Transcatheter aortic valve implantation (TAVI) has become an established treatment option for patients with severe, symptomatic aortic stenosis at high or intermediate surgical risk.1–4 From the very beginning of TAVI technology, valve preparation by performing pre-implantation balloon aortic valvuloplasty (pre-BAV) has been considered a necessary step to facilitate device implantation and ensure optimal valve expansion.5 However, as a result of procedural evolution over time and increasing operator experience, direct TAVI (without predilatation) has recently emerged as a new option to simplify the procedure and to avoid potential BAV-related complications (cerebrovascular events, conduction disturbances, severe acute aortic regurgitation and even annular rupture).6–9 After the first pioneering experience of Grube et al.,10 several observational studies and one small randomised study have evaluated procedural and clinical outcomes of TAVI with and without predilatation (Table 1). Therefore, this review aims to discuss the advantages of both direct TAVI and standard TAVI with pre-BAV, in light of the current published evidence.
Direct TAVI: Moving Toward a Simplified Approach
In an effort to obtain a more simplified and straightforward procedure, direct TAVI has increasingly been studied and performed (Table 1). Recent analysis of the UK TAVI Registry has shown a decreasing trend in the proportion of TAVI patients undergoing predilatation between 2007 and 2014; moreover, among centres with high experience (>200 TAVI performed), the rate of direct TAVI was around 50 %, decreasing to 11 % among centres with low experience (1–50 TAVI performed).11 These findings confirm the interest of more experienced operators in exploring a direct TAVI approach; simultaneously, they raise an interesting question regarding the potential (or demonstrated) advantages of removing predilatation.
Procedural Time and Contrast Use
As a logical result of removing one procedural step, this approach leads to shorter procedural time and lower total contrast volume use than TAVI with pre-BAV.12,13 This aspect may represent an interesting advantage for patients in whom a longer procedural time may be undesired, such as hemodynamically unstable patients, patients with severe left or right ventricular dysfunction or severe pulmonary hypertension (who, in addition, may not tolerate rapid pacing performed during balloon valvuloplasty). Furthermore, subjects at higher risk of contrast-induced nephropathy (e.g. those with pre-existing chronic kidney disease or diabetes mellitus) may benefit from lower contrast use during the procedure.14
Safety and Feasibility of Direct TAVI
The omission of one procedural step may be justified only if the simplified approach is demonstrated to be feasible, safe and not associated with adverse outcomes. The largest study exploring postprocedural outcomes after direct TAVI is the abovementioned UK TAVI Registry, that included 5,887 patients undergoing TAVI with vs without pre-BAV. In that study, direct TAVI was not associated with a higher rate of adverse short-term outcomes, especially when using the balloon-expandable SAPIEN (Edwards Lifesciences Inc., Irvine, CA) valve. For the self-expanding CoreValve (Medtronic, Minneapolis, MN) prosthesis, the use of predilatation was associated with lower odds of valve dysfunction before correction for multiple testing; however, this finding was not confirmed after multiplicity correction.11 These results are in line with a recent small prospective study that found similar early outcomes among 60 patients randomised to balloon-expandable TAVI (SAPIEN XT) with vs without predilatation.15 Moreover, recent large observational studies (including both balloon-expandable and selfexpanding valves) have confirmed that direct TAVI is associated with similar mid-term clinical outcomes compared to TAVI with pre-BAV.16–18 These real-world data, along with recent meta-analyses,12,13,19 suggest that simplified TAVI without predilatation is feasible, safe and achieves comparable clinical outcomes to standard TAVI with predilatation, justifying future research in this field.
Conduction Disturbances
The omission of pre-BAV SAPIEN may theoretically reduce the iatrogenic damage to the conduction system, leading to a lower risk of conduction disturbances and, consequently, of new permanent pacemaker implantation (PPI) after TAVI. Indeed, new conduction disturbances may not only occur during valve implantation, but also during the predilatation step, especially in the case of high balloon/aortic annulus ratio.20 Lange et al. have shown that moderate predilatation performed with smaller valvuloplasty balloons is associated with a reduced rate of PPI after CoreValve implantation; therefore, the authors proposed a two-hit model, where the first hit to the conduction system is given by a large valvuloplasty balloon and is usually insufficient to determine an advanced conduction disturbance, whereas the second hit is given by valve deployment and may eventually lead to the final damage requiring PPI.21 In line with these considerations, a recent analysis of the Brazilian Transcatheter Aortic Valve Replacement registry has reported that avoiding pre-BAV reduces the rate of new-onset persistent left bundle branch block after TAVI, particularly when implanting a CoreValve prosthesis.16 Although that study failed to demonstrate an association between omission of pre-BAV and reduction of PPI, recent meta-analyses and the large UK TAVI registry suggest a signal towards lower PPI rate with direct TAVI.11–13 Nevertheless, as the decision to perform pre-BAV was left to the operator’s discretion in most published studies, a significant selection bias may have influenced these findings; only large randomised studies will clarify the impact of predilatation on conduction disturbances and PPI rate after TAVI.
Pre-procedural Imaging Planning
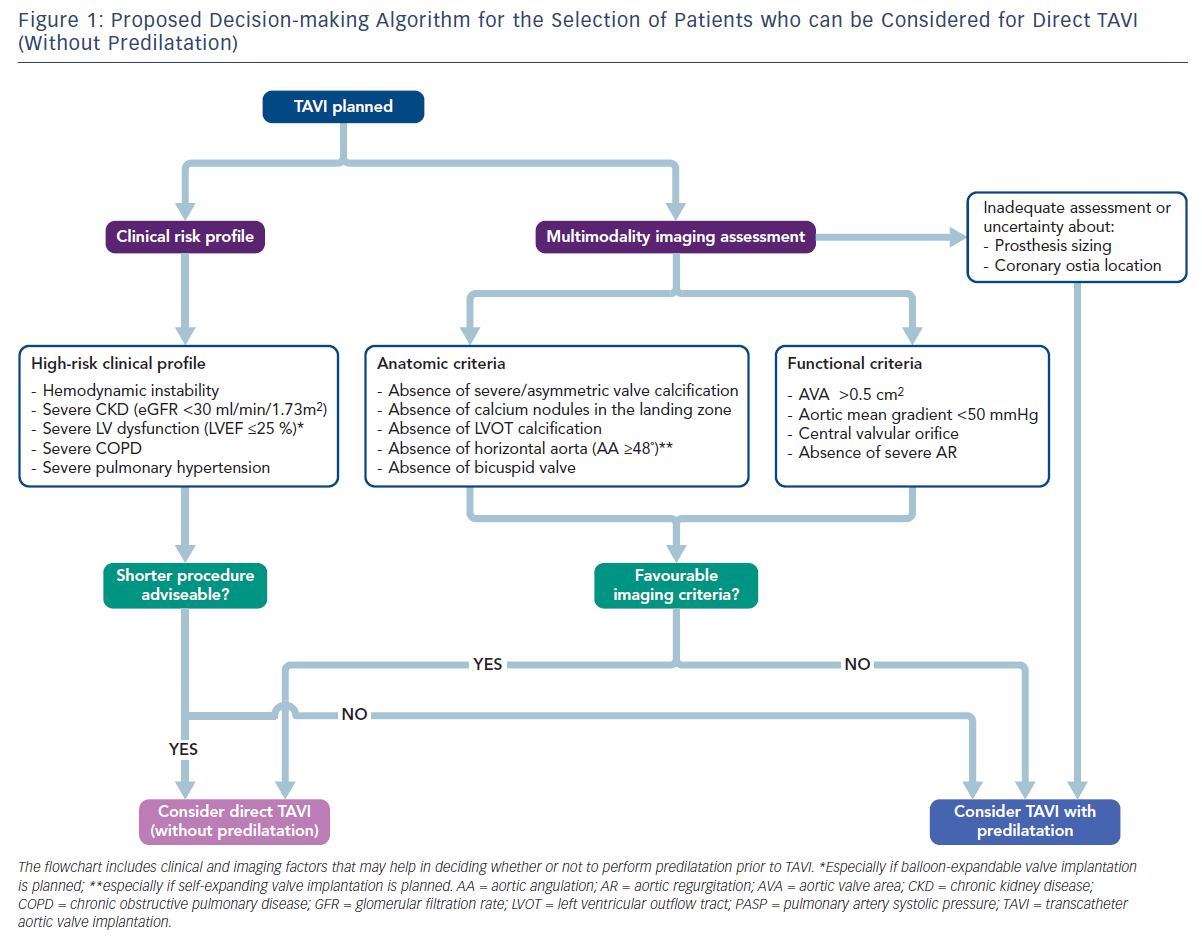
In the absence of definitive conclusions on the usefulness of predilatation during TAVI, the optimal strategy is probably to tailor the procedure to the individual patient. In daily practice, the interventional cardiologist should take into account all potential advantages of omitting pre-BAV and, therefore, avoiding its related complications (conduction disturbances, the risk of mobilising aortic debris potentially embolising to the brain, severe acute aortic regurgitation leading to haemodynamic compromise, and even annular rupture);6–9,22 however, in some patients predilatation may still be useful to optimise valve implantation and should be considered when planning the procedure. In this context, pre-procedural imaging techniques may be pivotal in deciding whether to predilate or not before TAVI. A recent study evaluated the usefulness of transoesophageal echocardiography (TEE) to select ideal candidates for direct TAVI with both balloonexpandable (SAPIEN XT) and self-expanding (CoreValve) prostheses. Among patients fulfilling all the proposed TEE criteria (valve area >0.4 cm2; central orifice; absence of severe valve calcification; mobility of aortic cusps not severely restricted; no left ventricular outflow tract calcification; absence of calcium nodules in the landing zone; absence of severe aortic regurgitation), TAVI without pre-BAV was performed with a high rate of success and low rates of postdilatation, paravalvular aortic regurgitation and PPI implantation.23 Combining anatomical and functional information obtained with a multimodality approach (TEE and multidetector computed tomography [MDCT]) could represent the ideal strategy to identify the subset of patients who can safely undergo simplified TAVI without predilatation.
TAVI with Predilatation: Potential Advantages of ‘Preparing the Ground’
A main concern regarding the omission of predilatation is the risk of technical challenges during TAVI. Pre-BAV may facilitate valve crossing and delivery of the prosthesis across the stenotic valve (especially in patients with very small aortic valve area), ensure optimal valve expansion (by reducing radial counterforces) and improve haemodynamic stability during valve deployment. Interestingly, a range of technical difficulties has been reported when adopting a direct TAVI approach, including haemodynamic instability during device positioning in severely stenotic valves, severe underexpansion of the prosthesis in heavily calcified valves, inability to cross the valve, trapping of the prosthesis inside the left ventricle and coaxiality issues.16 Furthermore, as already mentioned, the UK TAVI registry showed a signal towards increased valve dysfunction among patients receiving a CoreValve prosthesis without pre-BAV (a finding that was, however, not confirmed after Bonferroni correction for multiple testing).11 Although bailout predilatation may represent an option to overcome pre-implantation technical issues,16,24,25 careful selection of patients needing pre-BAV before TAVI is advisable to avoid the risk of severe procedural complications. Pre-procedural planning by means of combined imaging techniques (TEE and MDCT) allows the identification of basic anatomical features in which a standard TAVI approach (with predilatation) should be considered, such as severe or asymmetric aortic valve calcification, small aortic valve area (<0.5 cm2), horizontal aorta (especially when implanting self-expanding prostheses) or bicuspid aortic valve anatomy.18,23,26–29
Pre-BAV may be very helpful also in situations where prosthesis sizing is not completely established or the potential risk of coronary obstruction requires further assessment (especially when coronary artery takeoffs are low).30 As MDCT has become the standard technique for valve sizing and coronary ostia evaluation before TAVI, these two indications are limited to a very low number of patients in the contemporary TAVI era; nonetheless, pre-BAV represents a rescue option for patients in whom MDCT cannot be performed or is not conclusive.
Cerebral Embolic Risk
One of the proposed advantages of direct TAVI is to avoid the risk of debris embolisation during balloon valvuloplasty, potentially resulting in a lower rate of cerebrovascular events.10 However, a recent study evaluating cerebral ischemic lesions after TAVI has suggested the opposite idea. Bijuklic et al. have evaluated covert brain lesions detected by diffusion-weighted magnetic resonance imaging after TAVI in patients undergoing balloon-expandable valve (SAPIEN XT or SAPIEN 3) implantation with or without predilatation. The total lesion volume of cerebral ischemic lesions was significantly higher among patients undergoing direct TAVI; the incidence and number of cerebral lesions were only numerically (not significantly) higher in the direct TAVI group.31 These results seem in line with a recent study reporting a higher 30-day overt stroke rate in patients undergoing direct TAVI, especially when implanting self-expanding (CoreValve or Evolut R) prostheses; however, this finding was not confirmed after propensity score matching.17 The idea of a higher risk of cerebral damage when performing TAVI without predilatation seems counterintuitive as balloon dilatation of the aortic valve may per se be complicated by post-procedural stroke6,7,22,32 and one may suppose that pre-BAV before TAVI may confer an additive risk of neurological events. Conversely, it can be speculated that balloon inflation fragments calcific debris present on a heavily degenerated and diseased aortic valve, stabilises such debris through a homogeneous apposition onto aortic leaflets and the ascending aortic wall, and reduces the size of pieces that embolise during subsequent valve implantation. Studies evaluating transcranial Doppler-detected cerebral embolic load during TAVI have shown that only a limited number of high-intensity transient signals (a surrogate for microembolisation) occur during pre-BAV, while prosthesis positioning and deployment are the two phases associated with the highest embolic load,33–35 indirectly suggesting that the predilatation step does not significantly increase the cerebral embolic risk of the TAVI procedure. However, most published studies have shown a similar rate of clinical strokes in TAVI with and without pre-BAV11,12,15 and the reason for the higher risk of covert cerebral injury reported in the study of Bijuklic et al. remains completely speculative.31 Only future large, dedicated studies will definitively clarify the impact of predilatation on the risk of overt/covert cerebral injury after TAVI, especially if investigating the cerebral embolic load during all procedural steps.
Paravalvular Leakage
An expected limitation of direct TAVI is the risk of suboptimal valve expansion, potentially resulting in higher incidence and severity of residual paravalvular leakage (PVL) after the procedure. The occurrence of PVL immediately after valve implantation may lead to an increased need for postdilatation, a step that may theoretically impair prosthesis durability and that has been specifically associated with a higher risk of cerebrovascular events.36–38 In line with these considerations, a recent study has reported a higher rate of postdilatation in patients undergoing direct transfemoral TAVI,17 a finding that was confirmed in a meta-analysis.12 Unfortunately, the largest available study did not evaluate the need for postdilatation,11 preventing us from drawing more robust conclusions on this procedural aspect. With respect to the impact of direct TAVI on significant PVL, most published studies have reported similar rates of residual moderate or severe PVL after TAVI with or without predilatation (Table 1), including the large UK TAVI registry.11 Interestingly, two meta-analyses have suggested a reduced risk of moderate or severe PVL with direct TAVI (a result that was, however, not confirmed in a subanalysis evaluating only transfemoral TAVI).12,13 Although this counterintuitive finding seems to suggest an improved valve positioning with direct TAVI, the retrospective nature of the studies included may have determined a relevant selection bias. The choice of a predilatation strategy was left to the operators’ discretion, therefore it is likely that direct TAVI was performed more frequently in patients with favourable anatomical characteristics and the absence of severe or asymmetric aortic valve calcification. As the quantity and asymmetry of aortic valve calcification predict the severity of post-procedural PVL,39–44 this selection bias is of paramount importance. Moreover, other confounders such as device improvements and increasing the operator’s experience may have influenced these analyses. In the absence of high-quality, robust evidence on the incidence and severity of PVL after direct TAVI, definitive conclusions cannot be drawn; however, as PVL negatively affects clinical outcomes after TAVI,3,45–54 future large, randomised studies are needed to further address the risk of PVL when performing TAVI with or without predilatation.
‘Moderate’ Predilatation
An interesting option that has been recently proposed is performing TAVI with a moderate predilatation, i.e. prior balloon valvuloplasty with a smaller balloon size. This approach could preserve the advantages of pre-BAV in ‘preparing the ground’ before valve implantation, avoiding the risks of predilatation with a standard or larger balloon. As already mentioned, Lange et al. have shown that moderate predilatation (use of valvuloplasty balloons with ≤23 mm diameter) reduces the rate of PPI in patients undergoing TAVI with the CoreValve prosthesis, without affecting procedural success.21 Although the use of smaller balloons may conceivably reduce the iatrogenic damage to the conduction system, a concern regarding moderate pre-BAV is impaired valve expansion, mainly when using self-expanding devices and in cases of severe valve calcification. Moreover, recent studies have reported similar procedural success and clinical outcomes after TAVI with moderate predilatation (average balloon diameter of 15–16 mm; mean balloon diameter/MDCT mean annulus diameter ratio of 0.62–0.65) compared with direct TAVI with balloon-expandable valves.18,27 Of note, valve repositioning during implantation of newer self-expanding devices could arguably represent a form of mild predilatation; however, the relative role of this step with respect to pre-BAV is currently unknown. Future large, dedicated studies will better evaluate the potential advantages of moderate pre-BAV over standard or larger pre-BAV or direct TAVI in terms of procedural and clinical outcomes.
Conclusion
A growing interest in direct TAVI (without predilatation) has emerged during the last few years in an effort to obtain a more simplified procedure and to avoid the potential complications associated with pre-BAV. Recent real-world observational experiences and a small randomised study suggest that direct TAVI is safe, feasible and associated with outcomes similar to standard TAVI with pre-BAV (Table 1). In the absence of strong available evidence, several clinical and anatomical characteristics may help the interventional cardiologist in deciding whether or not to predilate before TAVI (Figure 1). However, future studies are needed to deeply investigate the safety and outcomes of direct TAVI and to identify the right subset of patients who can safely undergo this simplified approach. The results of ongoing dedicated randomised trials (The preDIlatation in tRanscathEter aortiC Valve implanTation Trial [DIRECT], NCT02448927; Implantation of the Transcatheter Aortic Prosthesis SAPIEN 3 With or Without Prior Balloon Predilatation [DIRECTAVI], NCT02729519; Transcatheter Aortic Valve Implantation Without Predilation [SIMPLIFy TAVI], NCT01539746) and prospective multicentre registries (Transfemoral Transcatheter Aortic Valve Implantation With or Without Predilation of the Aortic Valve [EASE-IT TF],55 NCT02760771; Balloon Expandable Transcatheter Aortic Valve Implantation Without Predilation of the Aortic Valve [EASE-IT],56 NCT02127580) will provide additional evidence on these unresolved issues.








