASS score at baseline was 0 to −1, suggesting that episodes may have been moderate hyperactivity or mild restlessness without being associated to any danger or distress to self or others and thus no indication for a pharmacologic intervention. The inclusion of patients with RASS episodes of 1 is questionable, because guidelines recommend that the use of benzodiazepines in delirium should be restricted to delirium due to withdrawal and episodes of severe agitation or anxiety (47,48).
Particularities: like in many palliative care trials, the patients enrolled in the trial were all suffering from advanced cancer, because the palliative care unit was associated to a large quartiary US cancer center. This may reduce the generalizability of the results, because palliative care patients suffer from a variety of diseases from different field of medicine (i.e., heart failure, chronic obstructive pulmonary disease, frailty, chronic progressive multiple sclerosis. Yet, many recommendations in palliative care rely on trials with a narrow patient population. Importantly it should be noted that no medication is currently licensed for use in the management of delirium, so the use of medications for the indication of delirium is ‘off-label’. In the trial, patients received haloperidol intravenously. Many clinicians avoid to give the substance via this route of administration, torsade du pointes and other cardiac arrhythmias have been reported, especially in the elderly and in case of preexisting QT-prolongations or other cardiac diseases (50). In palliative care, the subcutaneous route would be another well-tolerated option with slower onset of action (59). Of importance, Hui and co-workers administered daily doses of at least 12 mg haloperidol per day as a background medication for delirium. This is far more than most other clinicians would consider an average effective dose, far more than the neuroleptic dose used in the study of Agar and colleagues (40) and far beyond the 2 mg that has been reported to be effective in other trials (50). Moreover, patients received intravenous haloperidol 2 mg along with the study medication (or placebo) in case of agitation. This approach warrants attention and from the view of the authors of this manuscript, this should be viewed critically. First, Hui et al. chose relatively high doses of haloperidol. Second, they did not apply haloperidol according to its correct indication. This means, that haloperidol was given for the treatment of delirium (as background medication) and not for the treatment of severe psychotic symptoms. The high rate of mortality (less than 1 of 3 patients was discharged alive) may be explained with the high incidence of delirium in patients near the end of life, but an effect of the study medication cannot be excluded.
Conclusions
General: in 1996, a landmark randomized controlled trial compared haloperidol (N=11), chlorpromazine (N=13), and lorazepam (N=6) was conducted for the first-line management of delirium in HIV patients (60). The primary outcome, as assessed by the Delirium Rating Scale, improved with haloperidol (P<0.001) and chlorpromazine (P<0.001), and no significant differences were detected between the two neuroleptic arms (P=0.44). The lorazepam arm was terminated prematurely because of excessive drowsiness. Understandably, a 2012 Cochrane systematic review on drug therapy for delirium in terminally ill adult patients concluded that ‘there remains insufficient evidence to draw conclusions about the role of drug therapy in the treatment of delirium in terminally ill patients’ (61). In 2017, two landmark RCTs assessing pharmacotherapy of delirium in palliative care have become available (40,41). Thorough analysis of the evidence provided by these trials reveals that recommendations for the pharmacologic treatment of delirium can be adopted for palliative care (47,48,62). Key recommendations (Table 1) include that even though many clinicians still provide antipsychotics in mild forms of delirium or even as prophylaxis (50), delirium alone or disorientation or so called “biopsychosocial distress in dementia” (42) is no indication for the administration of drugs. Rather, these drugs are indicated in case of severe symptoms (hallucinations, delusions: neuroleptics; agitation and anxiety: benzodiazepines). If agitation is triggered by hallucinations, delusions or other psychotic symptoms, the combination of benzodiazepines and neuroleptics may be indicated.
Probably even more important than pharmacotherapy is the routine screening of patients at risk for delirium such as the Delirium Observational Screening Scale, the confusion assessment method for the intensive care unit (CAM-ICU) or the NuDESC and the provision of individualized non-pharmacologic (ecobiopsychosocial) interventions for patients and informal caregivers as prophylaxis and therapy (63-66). Though delirium is a life-threatening condition and especially in palliative care it is very often associated with imminent death and often irreversible (22,56), the high degree of distress for patients, caregivers and health-care professionals demands the meticulous identification and treatment of potentially reversible precipitants of delirium.
Palliative care setting: in patients in a palliative care situation, especially in the context of end-of-life- and comfort care, quality of life (the reduction and prevention of suffering) is the leading and often single therapeutic goal. Only a few guidelines focusing on the management of delirium at the end of life or in older adults (67). In these cases, issues of patient safety concerning the risk of arrhythmias may be less important than in other settings and therefore, electro-cardiographs are not mandatory prior to the use of haloperidol and other neuroleptics in many patients. Still, distressing extrapyramidal effects should always be anticipated and biperiden or benztropine should be readily available as antidotes. In contrast to patients who are treated in curative intent and where the fast tapering and stopping of neuroleptics and benzodiazepines is of upmost importance once the symptoms are no longer severe, it may be appropriate to continue the medication specifically in patients in the last days of life and patients with structural brain lesions (i.e., metastases) and severe symptoms such as agitation. In major trial registries (i.e., clinicaltrials.gov), a number of trials assessing melatonin (68-73) for the prophylaxis and treatment of delirium and have been registered and some are already recruiting (e.g., ClinicalTrials.gov Identifier: NCT02615340, NCT03013790, NCT02536417). Once data from these trials becomes available, these approaches may provide an interesting alternative to neuroleptics and benzodiazepines.
Future studies concerning delirium in palliative care must consider the importance of other agents such as melatonin or dexmedetomidine (31,33,74) as well as non-pharmacological interventions to prevent and treat delirium (44,45). Regarding delirium in palliative care the following questions and issues emerge from a clinical perspective and should be considered in further research:
- How well has the “palliative care” study population been described? Management of delirium in “palliative care” patients with expected survival of several months differs widely from patients with delirium in the dying phase;
- What are the underlying causes: management of delirium in patients with liver failure and or structural brain damage (e.g., metastasis) differ widely from treatment in patients with acute fever and dehydration;
- Which definition is used for diagnosing delirium: which clinical signs and symptoms are used (following ICD-code), which screening tool should be administered (including frequency of repetition) and which cut-off-point is regarded as clinically relevant;
- What are meaningful outcomes (connected to clinical cut-off-point definition) in the management of delirium in palliative care, i.e., which perspectives are taken into account: the patient’s, the family’s and the professionals’ perspective?
- Are new improved statistical methods in delirium research necessary given that e.g., delirium status changes over time, delirium cannot be assessed when patients are comatose, loss to follow-up is common to all longitudinal studies of older persons (75-78)?
- What are the most appropriate tools for the diagnosis of delirium in the dying phase, especially with elderly patients with dementia and comorbid delirium-dementia?
The authors argue that any meta-analysis based on observational studies or randomized trials will fail to provide clear guidance for clinical practice if these definitions have not been commonly agreed on, and used as a common basis, as long as evidence-based arguments for and against prescribing antipsychotics for the treatment of delirium exists (79-81).
Acknowledgments
We thank Dr. Walter Prikoszovich for his support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th edition. Arlington: American Psychiatric Association, 2013.
- Lawlor PG, Bush SH. Delirium diagnosis, screening and management. Curr Opin Support Palliat Care 2014;8:286-95. [Crossref] [PubMed]
- Adamis D, Meagher D, Murray O, et al. Evaluating attention in delirium: A comparison of bedside tests of attention. Geriatr Gerontol Int 2016;16:1028-35. [Crossref] [PubMed]
- Leonard M, O'Connell H, Williams O, et al. Attention, vigilance and visuospatial function in hospitalized elderly medical patients: Relationship to neurocognitive diagnosis. J Psychosom Res 2016;90:84-90. [Crossref] [PubMed]
- Maclullich AM, Anand A, Davis DH, et al. New horizons in the pathogenesis, assessment and management of delirium. Age Ageing 2013;42:667-74. [Crossref] [PubMed]
- Fong TG, Davis D, Growdon ME, et al. The interface between delirium and dementia in elderly adults. Lancet Neurol 2015;14:823-32. [Crossref] [PubMed]
- Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA 1996;275:852-7. [Crossref] [PubMed]
- Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014;383:911-22. [Crossref] [PubMed]
- Jackson TA, MacLullich AM, Gladman JR, et al. Diagnostic test accuracy of informant-based tools to diagnose dementia in older hospital patients with delirium: a prospective cohort study. Age Ageing 2016;45:505-11. [Crossref] [PubMed]
- Jackson TA, MacLullich AM, Gladman JR, et al. Undiagnosed long-term cognitive impairment in acutely hospitalised older medical patients with delirium: a prospective cohort study. Age Ageing 2016;45:493-9. [Crossref] [PubMed]
- Leonard M, McInerney S, McFarland J, et al. Comparison of cognitive and neuropsychiatric profiles in hospitalised elderly medical patients with delirium, dementia and comorbid delirium-dementia. BMJ Open 2016;6:e009212. [Crossref] [PubMed]
- Sepulveda E, Leonard M, Franco JG, et al. Subsyndromal delirium compared with delirium, dementia, and subjects without delirium or dementia in elderly general hospital admissions and nursing home residents. Alzheimers Dement (Amst) 2016;7:1-10. [Crossref] [PubMed]
- Bush SH, Kanji S, Pereira JL, et al. Treating an established episode of delirium in palliative care: expert opinion and review of the current evidence base with recommendations for future development. J Pain Symptom Manage 2014;48:231-48. [Crossref] [PubMed]
- McCaffrey N, Fazekas B, Cutri N, et al. How Accurately Do Consecutive Cohort Audits Predict Phase III Multisite Clinical Trial Recruitment in Palliative Care? J Pain Symptom Manage 2016;51:748-55. [Crossref] [PubMed]
- Wiffen PJ, Derry S, Moore RA. Impact of morphine, fentanyl, oxycodone or codeine on patient consciousness, appetite and thirst when used to treat cancer pain. Cochrane Database Syst Rev 2014.CD011056. [PubMed]
- Bush SH, Lacaze-Masmonteil N, McNamara-Kilian MT, et al. The preventative role of exogenous melatonin administration to patients with advanced cancer who are at risk of delirium: study protocol for a randomized controlled trial. Trials 2016;17:399. [Crossref] [PubMed]
- Lawley H, Hewison A. An integrative literature review exploring the clinical management of delirium in patients with advanced cancer. J Clin Nurs 2017;26:4172-83. [Crossref] [PubMed]
- Oh ES, Fong TG, Hshieh TT, et al. Delirium in Older Persons: Advances in Diagnosis and Treatment. JAMA 2017;318:1161-74. [Crossref] [PubMed]
- Harwood RH, Teale E. Where next for delirium research? Int J Geriatr Psychiatry 2018;33:1512-20. [Crossref] [PubMed]
- Bush SH, Tierney S, Lawlor PG. Clinical Assessment and Management of Delirium in the Palliative Care Setting. Drugs 2017;77:1623-43. [Crossref] [PubMed]
- Beller EM, van Driel ML, McGregor L, et al. Palliative pharmacological sedation for terminally ill adults. Cochrane Database Syst Rev 2015;1:CD010206. [PubMed]
- de la Cruz M, Noguera A, San Miguel-Arregui MT, et al. Delirium, agitation, and symptom distress within the final seven days of life among cancer patients receiving hospice care. Palliat Support Care 2015;13:211-6. [Crossref] [PubMed]
- Nakajima N, Satake N, Nakaho T. Indications and practice of artificial hydration for terminally ill cancer patients. Curr Opin Support Palliat Care 2014;8:358-63. [Crossref] [PubMed]
- Ferraz Goncalves JA, Almeida A, Costa I, et al. Comparison of Haloperidol Alone and in Combination with Midazolam for the Treatment of Acute Agitation in an Inpatient Palliative Care Service. J Pain Palliat Care Pharmacother 2016;30:284-8. [Crossref] [PubMed]
- Rose L. Development of core outcome sets for effectiveness trials of interventions to prevent and/or treat delirium (Del-COrS): study protocol. BMJ Open 2017;7:e016371. [Crossref] [PubMed]
- Prommer E. Olanzapine: palliative medicine update. Am J Hosp Palliat Care 2013;30:75-82. [Crossref] [PubMed]
- Bathula M, Gonzales JP. The pharmacologic treatment of intensive care unit delirium: a systematic review. Ann Pharmacother 2013;47:1168-74. [Crossref] [PubMed]
- Goldberg SE, Bradshaw LE, Kearney FC, et al. Care in specialist medical and mental health unit compared with standard care for older people with cognitive impairment admitted to general hospital: randomised controlled trial (NIHR TEAM trial). BMJ 2013;347:f4132. [Crossref] [PubMed]
- Heaven A, Cheater F, Clegg A, et al. Pilot trial of Stop Delirium! (PiTStop)--a complex intervention to prevent delirium in care homes for older people: study protocol for a cluster randomised controlled trial. Trials 2014;15:47. [Crossref] [PubMed]
- Perrar KM, Golla H, Voltz R. Pharmacological treatment of delirium in palliative care patients. A systematic literature review. Schmerz 2013;27:190-8. [Crossref] [PubMed]
- Prommer E. Review article: dexmedetomidine: does it have potential in palliative medicine? Am J Hosp Palliat Care 2011;28:276-83. [Crossref] [PubMed]
- Reston JT, Schoelles KM. In-facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med 2013;158:375-80. [Crossref] [PubMed]
- Dietz I, Schmitz A, Lampey I, et al. Evidence for the use of Levomepromazine for symptom control in the palliative care setting: a systematic review. BMC Palliat Care 2013;12:2. [Crossref] [PubMed]
- Maltoni M, Scarpi E, Rosati M, et al. Palliative sedation in end-of-life care and survival: a systematic review. J Clin Oncol 2012;30:1378-83. [Crossref] [PubMed]
- Bruera E, Hui D, Dalal S, et al. Parenteral hydration in patients with advanced cancer: a multicenter, double-blind, placebo-controlled randomized trial. J Clin Oncol 2013;31:111-8. [Crossref] [PubMed]
- Galanakis C, Mayo NE, Gagnon B. Assessing the role of hydration in delirium at the end of life. Curr Opin Support Palliat Care 2011;5:169-73. [Crossref] [PubMed]
- Currow DC, Shelby-James TM, Agar M, et al. Planning phase III multi-site clinical trials in palliative care: the role of consecutive cohort audits to identify potential participant populations. Support Care Cancer 2010;18:1571-9. [Crossref] [PubMed]
- Mercadante S, Porzio G, Valle A, et al. Palliative sedation in patients with advanced cancer followed at home: a systematic review. J Pain Symptom Manage 2011;41:754-60. [Crossref] [PubMed]
- Espolio-Desbaillet Y, Beauverd M. Evidence-based medicine and palliative medicine. Rev Med Suisse 2008;4:458-61. [PubMed]
- Agar MR, Lawlor PG, Quinn S, et al. Efficacy of Oral Risperidone, Haloperidol, or Placebo for Symptoms of Delirium Among Patients in Palliative Care: A Randomized Clinical Trial. JAMA Intern Med 2017;177:34-42. [Crossref] [PubMed]
- Hui D, Frisbee-Hume S, Wilson A, et al. Effect of Lorazepam With Haloperidol vs Haloperidol Alone on Agitated Delirium in Patients With Advanced Cancer Receiving Palliative Care: A Randomized Clinical Trial. JAMA 2017;318:1047-56. [Crossref] [PubMed]
- Maust DT, Kales HC. Medicating Distress. JAMA Intern Med 2017;177:42-3. [Crossref] [PubMed]
- Vieweg WV, Wood MA, Fernandez A, et al. Proarrhythmic risk with antipsychotic and antidepressant drugs: implications in the elderly. Drugs Aging 2009;26:997-1012. [Crossref] [PubMed]
- Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999;340:669-76. [Crossref] [PubMed]
- Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev 2016;3:CD005563. [PubMed]
- Clegg A, Siddiqi N, Heaven A, et al. Interventions for preventing delirium in older people in institutional long-term care. Cochrane Database Syst Rev 2014.CD009537. [PubMed]
- Barron JS, Duffey PL, Byrd LJ, et al. Informed consent for research participation in frail older persons. Aging Clin Exp Res 2004;16:79-85. [Crossref] [PubMed]
- Carlson RW, Larsen JK, McClure J, et al. International adaptations of NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2014;12:643-8. [Crossref] [PubMed]
- Croskerry P. From mindless to mindful practice--cognitive bias and clinical decision making. N Engl J Med 2013;368:2445-8. [Crossref] [PubMed]
- Daud ML. Drug management of terminal symptoms in advanced cancer patients. Curr Opin Support Palliat Care 2007;1:202-6. [Crossref] [PubMed]
- Bell ML, Fiero M, Horton NJ, et al. Handling missing data in RCTs; a review of the top medical journals. BMC Med Res Methodol 2014;14:118. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- Elie D, Gagnon P, Gagnon B, et al. Using psychostimulants in end-of-life patients with hypoactive delirium and cognitive disorders: A literature review. Can J Psychiatry 2010;55:386-93. [Crossref] [PubMed]
- Reville B, Axelrod D, Maury R. Palliative care for the cancer patient. Prim Care 2009;36:781-810. [Crossref] [PubMed]
- Breitbart W, Gibson C, Tremblay A. The delirium experience: delirium recall and delirium-related distress in hospitalized patients with cancer, their spouses/caregivers, and their nurses. Psychosomatics 2002;43:183-94. [Crossref] [PubMed]
- Pandharipande PP, Ely EW. Humanizing the Treatment of Hyperactive Delirium in the Last Days of Life. JAMA 2017;318:1014-5. [Crossref] [PubMed]
- Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Assessment Scale. J Pain Symptom Manage 1997;13:128-37. [Crossref] [PubMed]
- Dalal S, Del Fabbro E, Bruera E. Is there a role for hydration at the end of life? Curr Opin Support Palliat Care 2009;3:72-8. [Crossref] [PubMed]
- Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry 1996;153:231-7. [Crossref] [PubMed]
- Candy B, Jackson KC, Jones L, et al. Drug therapy for delirium in terminally ill adult patients. Cochrane Database Syst Rev 2012;11:CD004770. [PubMed]
- Savaskan E, Baumgartner M, Georgescu D, et al. Empfehlungen zur Prävention, Diagnostik und Therapie des Delirs im Alter. Praxis (Bern 1994) 2016;105:941-52.
- Marcantonio ER, Ngo LH, O'Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for. Ann Intern Med 2014;161:554-61. [Crossref] [PubMed]
- Neufeld KJ, Hayat MJ, Coughlin JM, et al. Evaluation of two intensive care delirium screening tools for non-critically ill hospitalized patients. Psychosomatics 2011;52:133-40. [Crossref] [PubMed]
- Neufeld KJ, Leoutsakos JS, Sieber FE, et al. Evaluation of two delirium screening tools for detecting post-operative delirium in the elderly. Br J Anaesth 2013;111:612-8. [Crossref] [PubMed]
- Shi Q, Warren L, Saposnik G, et al. Confusion assessment method: a systematic review and meta-analysis of diagnostic accuracy. Neuropsychiatr Dis Treat 2013;9:1359-70. [Crossref] [PubMed]
- Bush SH, Bruera E, Lawlor PG, et al. Clinical practice guidelines for delirium management: potential application in palliative care. J Pain Symptom Manage 2014;48:249-58. [Crossref] [PubMed]
- Al-Aama T, Brymer C, Gutmanis I, et al. Melatonin decreases delirium in elderly patients: a randomized, placebo-controlled trial. Int J Geriatr Psychiatry 2011;26:687-94. [Crossref] [PubMed]
- Chakraborti D, Tampi DJ, Tampi RR. Melatonin and melatonin agonist for delirium in the elderly patients. Am J Alzheimers Dis Other Demen 2015;30:119-29. [Crossref] [PubMed]
- de Rooij SE, van Munster BC. Melatonin deficiency hypothesis in delirium: a synthesis of current evidence. Rejuvenation Res 2013;16:273-8. [Crossref] [PubMed]
- de Rooij SE, van Munster BC, de Jonghe A. Melatonin prophylaxis in delirium: panacea or paradigm shift? JAMA Psychiatry 2014;71:364-5. [Crossref] [PubMed]
- Lawlor PG, Bush SH. Delirium in patients with cancer: assessment, impact, mechanisms and management. Nat Rev Clin Oncol 2015;12:77-92. [Crossref] [PubMed]
- Scholtens RM, van Munster BC, van Faassen M, et al. Plasma melatonin levels in hip fracture patients with and without delirium: A confirmation study. Mech Ageing Dev 2017;167:1-4. [Crossref] [PubMed]
- Rosenzweig AB, Sittambalam CD. A new approach to the prevention and treatment of delirium in elderly patients in the intensive care unit. J Community Hosp Intern Med Perspect 2015;5:27950. [Crossref] [PubMed]
- Davis DHJ, Kreisel SH, Muniz Terrera G, et al. The Epidemiology of Delirium: Challenges and Opportunities for Population Studies. Am J Geriatr Psychiatry 2013;21:1173-89. [Crossref] [PubMed]
- Pandharipande PP, Ely EW, Arora RC, et al. The intensive care delirium research agenda: a multinational, interprofessional perspective. Intensive Care Med 2017;43:1329-39. [Crossref] [PubMed]
- Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007;298:2644-53. [Crossref] [PubMed]
- Rondeau V, Mathoulin-Pelissier S, Jacqmin-Gadda H, et al. Joint frailty models for recurring events and death using maximum penalized likelihood estimation: application on cancer events. Biostatistics 2007;8:708-21. [Crossref] [PubMed]
- Girard TD, Exline MC, Carson SS, et al. Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness. N Engl J Med 2018;379:2506-16. [Crossref] [PubMed]
- Meagher D, Agar MR, Teodorczuk A. Debate article: Antipsychotic medications are clinically useful for the treatment of delirium. Int J Geriatr Psychiatry 2018;33:1420-7. [Crossref] [PubMed]
- Teodorczuk A, MacLullich A. New waves of delirium understanding. Int J Geriatr Psychiatry 2018;33:1417-9. [Crossref] [PubMed]


