-
PDF
- Split View
-
Views
-
Cite
Cite
Guillaume Chevrel, Anne‐Marie Schott, Elisabeth Fontanges, Jeanne E Charrin, Geneviève Lina‐Granade, François Duboeuf, Patrick Garnero, Monique Arlot, Claude Raynal, Pierre J Meunier, Effects of Oral Alendronate on BMD in Adult Patients With Osteogenesis Imperfecta: A 3‐Year Randomized Placebo‐Controlled Trial, Journal of Bone and Mineral Research, Volume 21, Issue 2, 1 February 2006, Pages 300–306, https://doi.org/10.1359/JBMR.051015
Close - Share Icon Share
Abstract
A 3‐year, randomized, double‐blind, placebo‐controlled trial evaluated the effect of oral alendronate on the BMD of 64 adult patients with osteogenesis imperfecta. The mean increases in the lumbar spine BMD were 10.1 ± 9.8% (p < 0.001) and 0.7 ± 5.7% in the alendronate and placebo groups, respectively. Oral alendronate increases BMD in adult patients with osteogenesis imperfecta.
Introduction: This study evaluated the effect of oral alendronate on the BMD of adult patients with osteogenesis imperfecta.
Materials and Methods: We carried out a 3‐year, randomized, double‐blind, placebo‐controlled trial of oral alendronate in 64 adult patients with osteogenesis imperfecta. The primary endpoint was the difference between the groups in the mean percent change in lumbar spine BMD at 3 years. Secondary outcomes included changes in BMD of total hip, vertebral and peripheral fracture incidence, pain, hearing loss, and bone turnover biochemical markers. Patients were treated daily with either placebo or 10 mg alendronate. All received 1 g of calcium and 800 IU of vitamin D daily.
Results: The mean ± SD increases in the lumbar spine BMD were 10.1 ± 9.8% (p < 0.001) and 0.7 ± 5.7% in the alendronate and placebo groups, respectively. Hip BMD increased in the alendronate group by 3.3 ± 0.5% (p = 0.001) and decreased in the placebo group by 0.3 ± 0.6%. The sample size was not sufficient to determine an effect of alendronate on fracture rate. A significant increase of the pain score was noted in the alendronate group (p = 0.04) in the intent‐to‐treat analysis but not in the per protocol analysis. There was no change in hearing in either group. Bone resorption and formation biochemical markers were significantly decreased in the alendronate group (p < 0.001). There were no differences in severe adverse effects between the groups, but there was an increase in nonsevere upper gastrointestinal effects in the alendronate group (p = 0.003).
Conclusions: Oral alendronate increases BMD and increase nonsevere gastrointestinal adverse effects but does not modify the hearing loss in adult patients with osteogenesis imperfecta. More studies are needed to evaluate an effect on the fracture rate.
INTRODUCTION
OSTEOGENESIS IMPERFECTA IS a rare heritable disorder of connective tissue characterized by bone fragility and osteopenia. (1, 2) Almost all cases of osteogenesis imperfecta are caused by mutations in one of the two genes that encode collagen type I α chains, COL1A1 and COL1A2. (3)
Among bisphosphonates, the effects of intravenous pamidronate have been mainly evaluated in children in several open trials. (4–12) More recently, olpadronate was evaluated in children in a randomized, double‐blind, 2‐year, placebo‐controlled trial. (13) This study showed a decrease of the peripheral fracture rate. In adult patients, fewer trials have been completed, and no randomized, double‐blind, placebo‐controlled trial is available. Only two open studies have been performed with an intravenous bisphosphonate. (14, 15) The main outcomes of the first study, involving eight adult patients with type IA osteogenesis imperfecta, were to determine the effect of treatment every 3 months with intravenous pamidronate on BMD and bone histomorphometry. (14) The second study, a 2‐year, randomized, controlled‐open trial of neridronate included 42 adult patients. (15) The control patients started neridronate treatment after 12 months of continued controlled calcium and vitamin D intake. Moreover, in adults, no study evaluating an oral bisphosphonate has been published.
Alendronate is a potent bisphosphonate that has been shown capable of increasing BMD of the spine and hip and lowering the incidence of vertebral, hip, and forearm fractures by ∼50 percent in postmenopausal women with osteoporosis. (16–18) In men with osteoporosis, alendronate increased spine and hip BMD. (19)
From April 1999 to October 2003, we initiated this 3‐year, randomized, double‐blind, placebo‐controlled trial of oral alendronate in adult patients with osteogenesis imperfecta. BMD of the lumbar spine was chosen as the primary endpoint. We did not consider the fracture rate as the primary endpoint, because in adult patients with osteogenesis imperfecta, the incidence of fractures that decreases after puberty remains low until menopause in women and the age of 70 in men. (20) The study also evaluated as secondary endpoints BMD of the hip, incidence of vertebral and peripheral fractures, pain index, hearing loss, and changes of biochemical markers of bone turnover.
MATERIALS AND METHODS
Patients
Sixty‐four patients (39 men and 25 women, pre‐ or postmenopausal) who were ≥20 years old were included in the study. Osteogenesis imperfecta was defined by the association of at least three criteria:
1. A typical personal history of bone fragility fractures (fractures in absence of car crash or major trauma) before 20 years of age with at least three fragility fractures
2. At least one criterion in the following list: blue sclerae, scoliosis (Cobb method and angle ≥10° but ≤40°), hearing loss defined by elevated pure‐tone thresholds >20 dB on at least two octave frequencies or mean Rinne >10 dB, abnormal laxity of ligaments defined by at least three criteria of Carter and Wilkinson, (21) dentinogenesis imperfecta defined by at least a yellow to brown tooth, and at least one family member with osteogenesis imperfecta
3. Low BMD of the lumbar spine or hip (total femur) measured by DXA with a T score −2.5 or below at one site
The exclusion criteria included evidence of concomitant malignant disease, primary hyperparathyroidism, osteomalacia, or other generalized bone diseases or disorders known to influence bone metabolism; abnormality of the esophagus delaying barium swallow, such as stenosis and achalasia; impossibility to remain seated or standing for <30 minutes; wheelchair bound; allergy to bisphosphonates; renal clearance <35 ml/min; pregnancy or lactation; bilateral hip prosthesis; glucocorticoids (≥5 mg of prednisone or equivalent/day), anabolic steroid, or calcitonin taken during the month before the study started; and estrogen replacement therapy, bisphosphonates, or fluoride salts during the 6 months before start of the study.
Mutations of the genes COLIA1 or COLIA2 have been determined in each patient by J Korkko and D Prockop from the Tulane Center for Gene Therapy (New Orleans, LA, USA). In 57 patients, the mutation was found.
The Ethic Committee (Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale‐Lyon B) approved the protocol and all patients gave written informed consent.
Treatment
Thirty‐one patients were randomly assigned to receive 10 mg of oral alendronate daily as a tablet taken 30 minutes before breakfast, and 33 patients received a matching placebo daily. Fifty‐eight patients were treated for 3 years. In addition, each patient received 1000 mg elemental calcium and 800 IU of vitamin D3 daily. A computer‐generated randomization list was drawn up by the pharmacy department of the Edouard Herriot Hospital. The researchers responsible for seeing the patients allocated the next available number on entry into the trial. The evaluation of alendronate treatment compliance was done by counting the number of tablets returned each year by the patients.
Measurements
The patients were seen at baseline and during the 3 years of the study: every year in Lyon with intermediary visits at months 6, 18, and 30 by their local physician. All study personnel and participants were blinded to treatment assignment for the duration of the study until the end of the data control for analysis.
The self‐reported baseline and final dietary calcium intake were estimated from a questionnaire that inquired about the consumption of various foods. (22) BMD of the lumbar spine and of both hips (total femur) was measured at baseline and at 12, 24, and 36 months by DXA with an Hologic device (Waltham, MA, USA). The same densitometer was used over the course of the study. With this device, the CVs were 0.86% for lumbar spine BMD and 1.04% for total hip BMD. Radiographs of the spine (anteroposterior and lateral views) were obtained at baseline and 36 months. Prevalent vertebral fractures were identified and graded by a semiquantitative scale. (23) Every new peripheral fracture was confirmed by X‐ray during the trial. Overall pain score and not only bone pain score was evaluated at baseline and every 6 months during 3 years with a visual analog scale score (0‐10). Each patient underwent audiometry and impedancometry at baseline and 36 months. The mean difference between air and bone thresholds at 250, 500, and 1000 Hz was calculated for each ear and named “mean Rinne.” Impedancometry included pure‐tone contralateral acoustic reflex threshold measurements. Patients with abnormal tympanometry (e.g., otitis media with effusion) were excluded of the hearing loss analysis. Serum and urine samples, obtained at baseline and 12, 24, and 36 months for measurement of biochemical markers of bone turnover (serum and urinary C‐telopeptides of type I collagen for bone resorption and serum N‐propeptide of type I collagen and serum osteocalcin for bone formation), were collected at a standardized time of day and were analyzed by Synarc laboratory (Lyon, France) in batches at the end of the study. Serum PTH, serum 25OH vitamin D concentrations, and serum and urinary calcium were collected and measured at baseline and 12, 24, and 36 months. Transiliac bone biopsies after tetracycline double labeling were taken at 36 months in five patients (two from the alendronate group and three from the placebo group).
Statistical analysis
The primary efficacy endpoint was the difference between groups in the percent change in lumbar spine BMD. Sample size was estimated on the basis of an expected difference of 0.07 g/cm2 between groups and an SD of 0.11 g/cm2 with an α risk of 0.05 and a β risk of 0.20 and under a unilateral hypothesis. This was 68 patients with 34 per group. Secondary efficacy endpoints were the percent changes in hip BMD, incidence of fractures, pain, hearing loss, and biochemical markers of bone turnover. The target sample size was not attained because of difficulties in recruiting subjects. The duration for recruitment was initially 1 year but was extended to 1.5 years.
Baseline characteristics of both groups were described using frequency for binary variables, mean, and SD for clinical continuous variables with Gaussian distribution. The distribution of biological parameters, not being Gaussian, were described by their median value and range. To avoid using too many statistical tests, only some of these characteristics, which had been determined a priori as the most likely to be confounding factors, were compared between groups with statistical tests. χ2 tests were used for binary variables. For continuous variables, t‐tests were performed to compare the mean values for clinical factors, and Wilcoxon tests were performed for biochemical parameters.
To estimate the efficacy of treatment, percent changes were calculated for continuous variables as the difference between baseline and 3‐year follow‐up over baseline value. Percent changes were calculated for lumbar spine BMD, the primary efficacy endpoint, for both hips BMD, and for biological parameters (calciotropic hormones and chemical markers of bone turnover). Mean percent changes were compared between groups with Student t‐tests. BMD values were not adjusted for the difference of baseline serum 25OH vitamin D, the only parameter significantly different between the two groups. To describe baseline characteristics of the paired organs (i.e., hip BMD and audiometry), separate analyses were performed. To estimate the percent change over time, values of right and left hip BMD were pooled at baseline and compared with pooled values at the 3‐year follow‐up visit. The same procedures were applied to audiometry. We did not take into account the fact that the paired organs were the left or the right one or the fact that two hips corresponded to the same patient. Pain was assessed with a visual analog scale score (0‐10), and for those patients scoring 0, the percent change from baseline could not be calculated. We thus calculated the absolute difference between values at baseline and values at 3‐year follow‐up instead of percent changes and compared those between groups with Wilcoxon tests. To estimate treatment efficacy on the risk of peripheral fractures, taking into account potential confounders, adjusted relative risks were estimated from a multivariate Cox proportional hazard model.
All analyses were performed using intent‐to‐treat criteria. For pain assessment, a per protocol analysis was also conducted. Analyses were performed with the use of SAS (SAS, Cary, NC, USA).
RESULTS
Sixty‐four patients were randomized and blindly allocated to either alendronate or placebo. The characteristics at baseline of the 64 patients are shown in Tables 1 and 2. None of them except serum 25OH vitamin D concentrations (p < 0.02) was significantly different between the alendronate and placebo groups. Serum 25OH vitamin D concentrations were lower in the placebo group. No women went through menopause during the study. BMD follow‐up was complete for 62 randomized patients, including the 3 in the alendronate group and 1 in the placebo group who withdrew from the study for personal reasons without drug‐related adverse effects.
Baseline Characteristics of the Study Patients: Main Efficacy and Secondary Efficacy Endpoints, Serum Parathormone, Serum 25OH Vitamin D Concentrations, and Urinary Calcium
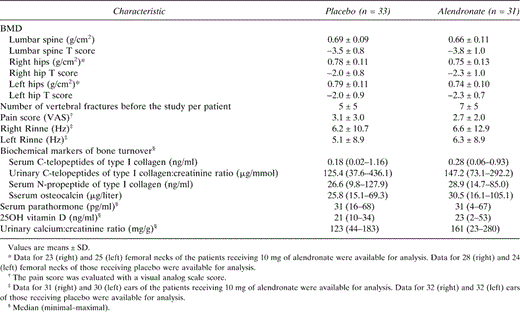
Baseline Characteristics of the Study Patients: Main Efficacy and Secondary Efficacy Endpoints, Serum Parathormone, Serum 25OH Vitamin D Concentrations, and Urinary Calcium

BMD
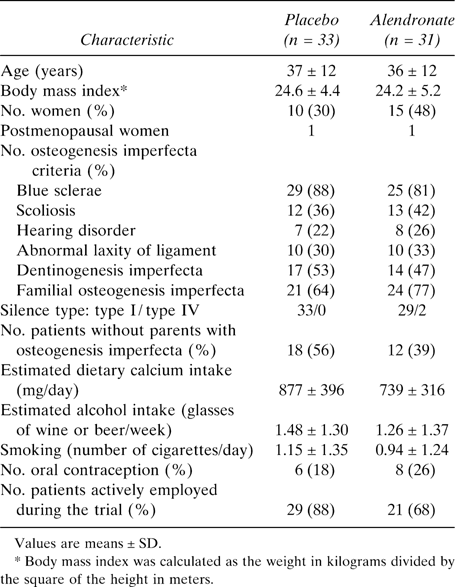
Lumbar spine BMD, the primary efficacy endpoint, was not different at baseline between the groups. At 36 months, BMD was significantly higher in the alendronate group than in the placebo group, with an absolute difference of 0.058 g/cm2, representing a difference of +9.4 ± 2.0% between groups (Fig. 1; Table 3). In the placebo group, the mean observed change of lumbar spine BMD over 3 years was 0.003 ± 0.039 (SD) g/cm2, representing an increase of +0.7 ± 5.7%, which was not statistically significant. In the alendronate group, the increase was much higher, reaching 10.1 ± 9.8% (i.e., an absolute change of 0.061 ± 0.041 g/cm2; p < 0.001). The BMD increase from baseline in the alendronate group was significant after 1 year (+7.5%; p < 0.001) and continued throughout the 3 years of the study without reaching a plateau, although the increase was much greater during the first year (Fig. 1).
Comparisons at 36 months Between Groups in the Changes of Main Efficacy and Secondary Efficacy Endpoints
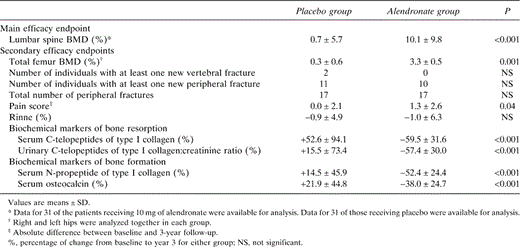
Comparisons at 36 months Between Groups in the Changes of Main Efficacy and Secondary Efficacy Endpoints

Effects of alendronate on lumbar and total femur BMD measured by DXA in adult patients with osteogenesis imperfecta. The curves show the mean percent ± SE changes from baseline to 3 years in the alendronate or placebo groups. Right and left femoral hips were analyzed together.20
Total femur BMD decreased in the placebo group by 0.3 ± 0.6% and increased in the alendronate group by 3.3 ± 0.5% (Fig. 1; Table 3). The mean observed change in the placebo and alendronate groups was −0.002 ± 0.005 and +0.024 ± 0.004 g/cm2, respectively. This increase in the alendronate group was significantly greater than that in the placebo group (p = 0.001).
Fractures
The incidence of vertebral and peripheral fractures was not significantly different between the alendronate and placebo groups. Two vertebral and 17 peripheral fractures occurred in 11 patients in the placebo group versus no vertebral and 17 peripheral fractures in 10 patients in the alendronate group. The distributions in each group were as follows (alendronate/placebo): foot, 1/4; tibia, 1/1; knee and patella, 1/1; femur shaft, 1/0; proximal femur, 4/0; hand and wrist, 3/1; elbow, 3/1; humerus, 1/0; rib, 2/7; scapula, 0/1; mandibula, 0/1. Patients with one fracture were seven and five, patients with two fractures were three and two, patients with three fractures were one and two, and patients with four fractures were one and zero in the alendronate and placebo groups, respectively.
Pain
The pain score was similar in both groups from month 0 to month 30. At month 36, in the intent‐to‐treat analysis, the absolute difference between baseline and 3‐year follow‐up was a significant increase in the alendronate group (1.3 ± 2.6; p = 0.04) but not in the placebo group (0.0 ± 2.1). This was not significant in the per protocol analysis, once adjusted for patients who stopped their treatment.
Hearing loss
The average Rinne was not significantly modified in patients receiving alendronate or placebo.
Biochemical markers of bone turnover
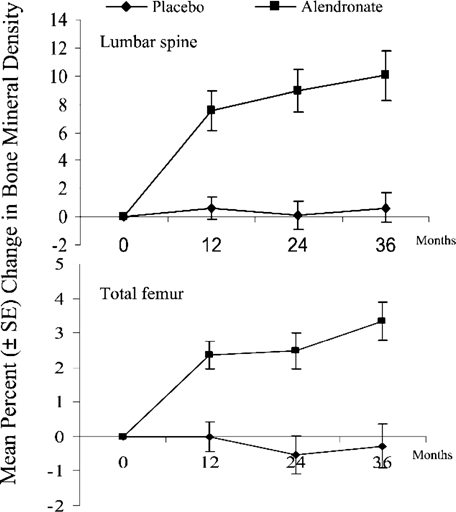
Biochemical markers of bone turnover significantly decreased in the alendronate group, whereas there were no significant changes in the placebo group (Fig. 2; Table 3). At 36 months, alendronate treatment was associated with significant decreases of serum and urinary C‐telopeptides of type I collagen of 59.5 ± 31.6% (p < 0.001) and 57.4 ± 30.0% (p < 0.001), respectively. Serum N‐propeptide of type I collagen and serum osteocalcin decreased, respectively, 52.4 ± 24.4% (p < 0.001) and 38.0 ± 24.7% (p < 0.001). These decreases tended to be more marked for the biochemical markers of bone resorption than for those of bone formation, especially for the first 2 years. The decrease of biochemical markers of bone turnover from baseline in the alendronate group was significant after 1 year. We do not have data about the early reductions in bone turnover during the first 3 and 6 months, because the samples were only obtained annually.
Effects of alendronate on bone resorption markers (serum and urinary C‐telopeptides of type I collagen) and bone formation markers (serum N‐propeptide of type I collagen and serum osteocalcin) from baseline values in adult patients with osteogenesis imperfecta. The curves show the mean percent ± SE changes from baseline to 3 years in the alendronate or placebo groups.20
Adverse effects
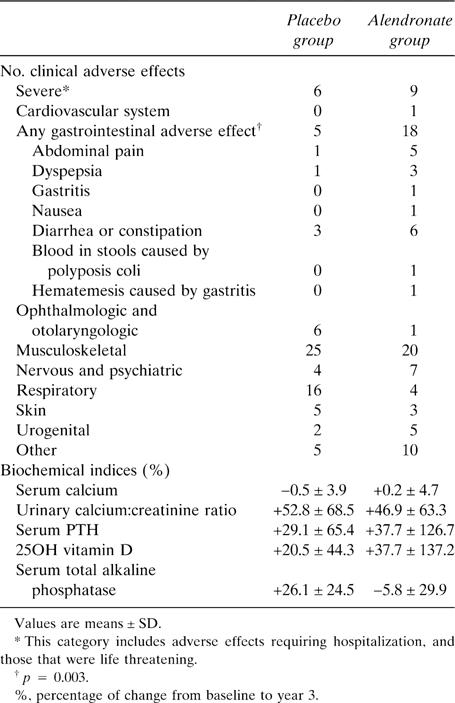
There was no significant difference in the overall incidence of clinical adverse effects and in laboratory abnormalities between the alendronate and placebo groups at the end of the study (Table 4). However, gastrointestinal adverse effects were more frequent in the alendronate group (p = 0.003). A hematemesis caused by gastritis was observed in a patient from the alendronate group taking a nonsteroidal anti‐inflammatory and, in the same patient, blood was found in the stools because of polyposis coli. No evidence of delayed mineralization was found in the biopsies from the two patients in the alendronate group. No patients withdrew from the study for drug‐related adverse effects or severe adverse effects.
DISCUSSION
Our study is the first double‐blinded study showing that daily treatment with oral alendronate for 3 years is able to increase BMD in adult patients with osteogenesis imperfecta. The magnitude of this change (+10.1% in the lumbar spine) was similar or even greater than the one observed in 3‐year trials run in patients with postmenopausal osteoporosis. (16–18) This effect was associated with a marked reduction of biochemical markers of bone turnover. The reduction in bone turnover in our study is consistent with the findings of other alendronate trials and provides evidence that alendronate slows osteoclastic bone resorption and bone remodeling in adult patients with osteogenesis imperfecta as pamidronate does in children. (7, 19, 24)
This study was not powered to show an effect of alendronate on fracture rate, and we did not observe any significant difference in the incidence of vertebral and peripheral fractures between the two groups. The data argue against a major effect on fracture incidence, although small effects are still possible, either positive or negative. Incident vertebral fracture rate in the treated group is lower than in the placebo. Inversely, the hip fracture rate is higher in the alendronate group than in the placebo group. Femoral fractures in adult patients with osteogenesis imperfecta are the consequence of bone fragility but also bone deformities, which may modify bone resistance. We did not take the femoral hip architecture at the moment of the inclusion into account. The size of our groups was too small to analyze the relationship between the femoral hip forms and the risk of fracture.
Osteogenesis imperfecta is a heritable disorder in contrast with the postmenopausal osteoporosis. Mutations in one of the two genes that encode collagen type I α chains, COL1A1 and COL1A2, are “private” for each family. Even if vertebral or femoral BMDs are identical for two patients from two different families, mutations introduce a probable difference in the bone strength. Collagen mutations are difficult to analyze. The mutation type for each patient introduces a noncontrolled variability. Conversely, the absence of a marked effect on the fracture incidence in adult patients contrasts with the study recently published about children treated with another oral bisphosphonate, olpadronate. (13) In children, pamidronate, the best evaluated bisphosphonate, inhibits only resorption and not formation in modeling bone, whereas resorption and formation are inhibited in remodeling bone, as in adult patients in our study. (25) This difference could explain the decrease of the risk of fracture in children and not in adult patients.
The pain score was slightly increased at month 36 in the alendronate group, but the placebo and alendronate curves were very similar during the study except for the last visit. We have no explanation for this observation. In the treated group, alendronate caused few adverse effects. Gastrointestinal symptoms were more common in the patients receiving alendronate than in those receiving placebo, although these symptoms were not responsible for treatment withdrawal. No severe adverse effect was associated with the alendronate treatment, indicating a good tolerance of the alendronate treatment.
Osteogenesis imperfecta is often regarded primarily as a disorder of childhood. However, many patients have a normal life expectancy and survive into late adult life. (20) New validated therapeutic approaches not requiring hospitalization are necessary in adult patients. Alendronate is now the best‐evaluated oral treatment in adult patients with osteogenesis imperfecta. To increase BMD may be an important objective of the treatment of adult patients with osteogenesis imperfecta if a relationship between this increase and a decrease of fracture rate could be shown. This relationship has not been established by the results of our study. We cannot conclude that alendronate use prevents bone fracture because our study was not powered to show an effect on fracture rate. More studies are needed.
Acknowledgements
We thank Pierre Verhaeghe for help. This study was supported by the Hospices Civils de Lyon, the Hospital Program for Clinical Research (P.H.R.C. 97.058), the Association de l'Ostéogenèse Imparfaite, Merck & Company, and the Institut National de la Santé et de la Recherche Médicale.
References
Author notes
Dr Meunier has received consulting fees from Servier, Nycomed, Amgen, Merck, and Aventis. All other authors have no conflict of interest