Abstract
Background
Recurrent, metastatic, and locally advanced gastrointestinal stromal tumors (GISTs) can be treated successfully with imatinib mesylate. Surgery for residual disease has been suggested for nonrefractory metastatic GISTs to reduce the probability of resistant recurrent clones, although no randomized Phase III trial has been performed to answer the question about its benefit. We carried out an analysis of the outcome of patients with recurrent unresectable locally advanced or metastatic imatinib-sensitive priamary GIST in 14 institutions in Spain. We compared two cohorts: treated or not treated with surgery after partial response or stabilization by imatinib.
Patients and Methods
Data were obtained from the online GIST registry of the Spanish Group for Research in Sarcomas. Selected patients were then divided into two groups: group A, treated initially only with imatinib, and group B, treated additionally with metastasectomy. Baseline characteristics between groups were compared, and univariate and multivariate analysis for progression-free survival and overall survival (OS) were performed.
Results
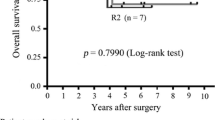
Analysis was undertaken in 171 patients considered nonrefractory to imatinib. The median follow-up time was 56.6 months. Focusing on OS, the Eastern Cooperative Oncology Group performance status different than 0, extent of disease limited to one metastatic organ, and comparison between groups A or B achieved statistical difference in the multivariate analysis. Median survival was 59.9 months in group A and 87.6 months in group B.
Conclusions
Based in its benefit in OS, our study supports surgery of metastatic disease in GIST patients who respond to imatinib therapy.

Similar content being viewed by others
References
Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course and prognostication in the pre-imatinib mesylate era: a population based study in western Sweden. Cancer. 2005;103(4):821–9.
Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80.
Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–10
Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard-versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing kit. J Clin Oncol. 2008;26:620–5.
Green S, Weiss GR. Southwest Oncology Group standard response criteria, end point definitions, and toxicity criteria. Invest New Drugs. 1992;10:239–53
Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–32.
Blay JY, Le CA, Ray-Coquard I, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol. 2007;25:1107–13.
Lux ML, Rubin BP, Biase TL, et al. Kit extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am J Pathol. 2000;156(3):791–5.
Heinrich MC, Owzar K, Corles CL, et al. Correlation of kinase genotype and clinical outcome in the North American intergroup phase III trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008;26:5360–7.
Wardelmann E, Merkelbach-Bruse S, Pauls K, et al. Polyclonal evolution of multiple secondary kit mutations in gastrointestinal stromal tumors under treatment with imatinib mesylate. Clin Cancer Res. 2006;12(6):1743–9.
Debiec- Rychter M, Cools J, Dumez H, et al. Mechanisms of resistance to imatinib mesylate in gastrointestinal stromal tumors and activity of the PKC412 inhibitor against imatinib-resistant mutants. Gastroenterology. 2005;128(2):270–9.
Van Glabbeke M, Verweij J, Casali PG, et al. Initial and late resistance to imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: a European Organisation for Research and Treatment of Cancer–Italian Sarcoma Group–Australasian Gastrointestinal Trials Group study. J Clin Oncol. 2005;23:5795–804.
Gronchi A, Fiore M, Miselli F, et al. Surgery of residual disease following molecular-targeted therapy with imatinib mesylate in advanced/metastatic GIST. Ann Surg. 2007;245:341–6.
DeMatteo RP, Maki RG, Singer S, et al. Results of tyrosine kinase inhibitor therapy followed by surgical resection for metastatic gastrointestinal stromal tumor. Ann Surg. 2007;245:347–52.
Bauer S, Hartmann JT, de Wit M, et al. Resection of residual disease in patients with metastatic gastrointestinal stromal tumors responding to treatment with imatinib. Int J Cancer. 2005;117:316–25
Andtbacka RH, Ng CS, Scaife CL, et al. Surgical resection of gastrointestinal stromal tumors after treatment with imatinib. Ann Surg Oncol. 2007;14:14–24.
Bonvalot S, Eldweny H, Péchoux CL, et al. Impact of surgery on advanced gastrointestinal stromal tumors (GIST) in the imatinib era. Ann Surg Oncol. 2006;13(12):1596–603.
Raut CP, Posner M, Desai J, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol. 2006;24:2325–31
Mussi C, Ronellenfitsch U, Jakib J, et al Post-imatinib surgery in advanced/metastatic GIST: is it worthwhile in all patients? Ann Oncol. 2010;21:403–8
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–9.
Bauer S, Rutkowski P, Hohenberger P, et al. Long-term follow-up of patients with GIST undergoing metastasectomy in the era of imatinib: analysis of prognostic factors (EORTC–STBSG collaborative study). Eur J Surg Oncol. 2014;40(4):412–9.
Disclosure
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Rubió-Casadevall, J., Martinez-Trufero, J., Garcia-Albeniz, X. et al. Role of Surgery in Patients with Recurrent, Metastatic, or Unresectable Locally Advanced Gastrointestinal Stromal Tumors Sensitive to Imatinib: A Retrospective Analysis of the Spanish Group for Research on Sarcoma (GEIS). Ann Surg Oncol 22, 2948–2957 (2015). https://doi.org/10.1245/s10434-014-4360-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-4360-8