Abstract
Epithelium, a highly dynamic system, plays a key role in the homeostasis of the intestine. However, thus far a human intestinal epithelial cell line has not been established in many countries. Fetal tissue was selected to generate viable cell cultures for its sterile condition, effective generation, and differentiated character. The purpose of the present study was to culture human intestinal epithelial cells by a relatively simple method. Thermolysin was added to improve the yield of epithelial cells, while endothelin-3 was added to stimulate their growth. By adding endothelin-3, the achievement ratio (viable cell cultures/total cultures) was enhanced to 60% of a total of 10 cultures (initiated from 8 distinct fetal small intestines), allowing the generation of viable epithelial cell cultures. Western blot, real-time PCR and immunofluorescent staining showed that cytokeratins 8, 18 and mouse intestinal mucosa-1/39 had high expression levels in human intestinal epithelial cells. Differentiated markers such as sucrase-isomaltase, aminopeptidase N and dipeptidylpeptidase IV also showed high expression levels in human intestinal epithelial cells. Differentiated human intestinal epithelial cells, with the expression of surface markers (cytokeratins 8, 18 and mouse intestinal mucosa-1/39) and secretion of cytokines (sucrase-isomaltase, aminopeptidase N and dipeptidylpeptidase IV), may be cultured by the thermolysin and endothelin-3 method and maintained for at least 20 passages. This is relatively simple, requiring no sophisticated techniques or instruments, and may have a number of varied applications.
Human intestinal epithelial cell; Cell culture; Thermolysin; Endothelin-3; Cytokeratin; Brush border enzyme
Braz J Med Biol Res, May 2010, Volume 43(5) 451-459
Culture of human intestinal epithelial cell using the dissociating enzyme thermolysin and endothelin-3
Z. Liu, P. Zhang, Y. Zhou,  Correspondence and Footnotes
Correspondence and Footnotes
 H. Qin and T. Shen
H. Qin and T. Shen
Department of Surgery, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
Epithelium, a highly dynamic system, plays a key role in the homeostasis of the intestine. However, thus far a human intestinal epithelial cell line has not been established in many countries. Fetal tissue was selected to generate viable cell cultures for its sterile condition, effective generation, and differentiated character. The purpose of the present study was to culture human intestinal epithelial cells by a relatively simple method. Thermolysin was added to improve the yield of epithelial cells, while endothelin-3 was added to stimulate their growth. By adding endothelin-3, the achievement ratio (viable cell cultures/total cultures) was enhanced to 60% of a total of 10 cultures (initiated from 8 distinct fetal small intestines), allowing the generation of viable epithelial cell cultures. Western blot, real-time PCR and immunofluorescent staining showed that cytokeratins 8, 18 and mouse intestinal mucosa-1/39 had high expression levels in human intestinal epithelial cells. Differentiated markers such as sucrase-isomaltase, aminopeptidase N and dipeptidylpeptidase IV also showed high expression levels in human intestinal epithelial cells. Differentiated human intestinal epithelial cells, with the expression of surface markers (cytokeratins 8, 18 and mouse intestinal mucosa-1/39) and secretion of cytokines (sucrase-isomaltase, aminopeptidase N and dipeptidylpeptidase IV), may be cultured by the thermolysin and endothelin-3 method and maintained for at least 20 passages. This is relatively simple, requiring no sophisticated techniques or instruments, and may have a number of varied applications.
Key words: Human intestinal epithelial cell; Cell culture; Thermolysin; Endothelin-3; Cytokeratin; Brush border enzyme
Introduction
Epithelial cells lining the intestinal mucosa play a key role in the homeostasis of the organisms (1,2). Depending on their location in the intestinal tract, they not only play a central role in absorptive and secretory functions, but also have the ability to protect the organism from various pathogens and toxins found even under the normal conditions in the intestine, providing an effective barrier to the complex antigenic load of intestinal contents (3). In addition, some of the most serious pathological conditions, such as inflammatory bowel disease (IBD), are known to be associated with alterations of the normal growth and functions of the intestinal epithelium (4). It is therefore of interest to investigate the physiology and pathophysiology of these cells and, not surprisingly, a large community of scientists in research fields such as intestinal physiology, intestinal immunology and cancer genesis are interested in the study of these cells (5). The epithelium of the small intestine is one of the most rapidly proliferating tissues in the body. It is continuously renewed by a process involving cell generation and migration, from stem cells located at the bottom of the crypt to the extrusion of the terminally differentiated cells at the tip of the villus (6). Epithelial cell proliferation, migration and differentiation are tightly regulated by various mechanisms because of their important role throughout these processes, which are primarily regulated by a variety of growth factors and macromolecules of the extracellular matrix (7). However, the mechanisms regulating their proliferation and differentiation have not been fully elucidated (8).
Although the colonic mucosa is also a highly dynamic system, a normal intestinal epithelial cell line is not available because of the difficulty of cell culture in vitro, and the study of human intestinal cell regulation has been limited to cell cultures generated from experimental animals (9) and human cancer tissues (10). Only short-term cultures have been obtained, including organ culture, monolayer culture, and suspension culture, which had limited success (11). Studies of the normal physiology of the intestinal epithelium have been performed on the whole organ and mucosal preparations, which are limited both by rapid necrosis and degeneration of the epithelium and by the impossibility of distinguishing between the functions of the different mucosal cytotypes (6).
Many methods have been tried to study normal function and to define the metabolic and growth alterations in IBD and other intestinal diseases. Rodent intestinal cell lines such as IEC-6 (12) have been very helpful in the study of various crypt cell activities including the regulation of proliferation, cell migration, synthesis of extracellular matrix molecules and, to a lesser extent, cell differentiation (6). However, it is also evident that an important limitation of these systems is that observations made with experimental animal models cannot always be transferred to humans, a fact possibly due to the difference of brush border enzyme expression and its regulation by hormones and growth factors and the fundamental differences between animal and human tissues in the composition of the epithelial basement membrane along the crypt-villus axis (13). The application of human cancer cell lines such as Caco-2, HT29 and T84, which can undergo a spontaneous and complete intestinal-like program of differentiation (14,15), has been limited by their cancerous nature (11). Caco-2, which presents obvious limitations because of the neoplastic origin of the cells, is particularly unsuitable for studies on the regulatory factors that influence normal intestinal functions.
On the whole, the availability of continuously growing cultures of pure, normal intestinal epithelial cells (IEC) in culture would therefore be very valuable. Many efforts have been made in recent years to culture human intestinal epithelial cells (HIEC), and several techniques have been described. In general, better cell preservation and longer survival times have been reported for cultures of human fetal intestinal epithelial cells, but success has been limited (16).
Indeed, successful ex-vivo cultures of IEC from human fetal intestine confirm that primary IEC cultures can be obtained more easily from fetal tissue (17). And after years of exploration, the Perreault and Jean-François method (6) to generate viable HIEC has proved to be very useful in studying their normal functions and in defining their metabolic and growth alterations (7,17). After analyzing a number of different methods, fetal tissue was selected for three reasons. First, fetal tissue can be obtained under sterile conditions. Second, fetal tissues seem to be more effective for the generation of epithelial cell cultures because of their rapid metabolism and self-renewing rate (18). Third, fetal cells in the small intestine have already expressed most of the adult differentiated markers in fetuses of 10-12 weeks of gestation (6). Thermolysin treatment, which was previously used for dermo-epidermal separation and keratinocyte isolation, could improve the yield of HIEC (6). Furthermore, it was also reported that endothelin-3 (ET-3) could affect the growth and differentiation of gastrointestinal epithelial cells by mediating the epithelial-mesenchymal interaction, a process that controls intestinal epithelial growth in developing mammalian fetuses (19). Consequently, ET-3 could be added to stimulate the growth of HIEC.
However, thus far, the HIEC line has not been established in many countries. Because it is easier to obtain human fetal tissues in our country, the thermolysin and ET-3 method can be used in our studies to isolate and generate viable HIEC from the human small intestine, and long-surviving crypt cells, which can be sub-cultured for a number of passages, have been obtained. The details of the methods used are described below.
Material and Methods
Tissues
Specimens of small intestines (jejunum and ileum) from fetuses of 18 weeks of gestation (post-fertilization) were obtained from legal abortions in accordance with a protocol on the use of human material approved by the Institutional Ethics Committee of Shanghai Sixth People’s Hospital Affiliated with Shanghai Jiao Tong University. Written informed consent was obtained from the donors before the tissues were collected from the fetuses. Only immediately obtained specimens were used in the present study.
Primary cell culture
The culture medium was high-glucose Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA) supplemented with 4 mM glutamine, 20 mM HEPES, 50 U/mL penicillin, 50 mg/mL streptomycin, 10 ng/mL recombinant human epidermal growth factor (all obtained from Gibco), 0.2 IU/mL insulin (Shanghai BASF Polyurethane Co., Ltd., China), 30 µm/L ET-3 (Sigma, USA), and 5% fetal bovine serum (Gibco), referred to as the complete DMEM. Cells were grown in 25-cm2 plastic culture dishes (Corning, USA) kept in a humidified atmosphere of 5% CO2 at 37°C. Cell passage was at pre-confluent densities using 0.05% trypsin (Invitrogen, USA) and 0.5 mm EDTA (Invitrogen).
Thermolysin method for the isolation of HIECs
Thermolysin (0.005%, type X, lot 23C-0280, Sigma), which could specifically catalyze the hydrolysis of peptide bonds containing hydrophobic amino acids, was used for the isolation of HIEC. Segments 5- to 10-cm long of 18-week fetal ileum were used. The muscular layer was removed with a forceps and the intestine was opened longitudinally and cut into 5-mm fragments, which were washed twice in HEPES buffer (6.7 mM KCl, 142 mM NaCl, 10 mM HEPES, 1 mM CaCl2, 0.45 mM NaOH, pH 7.4). Fragments were then incubated at 37°C with shaking for 3 h in the presence of thermolysin (0.005% protease type X in HEPES buffer; Sigma). The incubation solutions were carefully removed and centrifuged at 1000 g for 5 min and the pellets were re-suspended and incubated in complete DMEM. The medium with the non-adhering cells was recovered after 90 min and plated onto a new dish. Medium was changed every 24 h and, when successful, confluence was reached within approximately 2 weeks. Subcultures were performed after trypsinization (0.5% trypsin, 0.54 mM EDTA in PBS, 5 min at 23°C). Cell populations were expanded and then kept frozen at passage 8 or 9 in liquid nitrogen. The achievement ratio was calculated as viable cell cultures/total cultures.
Caco-2 and human intestinal mesenchymal cell cultures
The human colon carcinoma cell line Caco-2 is a clone of the parent Caco-2 cell line (HBT-37; ATCC, China), which has been characterized elsewhere, with its culture conditions having been defined (20). Cells were used between passages 60 and 80. Human intestinal mesenchymal (HIM) cells were generated and isolated as described elsewhere (6), and were also derived from the 18-week-old fetal small intestine and cultured.
Primary antibodies
The monoclonal antibodies used in the present study were directed against human cytokeratins 8 (ab9023; Abcam, USA), 18 (ab668; Abcam), 19 (ab9221; Abcam), human intestinal brush border hydrolase sucrase-isomaltase (SI, sc-27605, Abcam), dipeptidylpeptidase IV (DPPIV, ab28341; Abcam), aminopeptidase N (APN, ab7417; Abcam), and maltase-glucoamylase (MGA, sc-98598, Abcam). Production of a rabbit-specific anti-mouse intestinal mucosa (MIM) -1/39 antibody has been described elsewhere (21-23).
Gel electrophoresis and Western
blotting
Samples of fetal small intestine mucosal scrapings were obtained as described. A cell monolayer was collected and immediately snap-frozen in liquid nitrogen. For the preparation of SDS-PAGE, cells were thawed to 4°C and homogenized in chilled RIPA buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 0.5% sodium deoxycholate, 1% Triton X-100, 1 mM EDTA), including protease and phosphatase inhibitors (1 mM PMSF, 1 mM Na3VO4, 1 mM NaF, and 5 g/mL each of aprotinin, leupeptin, and pepstatin). After centrifugation at 10,000 g for 10 min at 4°C, the supernatant was recovered and assayed for protein content (DC protein assay; Bio-Rad, USA). Equal amounts of total protein were separated on 10% SDS-polyacrylamide gels and then transferred to a nitrocellulose membrane. After blocking overnight in Tris-buffered saline (TBS) containing 0.05% Tween (TBS-T) and 5% dry powdered milk, membranes were washed three times for 5 min each time with TBS-T and incubated for 2 h at room temperature with primary antibodies. After three washes with TBS-T, the membranes were incubated for 1 h with horseradish peroxidase-conjugated secondary antibody. Following two washes with TBS-T and one wash with TBS, the membranes were developed for visualization of proteins by the addition of enhanced chemiluminescence reagent (Amersham, USA).
Quantitative real-time PCR
Total RNA of different cells was extracted with the RNeasy Mini Kit (Qiagen, Germany) and quantified spectrophotometrically (Gene Quant II; Amersham Pharmacia Biotech, USA). Primer sequences for target proteins are shown in Table 1. First-strand cDNA was synthesized from 1 µg total RNA by reverse transcription using oligo-dT primers and reverse transcriptase (superscript II; Invitrogen) according to manufacturer instructions. Real-time PCR was performed in a 20-µL mixture containing 1 µL of the cDNA preparation, 10X PCR mix (iQ SYBR Green Supermix; Bio-Rad) and 500 nm of each primer in a thermocycler (iCycler iQ system; Bio-Rad) using the following PCR parameters: 95°C for 5 min followed by 50 cycles at 95°C for 15 s, 6°C for 15 s, and 72°C for 15 s. The fluorescence threshold (Ct) was calculated with the appropriate Bio-Rad software. The absence of nonspecific products was confirmed by the analysis of the melting point curves. GAPDH served as an internal standard of mRNA expression.
Fluorescence staining
Briefly, monolayers were fixed and permeabilized with methanol at -20°C and then incubated overnight at 4°C with primary antibodies, followed by 2-h incubation with FITC-conjugated specific secondary antibody (Sigma) at room temperature in the dark. Subsequently, monolayers were washed several times with 10 mM phosphate-buffered saline (pH 7.4, 136 mM NaCl, 2.6 mM KCl, 8.1 mM Na2HPO4, 1.4 mM KH2PO4), and then detached with the Anocell inserts and mounted with Vectashield (Vector Laboratories, Inc., USA). Cell staining was detected by confocal laser scanning microscopy (MRC 1024, Bio-Rad). To allow comparison between the HIEC, HIM and Caco-2 groups, the microscopic examination of both groups was done in the same experimental session.
Statistical analysis
Data were analyzed statistically by one-way ANOVA with Tukey Kramer post hoc comparison. For each variable at least three independent experiments were carried out. Data are reported as means ± SEM. All analyses were done using the GraphPad Prism 5 Software (USA); differences were considered to be significant at P < 0.05.
Results
Generation of HIEC cultures by the thermolysin and ET-3 method
By adding ET-3, the achievement ratio (viable cell cultures/total cultures) was enhanced again to 60% of a total of 10 cultures (we achieved 6 viable cultures from the initial total 10 cultures, initiated from 8 distinct fetal small intestines), allowing the generation of epithelial cell cultures, as compared with the achievement ratio of only 38% of a total of 34 cultures (initiated from 26 distinct fetal small intestines) reported with the use of the Perreault and Jean-François method (6). The remaining cultures were either overgrown by mesenchymal cells or were not successful (no cell attachment or growth). Both proliferative and non-proliferative epithelial cells were steadily observed. Non-proliferative epithelial colonies consisted of islands of condensed epithelial cells (Figure 1A) after 48 h of culture. These cells gradually spread extensively on the substrate after 72 h (Figure 1B) for up to 7 days (Figure 1C) before they began to degenerate. By 14 days, all the cells composing the colonies had degenerated and detached from the substrate (Figure 1D). In contrast, proliferative epithelial colonies consisted of condensed epithelial aggregates (Figure 2A) after 48 h and these cells started expanding after 72 h (Figure 2B). After 7 days, the multiple colonies had expanded considerably and still exhibited an epithelial morphology (Figure 2C). After 14 days, cell confluence had been reached, and a more typical epithelial morphology was achieved (Figure 2D). These primary cultures have now been maintained for up to 20 passages.
Human intestinal epithelial cells were identified
by epithelial and differentiated characteristics by Western blotting
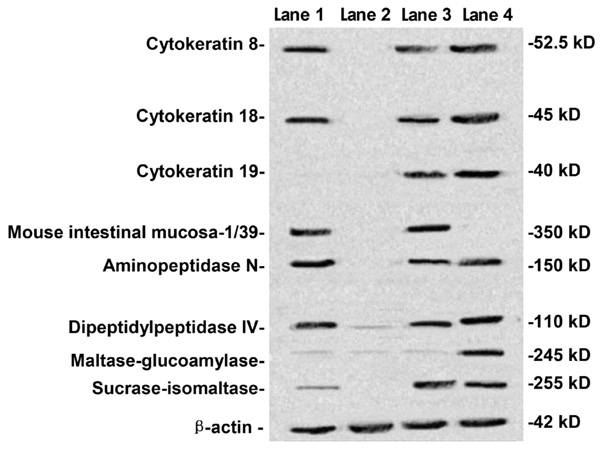
Cells were tested at passages 8-10. Surface markers (cytokeratins 8, 18, 19 and MIM-1/39) and differentiated secreted functional markers (SI, APN, DPPIV, MGA) were investigated by Western blotting. HIEC, HIM, 18-week fetal small intestine, and Caco-2 cells were determined by Western blotting (Figure 3). Cytokeratins 8 and 18 were detected in HIEC (lane 1), 18-week fetal small intestine (lane 3), and Caco-2 cells (lane 4) but not in HIM (lane 2), while cytokeratin 19 was not detected in HIEC (lane 1). MIM-1/39, an intestinal functional marker specific for intestinal crypt cells, was detected in HIEC (lane 1) and 18-week fetal small intestine (lane 3) but not in HIM (lane 2) or Caco-2 cells (lane 4). Furthermore, SI, APN, DPPIV, and MGA were also determined. SI, APN, and DPPIV were expressed in HIEC (lane 1), 18-week fetal small intestine (lane 3), and Caco-2 cells (lane 4) but not in HIM (lane 2), while MGA was detected only in Caco-2 cells (lane 4).
Human intestinal epithelial cells were identified by epithelial and differentiated characteristics at the mRNA level by real-time PCR
Real-time PCR showed results similar to those obtained with Western blot, i.e., that the epithelial markers, cytokeratins 8 and 18, had higher mRNA levels in HIEC, 18-week fetal small intestine, and Caco-2 cells compared with HIM (Figure 4A,B). Cytokeratin 19 showed lower mRNA levels in HIEC (Figure 4C). The mRNA levels of GAPDH in different groups were statistically similar (Figure 4D). The differentiated markers SI, APN and DPPIV had higher mRNA levels in HIEC, 18-week fetal small intestine, and Caco-2 cells (Figure 4E,F,H). However, the expression of MGA was lower in HIEC, 18-week fetal small intestine and HIM than Caco-2 cells (Figure 4G).
Human intestinal epithelial cells were identified by epithelial and differentiated characteristics by
immunofluorescence
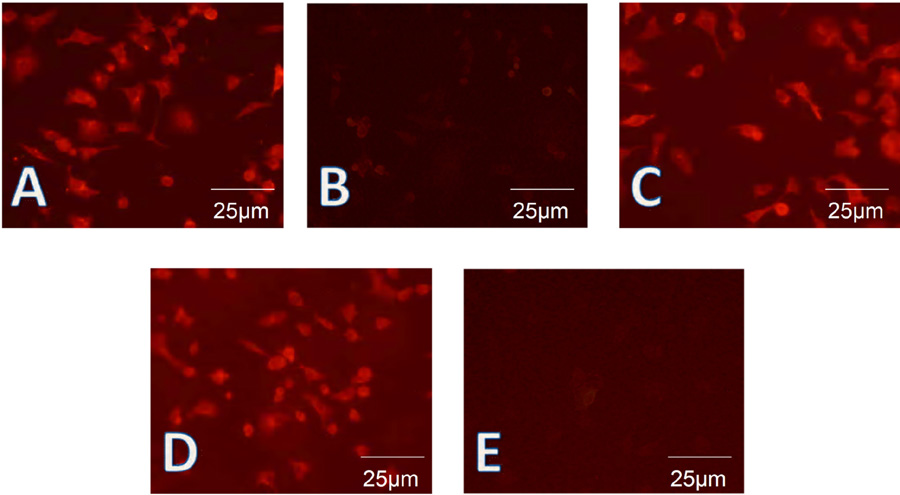
Cells were tested at passages 8-10. To further confirm the presence of these cytokeratins, HIEC, HIM, and Caco-2 cells were subjected to fluorescence staining. Cytokeratin 8 was found to be expressed in the majority of the cells (Figure 5A) while cytokeratin 19 (Figure 5B) was not detected in most cells, as expected from the results of Western blot and real-time PCR. Cytokeratin 18 and cytokeratin 8 were expressed (Figure 5C). Similar to the results of Western blotting, MIM-1/39, an intestinal functional marker specific for intestinal crypt cells, was detected in HIEC (Figure 5D), but not in Caco-2 cells (Figure 5E). SI, APN, and DPPIV showed a staining pattern similar to that of cytokeratin 8, while MGA was detected only in Caco-2 cells (data not shown).
Non-proliferative epithelial cells in different stages. A, Non-proliferative epithelial colonies consisted of islands of condensed epithelial cells after 48 h of culture. B, Non-proliferative epithelial cells gradually spread extensively on the substrate after 72 h. C, Non-proliferative epithelial cells spread extensively for up to 7 days before they began to degenerate. D, All the non-proliferative epithelial cells composing the colonies had degenerated and detached from the substrate after 14 days.
Proliferative epithelial cells in different stages. A, Proliferative epithelial colonies consisted of condensed epithelial aggregates after 48 h. B, Proliferative epithelial cells started to expand after 72 h. C, Multiple colonies had expanded considerably and still exhibited epithelial morphology after 7 days. D, Cell confluence was reached, and a more typical epithelial morphology was achieved after 14 days.
Human intestinal epithelial cells were identified in terms of their epithelial and differentiated character by Western blot. Human intestinal epithelial cells (HIEC, lane 1), human intestinal mesenchymal cells (HIM, lane 2), 18-week fetal small intestine (lane 3), and Caco-2 cells (lane 4) were determined by Western blotting for the detection of cytokeratins 8, 18, 19, mouse intestinal mucosa (MIM)-1/39, aminopeptidase N, dipeptidylpeptidase IV, maltase-glucoamylase and sucrase-isomaltase. Cytokeratins 8 and 18 were detected in HIEC (lane 1), 18-week fetal small intestine (lane 3), and Caco-2 cells (lane 4) but not in HIM (lane 2), while cytokeratin 19 was not detected in HIEC (lane 1). MIM-1/39 was detected in HIEC (lane 1) and 18-week fetal small intestine (lane 3) but not in HIM cells (lane 2) and Caco-2 cells (lane 4). Aminopeptidase N, dipeptidylpeptidase IV and sucrase-isomaltase were expressed at HIEC (lane 1), 18-week fetal small intestine (lane 3), and Caco-2 cells (lane 4) but not in HIM (lane 2), while maltase-glucoamylase was detected only in Caco-2 cells (lane 4).
Human intestinal epithelial cells (HIEC) were identified on the basis of their epithelial and differentiated character at the mRNA level by real-time PCR. A, B, Real-time PCR showed that cytokeratins 8 and 18 had higher mRNA levels in HIEC, 18-week fetal small intestine, and Caco-2 cells compared with human intestinal mesenchymal cells (HIM). C, Cytokeratin 19 showed lower mRNA levels in HIEC. D, mRNA levels of GAPDH were nonsignificant in different groups. E, F, H, mRNA levels of aminopeptidase N, dipeptidylpeptidase IV and sucrase-isomaltase had higher mRNA levels in HIEC, 18-week fetal small intestine, and Caco-2 cells. G, Maltase-glucoamylase had lower expression in HIEC, 18-week fetal small intestine and HIM than Caco-2 cells. P < 0.05 * vs #. One-way ANOVA was performed with Tukey Kramer post hoc comparison. Values were calculated by the Student t-test. All data are reported as means ± SEM.
Human intestinal epithelial cells were identified on the basis of their epithelial and differentiated character by fluorescence staining. A, Fluorescence staining showed that cytokeratin 8 was expressed in the vast majority of human intestinal epithelial cells (HIEC). B, Fluorescence staining showed that cytokeratin 19 was not strongly detected in most HIEC preparations. C, Fluorescence staining showed that cytokeratin 18 was expressed in the vast majority of HIEC. D, Mouse intestinal mucosa (MIM)-1/39, an intestinal functional marker specific for intestinal crypt cells, was detected only in HIEC. E, MIM-1/39 was not detected in Caco-2 cells.
Discussion
The epithelium of the small intestine is continuously and rapidly renewed in a process involving cell generation and migration from the multi-potential stem cells, housed in specific "niches" within the crypts, to the extrusion of the terminally differentiated cells at the tips of the villi, within a period of 3-5 days (24). HIEC play a central role in absorptive and secretory functions, protecting the organisms from bacteria and toxins, providing an effective barrier against the complex antigenic load of intestinal contents (3,25). However, difficulties still exist in establishing an HIEC cell line in China.
Thermolysin contributed to the isolation of epithelial cells from the lung and trachea, and of keratinocytes in the past (20,26). In recent years, it has also been applied to the isolation of many other high-yield homogenous and viable cells (27). Because of the persistent renewal of human fetal intestinal cells, continuous epithelial cell cultures can be generated relatively easily from the human fetal intestine in a reproducible manner (17,19). Furthermore, the thermolysin method of generating viable HIEC cultures has proven to be particularly useful in the study of normal function and of metabolic and growth mechanisms (7,17,28). Since ET-3 affects the growth and differentiation of gastrointestinal epithelial cells by mediating epithelial-mesenchymal interactions, which control intestinal epithelial growth in the developing mammalian fetus (19), ET-3 was added to stimulate the growth of HIEC. By adding ET-3, the achievement ratio was enhanced again to 60% from only 38% in a previous study (6).
The main advantages of this method include using thermolysin as a dissociating enzyme under the selected conditions due to its ability to release epithelial cells free of mesenchymal cells, while ET-3 enhances the ability of thermolysin to promote the release of clumps of both proliferative and non-proliferative epithelial cells. Above all, this method considerably improved the yield of epithelial cells in general and preserved cell viability, without destruction by the underlying stromal cells (16,21).
The crypts were attached to the growth surface within 3 to 6 h after being placed in culture. And after an overnight incubation, the original residues were surrounded by cell colonies of variable sizes. The colonies were filled with epithelial cells as shown by morphology and by the expression of cell-type-specific intermediate filaments. The contamination by mesenchymal cells remained low throughout the culture period. Lymphocytes were very few and only observed in the first 24 h, being absent after the first change of the medium, possibly being washed away.
The epithelial origin and nature of HIEC have been confirmed by analyzing the expression of specific intestinal markers such as keratins 8, 18, and 19 by Western blot, real-time PCR and immunofluorescence (29,30). As mentioned above, the detection of cytokeratins 8 and 18 by means of immunofluorescent staining and Western blot analysis confirmed the epithelial origin and nature of HIEC. The absence of cytokeratin 19 in HIEC agreed with the result reported by Perreault and Jean-François (6), which requires further investigation. The expression and distribution of cytokeratins 8 and 18 were important to distinguish HIEC from HIM cells (29).
Concerning the investigation of the expression of brush border enzymes, the expression of intestinal functional markers specific for HIEC such as SI, APN and DPPIV also supported the view that HIEC retain their epithelial origin and nature. And the presence of these intestinal functional markers in HIEC indicated that these cells have some potential for functional differentiation. As a crypt cell marker, the detection of the MIM-1/39 antigen provided strong evidence that serially passaged HIEC still retained their epithelial character, a feature that was absent in Caco-2 and HIM cells (21,23). This may also indicate that HIEC had the characteristics of crypt cells (31). The absence of MGA expression in HIEC was also demonstrated in previous experiments (6); this was expected since the enzymatic complex has been reported to be absent from the human fetal intestine (32). Interestingly, in our study, SI was also detected in HIEC, in contrast to the study by Perreault and Jean-François (6). Therefore, although APN and DPPIV are present on the luminal surface of both crypt and villus cells, which cannot be strictly considered as differentiation markers (33), expression of SI confirmed the differentiated character of HIEC (34). This indicated that, compared with the Perreault and Jean-François study (6), human fetal intestinal epithelial cells showed a more differentiated character in our study, although they may still belong to crypt cells. However, it should be mentioned that tight junctions were poorly organized in these cells, with their location more variable in the cytoplasm or at the cell periphery, suggesting that the function of HIEC still needed to develop and mature (35). Consequently, by analyzing the expression of intestinal markers of HIEC, further characteristics of HIEC may be discovered.
The method described here is relatively simple, requiring no sophisticated techniques or instruments. And HIEC may have a number of different applications (36). For example, they can be used to evaluate the effects of growth or maturation factors on the expression of membrane receptors and antigens, enzymatic activities, transport and secretory functions (37), to study drug metabolism, and to investigate carcinogenic factors and agonists (38). Furthermore, they contribute to growth and functional alterations of the colonic epithelium in intestinal cancers, IBD and other diseases and to the understanding of the mechanisms of interactions of intestinal pathogens with the mucosal surface (39) and the intracellular signaling transduction (40).
We conclude that ET-3 may enhance the ability of thermolysin to isolate epithelial cells from the human fetal small intestine and then produce continuously growing HIEC cultures, which may retain the ability to express specific cytokeratins as well as a number of intestinal functional cell markers. The isolation and culture of HIEC using thermolysin and ET-3 facilitate the research of epithelial cell proliferation, migration, differentiation, and its various mechanisms.
References
1. Bloushtain-Qimron N, Yao J, Shipitsin M, Maruyama R, Polyak K. Epigenetic patterns of embryonic and adult stem cells. Cell Cycle 2009; 8: 809-817.
2. Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest 2009; 119: 1159-1166.
3. Podolsky DK, Gerken G, Eyking A, Cario E. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology 2009; 137: 209-220.
4. Gradel KO, Nielsen HL, Schonheyder HC, Ejlertsen T, Kristensen B, Nielsen H. Increased short- and long-term risk of inflammatory bowel disease after Salmonella or Campylobacter gastroenteritis. Gastroenterology 2009; 137: 495-501.
5. Press B, Di Grandi D. Permeability for intestinal absorption: Caco-2 assay and related issues. Curr Drug Metab 2008; 9: 893-900.
6. Perreault N, Jean-François B. Use of the dissociating enzyme thermolysin to generate viable human normal intestinal epithelial cell cultures. Exp Cell Res 1996; 224: 354-364.
7. Podolsky DK. Regulation of intestinal epithelial proliferation: a few answers, many questions. Am J Physiol 1993; 264: G179-G186.
8. Fukamachi H, Narita T, Yahagi N, Takeda H, Ichinose M. Endothelin-3 controls growth of colonic epithelial cells by mediating epithelial-mesenchymal interaction. Dev Growth Differ 2005; 47: 573-580.
9. Evans GS, Flint N, Potten CS. Primary cultures for studies of cell regulation and physiology in intestinal epithelium. Annu Rev Physiol 1994; 56: 399-417.
10. Rousset M. The human colon carcinoma cell lines HT-29 and Caco-2: two in vitro models for the study of intestinal differentiation. Biochimie 1986; 68: 1035-1040.
11. Whitehead RH, van Eeden PE. A method for the prolonged culture of colonic epithelial. Methods Cell Sci 2005; 13: 103-106.
12. Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol 1979; 80: 248-265.
13. Beaulieu JF, Vachon PH. Reciprocal expression of laminin A-chain isoforms along the crypt-villus axis in the human small intestine. Gastroenterology 1994; 106: 829-839.
14. Wu CC, Hsu CW, Chen CD, Yu CJ, Chang KP, Tai DI, et al. Candidate serological biomarkers for cancer identified from the secretomes of 23 cancer cell lines and the human protein atlas. Mol Cell Proteomics 2010 (in press).
15. Moberg KH, Bell DW, Wahrer DC, Haber DA, Hariharan IK. Archipelago regulates cyclin E levels in Drosophila and is mutated in human cancer cell lines. Nature 2001; 413: 311-316.
16. Germain L, Rouabhia M, Guignard R, Carrier L, Bouvard V, Auger FA. Improvement of human keratinocyte isolation and culture using thermolysin. Burns 1993; 19: 99-104.
17. Grossmann J, Walther K, Artinger M, Kiessling S, Steinkamp M, Schmautz WK, et al. Progress on isolation and short-term ex-vivo culture of highly purified non-apoptotic human intestinal epithelial cells (IEC). Eur J Cell Biol 2003; 82: 262-270.
18. Ma Y, Peng J, Liu W, Zhang P, Huang L, Gao B, et al. Proteomics identification of desmin as a potential oncofetal diagnostic and prognostic biomarker in colorectal cancer. Mol Cell Proteomics 2009; 8: 1878-1890.
19. Kalabis J, Li G, Fukunaga-Kalabis M, Rustgi AK, Herlyn M. Endothelin-3 stimulates survival of goblet cells in organotypic cultures of fetal human colonic epithelium. Am J Physiol Gastrointest Liver Physiol 2008; 295: G1182-G1189.
20. Vachon PH, Beaulieu JF. Transient mosaic patterns of morphological and functional differentiation in the Caco-2 cell line. Gastroenterology 1992; 103: 414-423.
21. Calvert R, Millane G, Pothier P, Beaulieu JF. An intestinal secretory protein is found in most glands associated with the gastrointestinal tract: von Ebner’s and salivary glands, gallbladder, and pancreas. J Histochem Cytochem 1993; 41: 1223-1231.
22. Golaz JL, Vonlaufen N, Hemphill A, Burgener IA. Establishment and characterization of a primary canine duodenal epithelial cell culture. In Vitro Cell Dev Biol Anim 2007; 43: 176-185.
23. Beaulieu JF, Millane G, Calvert R. Developmental expression of two antigens associated with mouse intestinal crypts. Dev Dyn 1992; 193: 325-331.
24. Fernandes MI, Galvao LC, Bortolozzi MF, Oliveira WP, Zucoloto S, Bianchi ML. Disaccharidase levels in normal epithelium of the small intestine of rats with iron-deficiency anemia. Braz J Med Biol Res 1997; 30: 849-854.
25. Sherman PM, Ossa JC, Johnson-Henry K. Unraveling mechanisms of action of probiotics. Nutr Clin Pract 2009; 24: 10-14.
26. Goldman WE, Baseman JB. Selective isolation and culture of a proliferating epithelial cell population from the hamster trachea. In Vitro 1980; 16: 313-319.
27. Brandhorst H, Friberg A, Andersson HH, Felldin M, Foss A, Salmela K, et al. The importance of tryptic-like activity in purified enzyme blends for efficient islet isolation. Transplantation 2009; 87: 370-375.
28. Vedina LA, Sennikov SV, Trufakin VA, Kozlov VA. Stem cells of small intestinal epithelium. Bull Exp Biol Med 2008; 145: 495-498.
29. Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982; 31: 11-24.
30. Arin MJ. The molecular basis of human keratin disorders. Hum Genet 2009; 125: 355-373.
31. Pageot LP, Perreault N, Basora N, Francoeur C, Magny P, Beaulieu JF. Human cell models to study small intestinal functions: recapitulation of the crypt-villus axis. Microsc Res Tech 2000; 49: 394-406.
32. Quinlan JM, Yu WY, Hornsey MA, Tosh D, Slack JM. In vitro culture of embryonic mouse intestinal epithelium: cell differentiation and introduction of reporter genes. BMC Dev Biol 2006; 6: 24.
33. Gorvel JP, Ferrero A, Chambraud L, Rigal A, Bonicel J, Maroux S. Expression of sucrase-isomaltase and dipeptidylpeptidase IV in human small intestine and colon. Gastroenterology 1991; 101: 618-625.
34. Gu N, Adachi T, Takeda J, Aoki N, Tsujimoto G, Ishihara A, et al. Sucrase-isomaltase gene expression is inhibited by mutant hepatocyte nuclear factor (HNF)-1alpha and mutant HNF-1beta in Caco-2 cells. J Nutr Sci Vitaminol 2006; 52: 105-112.
35. Li Q, Zhang Q, Wang C, Li Y, Li Y, Li N, et al. Alteration of tight junctions in intestinal transplantation induced by Campath-1H. Clin Immunol 2009; 132: 141-143.
36. Wang J, Lopez-Fraga M, Rynko A, Lo DD. TNFR and LTbetaR agonists induce follicle-associated epithelium and M cell specific genes in rat and human intestinal epithelial cells. Cytokine 2009; 47: 69-76.
37. Amin MR, Ghannad L, Othman A, Gill RK, Dudeja PK, Ramaswamy K, et al. Transcriptional regulation of the human Na+/H+ exchanger NHE3 by serotonin in intestinal epithelial cells. Biochem Biophys Res Commun 2009; 382: 620-625.
38. Haslam IS, Jones K, Coleman T, Simmons NL. Induction of P-glycoprotein expression and function in human intestinal epithelial cells (T84). Biochem Pharmacol 2008; 76: 850-861.
39. Freour T, Jarry A, Bach-Ngohou K, Dejoie T, Bou-Hanna C, Denis MG, et al. TACE inhibition amplifies TNF-alpha-mediated colonic epithelial barrier disruption. Int J Mol Med 2009; 23: 41-48.
40. Bandyopadhaya A, Das D, Chaudhuri K. Involvement of intracellular signaling cascades in inflammatory responses in human intestinal epithelial cells following Vibrio cholerae infection. Mol Immunol 2009; 46: 1129-1139.
Acknowledgments
The authors thank Shanghai Jiao Tong University Affiliated Sixth People’s Hospital for technical assistance during this study. Research supported by the National Natural Science Foundation of China (#30672044), and the National Basic Research Program of China (#2008CB517403).
 Address for correspondence: H. Qin, Department of Surgery, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, 600 Yishan Road, Shanghai 200233, China. Fax: +86-021-6436-8920. E-mail: hlqin@live.cn
Address for correspondence: H. Qin, Department of Surgery, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, 600 Yishan Road, Shanghai 200233, China. Fax: +86-021-6436-8920. E-mail: hlqin@live.cn
Received August 20, 2009. Accepted April 6, 2010. Available online April 30, 2010. Published May 14, 2010.
The Brazilian Journal of Medical and Biological Research is partially financed by
- 1. Bloushtain-Qimron N, Yao J, Shipitsin M, Maruyama R, Polyak K. Epigenetic patterns of embryonic and adult stem cells. Cell Cycle 2009; 8: 809-817.
- 2. Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest 2009; 119: 1159-1166.
- 3. Podolsky DK, Gerken G, Eyking A, Cario E. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology 2009; 137: 209-220.
- 4. Gradel KO, Nielsen HL, Schonheyder HC, Ejlertsen T, Kristensen B, Nielsen H. Increased short- and long-term risk of inflammatory bowel disease after Salmonella or Campylobacter gastroenteritis. Gastroenterology 2009; 137: 495-501.
- 5. Press B, Di Grandi D. Permeability for intestinal absorption: Caco-2 assay and related issues. Curr Drug Metab 2008; 9: 893-900.
- 6. Perreault N, Jean-François B. Use of the dissociating enzyme thermolysin to generate viable human normal intestinal epithelial cell cultures. Exp Cell Res 1996; 224: 354-364.
- 7. Podolsky DK. Regulation of intestinal epithelial proliferation: a few answers, many questions. Am J Physiol 1993; 264: G179-G186.
- 8. Fukamachi H, Narita T, Yahagi N, Takeda H, Ichinose M. Endothelin-3 controls growth of colonic epithelial cells by mediating epithelial-mesenchymal interaction. Dev Growth Differ 2005; 47: 573-580.
- 9. Evans GS, Flint N, Potten CS. Primary cultures for studies of cell regulation and physiology in intestinal epithelium. Annu Rev Physiol 1994; 56: 399-417.
- 10. Rousset M. The human colon carcinoma cell lines HT-29 and Caco-2: two in vitro models for the study of intestinal differentiation. Biochimie 1986; 68: 1035-1040.
- 11. Whitehead RH, van Eeden PE. A method for the prolonged culture of colonic epithelial. Methods Cell Sci 2005; 13: 103-106.
- 12. Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol 1979; 80: 248-265.
- 13. Beaulieu JF, Vachon PH. Reciprocal expression of laminin A-chain isoforms along the crypt-villus axis in the human small intestine. Gastroenterology 1994; 106: 829-839.
- 14. Wu CC, Hsu CW, Chen CD, Yu CJ, Chang KP, Tai DI, et al. Candidate serological biomarkers for cancer identified from the secretomes of 23 cancer cell lines and the human protein atlas. Mol Cell Proteomics 2010 (in press).
- 15. Moberg KH, Bell DW, Wahrer DC, Haber DA, Hariharan IK. Archipelago regulates cyclin E levels in Drosophila and is mutated in human cancer cell lines. Nature 2001; 413: 311-316.
- 16. Germain L, Rouabhia M, Guignard R, Carrier L, Bouvard V, Auger FA. Improvement of human keratinocyte isolation and culture using thermolysin. Burns 1993; 19: 99-104.
- 17. Grossmann J, Walther K, Artinger M, Kiessling S, Steinkamp M, Schmautz WK, et al. Progress on isolation and short-term ex-vivo culture of highly purified non-apoptotic human intestinal epithelial cells (IEC). Eur J Cell Biol 2003; 82: 262-270.
- 18. Ma Y, Peng J, Liu W, Zhang P, Huang L, Gao B, et al. Proteomics identification of desmin as a potential oncofetal diagnostic and prognostic biomarker in colorectal cancer. Mol Cell Proteomics 2009; 8: 1878-1890.
- 19. Kalabis J, Li G, Fukunaga-Kalabis M, Rustgi AK, Herlyn M. Endothelin-3 stimulates survival of goblet cells in organotypic cultures of fetal human colonic epithelium. Am J Physiol Gastrointest Liver Physiol 2008; 295: G1182-G1189.
- 20. Vachon PH, Beaulieu JF. Transient mosaic patterns of morphological and functional differentiation in the Caco-2 cell line. Gastroenterology 1992; 103: 414-423.
- 21. Calvert R, Millane G, Pothier P, Beaulieu JF. An intestinal secretory protein is found in most glands associated with the gastrointestinal tract: von Ebner’s and salivary glands, gallbladder, and pancreas. J Histochem Cytochem 1993; 41: 1223-1231.
- 22. Golaz JL, Vonlaufen N, Hemphill A, Burgener IA. Establishment and characterization of a primary canine duodenal epithelial cell culture. In Vitro Cell Dev Biol Anim 2007; 43: 176-185.
- 23. Beaulieu JF, Millane G, Calvert R. Developmental expression of two antigens associated with mouse intestinal crypts. Dev Dyn 1992; 193: 325-331.
- 24. Fernandes MI, Galvao LC, Bortolozzi MF, Oliveira WP, Zucoloto S, Bianchi ML. Disaccharidase levels in normal epithelium of the small intestine of rats with iron-deficiency anemia. Braz J Med Biol Res 1997; 30: 849-854.
- 25. Sherman PM, Ossa JC, Johnson-Henry K. Unraveling mechanisms of action of probiotics. Nutr Clin Pract 2009; 24: 10-14.
- 26. Goldman WE, Baseman JB. Selective isolation and culture of a proliferating epithelial cell population from the hamster trachea. In Vitro 1980; 16: 313-319.
- 27. Brandhorst H, Friberg A, Andersson HH, Felldin M, Foss A, Salmela K, et al. The importance of tryptic-like activity in purified enzyme blends for efficient islet isolation. Transplantation 2009; 87: 370-375.
- 28. Vedina LA, Sennikov SV, Trufakin VA, Kozlov VA. Stem cells of small intestinal epithelium. Bull Exp Biol Med 2008; 145: 495-498.
- 29. Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982; 31: 11-24.
- 30. Arin MJ. The molecular basis of human keratin disorders. Hum Genet 2009; 125: 355-373.
- 31. Pageot LP, Perreault N, Basora N, Francoeur C, Magny P, Beaulieu JF. Human cell models to study small intestinal functions: recapitulation of the crypt-villus axis. Microsc Res Tech 2000; 49: 394-406.
- 32. Quinlan JM, Yu WY, Hornsey MA, Tosh D, Slack JM. In vitro culture of embryonic mouse intestinal epithelium: cell differentiation and introduction of reporter genes. BMC Dev Biol 2006; 6: 24.
- 33. Gorvel JP, Ferrero A, Chambraud L, Rigal A, Bonicel J, Maroux S. Expression of sucrase-isomaltase and dipeptidylpeptidase IV in human small intestine and colon. Gastroenterology 1991; 101: 618-625.
- 34. Gu N, Adachi T, Takeda J, Aoki N, Tsujimoto G, Ishihara A, et al. Sucrase-isomaltase gene expression is inhibited by mutant hepatocyte nuclear factor (HNF)-1alpha and mutant HNF-1beta in Caco-2 cells. J Nutr Sci Vitaminol 2006; 52: 105-112.
- 35. Li Q, Zhang Q, Wang C, Li Y, Li Y, Li N, et al. Alteration of tight junctions in intestinal transplantation induced by Campath-1H. Clin Immunol 2009; 132: 141-143.
- 36. Wang J, Lopez-Fraga M, Rynko A, Lo DD. TNFR and LTbetaR agonists induce follicle-associated epithelium and M cell specific genes in rat and human intestinal epithelial cells. Cytokine 2009; 47: 69-76.
- 37. Amin MR, Ghannad L, Othman A, Gill RK, Dudeja PK, Ramaswamy K, et al. Transcriptional regulation of the human Na+/H+ exchanger NHE3 by serotonin in intestinal epithelial cells. Biochem Biophys Res Commun 2009; 382: 620-625.
- 38. Haslam IS, Jones K, Coleman T, Simmons NL. Induction of P-glycoprotein expression and function in human intestinal epithelial cells (T84). Biochem Pharmacol 2008; 76: 850-861.
- 39. Freour T, Jarry A, Bach-Ngohou K, Dejoie T, Bou-Hanna C, Denis MG, et al. TACE inhibition amplifies TNF-alpha-mediated colonic epithelial barrier disruption. Int J Mol Med 2009; 23: 41-48.
- 40. Bandyopadhaya A, Das D, Chaudhuri K. Involvement of intracellular signaling cascades in inflammatory responses in human intestinal epithelial cells following Vibrio cholerae infection. Mol Immunol 2009; 46: 1129-1139.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
07 May 2010 -
Date of issue
May 2010
History
-
Accepted
06 Apr 2010 -
Received
20 Aug 2009