Abstract
Interdigital foot infections are mostly caused initially by dermatophytes, yeasts and less frequently by bacteria. Erythrasma caused by Corynebacterium minutissimum can be confused with superficial mycoses. The aim of the study was to determine the prevalence of the etiologic agents of superficial mycoses and the frequency of Corynebacterium minutissimum in interdigital foot infections. All the samples obtained from the 121 patients with interdigital foot infections were examined directly with the use of 20% potassium hydroxide mounts and Gram stain under the microscope and cultured on Sabouraud's dextrose agar plates. In identification of superficial mycoses, the rate was found to be 14% with the cultural method and 14% with direct microscopic examination. Using a combination of direct microscopic examination and culture, a 33.8% ratio was achieved. In the culture of these samples, the most isolated factor was Trichophyton rubrum (33.7%). In 24 of the patients (19.8%) Corynebacterium minutissimum was detected by Gram staining, in 6 of these patients Trichophyton rubrum was found, Trichophyton mentagrophytes was found in 2 and Trichosporon spp. was found in 1. The examination of interdigital foot lesions in the laboratory, the coexistence of erythrasma with dermatophytes and yeast should be considered.
interdigital foot infections; superficial mycoses; dermatophytosis; yeast; Corynebacterium minutissimum
RESEARCH PAPER
Interdigital foot infections: Corynebacterium minutissimum and agents of superficial mycoses
Fatma Mutlu SariguzelI; A. Nedret KocII; Gülhan YagmurI; Elife BerkI
IDepartment of Microbiology, Kayseri Education and Research Hospital, Kayseri, Turkey
IIDepartment of Microbiology, Erciyes University Medical School, Kayseri, Turkey
Correspondence Correspondence: F.M. Sariguzel Department of Microbiology Kayseri Education and Research Hospital TR-38010 Kayseri, Turkey E-mail: fmutluguzel@gmail.com
ABSTRACT
Interdigital foot infections are mostly caused initially by dermatophytes, yeasts and less frequently by bacteria. Erythrasma caused by Corynebacterium minutissimum can be confused with superficial mycoses. The aim of the study was to determine the prevalence of the etiologic agents of superficial mycoses and the frequency of Corynebacterium minutissimum in interdigital foot infections. All the samples obtained from the 121 patients with interdigital foot infections were examined directly with the use of 20% potassium hydroxide mounts and Gram stain under the microscope and cultured on Sabouraud's dextrose agar plates. In identification of superficial mycoses, the rate was found to be 14% with the cultural method and 14% with direct microscopic examination. Using a combination of direct microscopic examination and culture, a 33.8% ratio was achieved. In the culture of these samples, the most isolated factor was Trichophyton rubrum (33.7%). In 24 of the patients (19.8%) Corynebacterium minutissimum was detected by Gram staining, in 6 of these patients Trichophyton rubrum was found, Trichophyton mentagrophytes was found in 2 and Trichosporon spp. was found in 1. The examination of interdigital foot lesions in the laboratory, the coexistence of erythrasma with dermatophytes and yeast should be considered.
Key words: interdigital foot infections, superficial mycoses, dermatophytosis, yeast, Corynebacterium minutissimum.
Introduction
Interdigital foot infections are frequently seen in humans and, although not life-threatening, affect the quality of life of sufferers in our country and all over the world. Several pathogens and factors may play a role in toe web infections. The etiologic agents of infection between the toes are most frequently dermatophytes, followed by yeast, saprobes molds and less frequently bacteria. Superficial mycoses are infections of the keratinous tissue caused by dermatophytes, yeasts and environmental fungi. Dermatophytes follow a course such as skin scaling, vesicle formation and sometimes inflammation and it is possible for them to spread from human to human, animal to human, and nature to human (Summerbell et al., 2007). The determination of superficial mycotic agents according to region will provide data for epidemiology, as well as increase the success of treatment.
Tinea pedis which is also known as athlete's foot, is a chronic fungal infection of the feet. Tinea pedis is a common fungal infection in adults, affecting between 30 and 70% of the population (Al Hasan et al., 2004).
Erythrasma is a superficial infection caused by Corynebacterium minutissimum and affects the major interdigital regions of the feet. In 1961, it was found for the first time that C. minutissimum was a factor for erythrasma (Brown, 1995). It is characterized by erythematous plaques, brown, scaly patches, vesicles, blisters and maceration. The course of the disease is chronic and remission does not tend to occur. C. minutissimum often coexists with other bacteria, dermatophytes and yeast (Summerbell et al., 2007, Svejgaard et al., 1986).
Determination of C. minutissimum is usually made by direct examination of skin scrapings from the infected site. Erythrasma exhibits coral-red fluorescence under a Wood's lamp. Due to the coexistence of erythrasma with agents of superficial mycoses, interdigital foot infections should be viewed by direct microscopy. Direct microscopic examination (Gram stain) shows clusters of long, large bacillus such as are common in epithelial cells. Cultures are usually unsuccessful and not required to establish a diagnosis of erythrasma (Henry, 2001).
The purpose of this study was to investigate the agents of superficial mycoses and determine the frequency of C. minutissimum in patients with interdigital foot lesions.
Material and Method
Specimens were taken from the interdigital foot lesions of 121 patients with pre-identification of superficial mycoses and/ or erythrasma which were sent to the Mycology Laboratory from the dermatology clinics in the Hospital of Erciyes University Medical Faculty. Specimens consisting of epidermal scale and macerated skin were scraped from the rim of lesions using a sterile scalpel blade following the cleaning of the affected sites with 70 v/v isopropyl alcohol. The scrapings were collected sterile petri dish. Moist cotton swabs were used to collect pus from inflammatory lesions. The samples were divided into two portions: one for microscopic examination and one for culture. The direct examination of the samples was performed by using potassium hydroxide (KOH, 20%) for the fungus and the specimen for C. minutissimum was fixed with ether on a slide and then Gram staining was performed. Sabouraud dextrose agar (SDA) (Acumedia) and SDA with chloramphenical (Sigma) and cycloheximide plus chloramphenical (Oxoid) were used for culture. Specimens were incubated at 37 °C and 25 °C for three weeks. Molds growing in culture were evaluated with respect to their rate of growth, temperature tolerance and temperature enhancement, surface topography, urea hydrolysis, special nutritional requirements, existence of pigments in the colony and in vitro hair perforation. Yeast colonies were identified according to assimilation of carbonhydrate with API AUX C 20 (Biomerieux, France) kits, with respect to the microscopic and macroscopic morphologies of the colony, germ tube test, capability of growing at 37 °C, urea hydrolysis and sensitivity for cycloheximide.
Result
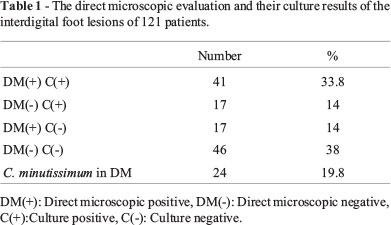
Direct microscopic findings: The rate was found to be 14% with direct microscopic examination. Gram positive rods related to C. minutissimum were determined in 24 (19.8%) of the specimens (Table 1).
Culture findings: The fungus was isolated in 58 of the total cultures (47.9%). Direct microscopic examination was found to be positive in 41(33.8%) of the growing cultures. However, the microscopic examination of the other 17 samples (14%) were found to be negative (Table 1).
The isolated strains were identified as dermatophytes in 46 (38%), as yeast in 9 (7.4%), as yeast and dermatophytes in 1(0.8%) and as mold in 3 (2.4%). The isolated dermatophytes were identified as T. rubrum in 41 and as T. mentagrophytes in 5. The isolated yeasts were identified as C.albicans in 3, as C. glabrata in 1, as C. parapsilosis in 1, as Rhodotorula spp. in 1, as Trichosporon spp. in 4. One of the mold strains was identified as Aspergillus spp., 1 as Fusarium spp. and 1 as Acremonium spp.
T. rubrum was isolated from 6 of the 24 patients who had C. minutissimum. T. mentagrophytes and Trichosporon spp. were also isolated from 2 and 1 of these 24 patients, respectively (Table 2).
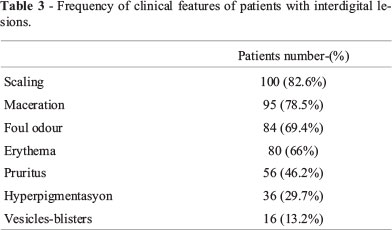
Clinical Features: The most common clinical sign was scaling,which affected 82.6% of patients (Table 3).
Discussion
Superficial mycoses are quite common infections and their prevalence changes according to different countries and regions. Also, in the same area, the prevalence may change over time. Direct microscopy and culture methods are used for the laboratory identification of superficial mycoses. Due to the fact that culture results are obtained in 2-3 weeks, direct microscopic examination is of importance in pre-diagnosis. Using a combination of these methods increases the rate of diagnosis. In the literature related to to be direct microscopic investigation and culture growth, the percentage of positivity was found to be between 22.3 and 66.7% (Kiraz et al., 1999, Khosravi et al., 1994, Manzano-Gayosso et al., 1994). Koksal et al. (2009) reported that when using a combination of direct microscopic examination and culture, a 97% ratio was achieved. However, only 3% of the samples were determined using cultures without direct microscopy. The ratio obtained from this study was in the range of the literature values (33.8%).
Although direct microscopic evaluation provides information about pre-identification, culture increases the ratio of identification. In studies carried out in our country, it was found that although specimens were found to be negative in direct inspection, the rate of subjects who were determined to have culture growth was between 3.4%- 16.6% (Karaarslan et al., 1998, Kiraz et al., 1999, Khosravi et al., 1994, Manzano-Gayosso et al., 1994). Tanis et al. (2010) reported that patients that were pre-diagnosed with superficial mycoses were determined at ratios of 44.7% and 22% with direct diagnosis and the culture method, respectively. In this study the rate was found to be 14% with the culture method and 14% positivity was detected with direct microscopic examination. It was estimated that factors which affect direct microscopic examination and culture are the collection of specimens, sending them to the laboratory, the stages of laboratory investigation, and the previous treatment of patients.
According to various studies conducted in Turkey, dermatophytes are the most common isolated microorganisms as agents of superficial mycoses, followed by yeasts (Aydin et al., 2001, Baysal et al., 1997, Pekbay et al., 2000). The etiologic pathogens most commonly associated with Tinea pedis are Trichophyton rubrum, T. mentagrophytes and Epidermatophyton floccosum (Noble et al., 1998, Zuber and Baddam, 2001). In addition,Tinea pedis is not only caused by dermatophytes but also can cause dermatomycosis (Hainer, 2003,Vander Straten et al., 2003).
In a study, conducted by Koksal et al. (2009), in which 9150 clinical specimens taken from 8200 patients were examined in terms of superficial mycoses, 5722 clinical strains (70%) were isolated. Seventy-four percent of these isolates were identified as dermatophytes, 21% as Candida spp., 3% as Malassezia furfur, and 2% as Trichosporon spp. In the dermatophytes, the most commonly found isolates were T. rubrum, T. mentagrophytes and other Trichophyton spp., and the least frequently reported were E. floccosum and Microsporum sp.. In this study, the most isolated factor was T. rubrum (33.7%). T. mentagrophytes isolates were identified in 5 specimens.
Fusarium spp. is a rare infection causing interdigital foot lesions (Romano and Gianni, 2002). Romano et al. (Romano et al., 1998b, Romanno et al., 1999c) found that only four cases of interdigital intertrigo resulting from Fusarium spp. have been reported. In this study, from molds out of dermatophyte, Fusarium spp, Aspergillus spp. and Acremonium spp. were isolated in 1 specimen each, in 3 of them C. albicans, in 1 C. glabrata, in one Rhodotorula spp., in 4 Trichosporon spp. and in 1 specimen both C. parapsilosis and T. rubrum were isolated.
There is a lack of epidemiologic data on interdigital erythrasma and the reported prevalence varies greatly from one study to another. Svejgaard et al. (1986) reported a prevalence of 77.1% in a group of military recruits. Morales-Trujillo et al. (2008) reported a prevalence of 32.8% in their study. In one study with a group of 300 patients, 42 (14%) were determined to have erythrasma alone, 42 (14%) had evidence of a dermatophyte infection alone, 12 (4%) were infected with a dermatophyte and C. minutissimum, 5(1.7%) had C. albicans alone, 2(0.7%) had both C. albicans and C. minutissimum and 6(2%) failed to grow any organisms (Allen et al., 1990). In this study, 15(12.3%) were infected with C. minutissimum, 6(4.9%) were infected with C. minutissimum and T. rubrum, 2 (1.6%) were infected with T. mentographytes and C. minutissimum, and 1 (0.8%) was infected with Trichosporon spp. and C. minutissimum.
In conclusion, interdigital erythrasma is a common condition and can be easily confused with interdigital tinea. In interdigital foot lesions the combined use of direct microscopy (with KOH), Gram staining and culture increases the possibility of fungus and C. minutissimum determination. The condition persists if not treated appropriately. Therefore, Gram staining direct microscopy (with KOH), and culture can determine the choice of the correct treatment. Hence in the mycology laboratory during the examination of interdigital foot lesions, C. minutissimum should be observed together with the etiological agents of superficial mycoses.
Submitted: December 13, 2012
Approved: March 14, 2014
All the content of the journal, except where otherwise noted, is licensed under a Creative Commons License CC BY-NC.
- Al Hasan M, Fitzgerald SM, Saoudion M, Krishnaswam G (2004) Dermatology for the practicing allergist: Tinea pedis and its complications. Clin Mol Allergy 2:5.
- Allen S, Christmas TI, Mc Kinney W, Parr D, Oliver GF (1990) The Auckland skin clinic tinea pedis and erythrasma study. N Z Med J 103:391-393.
- Aydin N, Hilmioglu S, Gultekin B, Bozkurt E, Aydemir S, Gürel M (2001) The agents isolated from superficial mycoses. Turk J Infect 15:47-50 (In Turkish).
- Baysal V, Ozcelik N, Yildirim M (1997) Clinical and mycological features of dermatophytoses in Isparta region. Med J Suleyman Demirel Univ 4:31-35 (In Turkish).
- Brown AE (1995) Other Corynebacteria and Rhodococcus In: Mandell GL, Bennett JE, Polin R(eds). Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases. Vol. 2. 4th ed. Churchill Livingstone, New York, pp 1872-1880.
- Hainer BL (2003) Dermatophyte infections. Am Fam Physician 67:101-108.
- Henry JB (2001) Clinical, Diagnosis and Management by Laboratory Methods. 20th ed. Saunders, Philadelphia, pp 1096-1097.
- Karaarslan A, Karaarslan F, Cengiz AT (1998) The dermatomycosis agents isolated from Kecioren region, Ankara. Turkish J Infect 12:93-96 (In Turkish);
- Kiraz M, Yegenoglu Y, Erturan Z, Ang O (1999) The epidemiology of onychomycoses in Istanbul, Turkey. Mycoses 42:323-329.
- Khosravi AR, Aghamirian MR, Mahmoudi M (1994) Dermatophytoses in Iran. Mycoses 37:43-48.
- Koksal F, Er E, Samasti M (2009) Causative agents of superficial mycoses in Istanbul, Turkey: retrospective study. Mycopathologia 168:117-123.
- Manzano-Gayosso P, Mendez-Tovar LJ, Hernandez- Hernandez F, López-Martínez R (1994) Dermatophytoses in Mexico City. Mycoses 37:49-52.
- Morales-Trujillo ML, Arenas R, Arroyo S (2008) Interdigital Erythrasma: Clinical, epidemiologic and microbiologic findings. Actas Dermosifiliogr 99:469-473.
- Noble SL, Forbes RC, Stomm PL (1998) Diagnosis and management of common tinea infections. Am Fam Physician 58:163-174.
- Okenke CN, Tsuboi R, Kawai M, Ogawa H (2000) Fluorometric assessment of in vitro antidermatophytic activities of antimycotics based on their keratin-penetrating power. J Clin Microbiol 38:489-491.
- Pekbay A, Sanic A, Yenigun A (2000) Determine of superficial mycoses prevalence and causative pathogens at workers. J Exp Clin Med 17:45-49.
- Romano C, Gianni C (2002) Tinea pedis resulting from Fusarium spp. Int J Dermatol 41:360-362.
- Romano C, Miracco C, Difonzo EM (1998) Skin and nail infections due to Fusarium oxysporum in Tuscany, Italy. Mycoses 41:433-437.
- Romano C, Presenti L, Massai L (1999) Interdigital intertrigo of the feet due to therapy resistant Fusarium solani. Dermatology 199:177-179.
- Summerbell RC, Weitzman I, Padhye A (2007) Trichophyton, Microsporum, Epidermophyton, and agents of superficial mycoses. In: Murray, P.R., Baron, E.J., Pfaller, M.A., Jorgensen, J.H., Landry, M.L. (eds) Manual of Clinical Microbiology, Vol. 2. 9th ed. Washington DC, pp 1874-1897.
- Svejgaard E, Christophersen J, Jelsdorf HM (1986) Tinea pedis and erythrasma in Danish recruits. J Am Aca. Dermatol 14:993-999.
- Tanis H, Toraman ZA, Cihangir N, Sasmaz S (2010) The Causative Agents of Superficial Fungal Infections at Kahramanmaras, Turkey. Turk Microbiol Soc 40:48-53 (in Turkish).
- Vander Straten MR, Hassain MA, Ghannaum MA (2003) Cutaneous infections dermatophytosis onychomycosis and tinea versicolor. Infect Dis Clin North Am 17:87-112.
- Zuber TJ, Baddam K (2001) Superficial fungal infection of the skin. Where and how it appears help determine therapy. Postgrad Med 109:17-132.
Publication Dates
-
Publication in this collection
04 Nov 2014 -
Date of issue
Sept 2014
History
-
Received
13 Dec 2012 -
Accepted
14 Mar 2014