Abstract
Background
In patients with metastatic gastrointestinal stromal tumor (GIST) on first-line imatinib (IM) undergoing cytoreductive surgery, response to IM at time of surgery correlates with completeness of resection and progression-free and overall survival (PFS, OS). Impact of surgery in IM-resistant patients on second-line sunitinib (SU) is unknown.
Methods
Patients on SU undergoing surgery for metastatic GIST at our institution were reviewed. Response to SU at time of surgery was categorized as responsive disease (RD), limited progression (LP) or generalized progression (GP).
Results
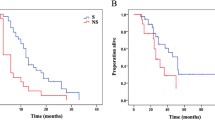
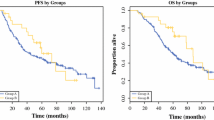
Fifty patients underwent surgery after a median 6.7 months of SU. Forty patients (80%) had prior surgery at initial presentation of GIST; 16 (32%) underwent prior surgery on IM. At time of surgery on SU, 10 patients (20%) had RD, 22 (44%) had LP, and 18 (36%) had GP. Resections were macroscopically complete in 25 patients (50%); completeness of resection did not correlate with response to SU. Complication rate was 54%; reoperations were required in 16%. Median PFS after surgery and start of SU was 5.8 and 15.6 months, respectively (median follow-up 15.2 months). Corresponding median OS was 16.4 and 26.0 months, respectively. Differences in PFS and OS based on response to SU were not significant. Younger age was prognostic of survival.
Conclusion
Surgery is feasible in patients with metastatic GIST on SU, but incomplete resections are frequent and complication rates are high. Relevance of survival rates is difficult to assess given the selection bias. Benefits of surgery should be weighed against symptoms and alternative treatments.


Similar content being viewed by others
References
Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–32.
Andtbacka RH, Ng CS, Scaife CL, et al. Surgical resection of gastrointestinal stromal tumors after treatment with imatinib. Ann Surg Oncol. 2007;14:14–24.
Bonvalot S, Eldweny H, Pechoux CL, et al. Impact of surgery on advanced gastrointestinal stromal tumors (GIST) in the imatinib era. Ann Surg Oncol. 2006;13:1596–603.
DeMatteo RP, Maki RG, Singer S, et al. Results of tyrosine kinase inhibitor therapy followed by surgical resection for metastatic gastrointestinal stromal tumor. Ann Surg. 2007;245:347–52.
Gronchi A, Fiore M, Miselli F, et al. Surgery of residual disease following molecular-targeted therapy with imatinib mesylate in advanced/metastatic GIST. Ann Surg. 2007;245:341–6.
Raut CP, Posner M, Desai J, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol. 2006;24:2325–31.
Rutkowski P, Nowecki Z, Nyckowski P, et al. Surgical treatment of patients with initially inoperable and/or metastatic gastrointestinal stromal tumors (GIST) during therapy with imatinib mesylate. J Surg Oncol. 2006;93:304–11.
Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–38.
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Blay JY, Le Cesne A, Ray-Coquard I, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol. 2007;25:1107–13.
Adenis A, Cassier PA, Bui BN, et al. Does interruption of imatinib (IM) in responding patients after three years of treatment influence outcome of patients with advanced GIST included in the BFR14 trial?. J Clin Oncol. 2008;26:A10522–00000.
Disclosures
Chandrajit P. Raut—Novartis (honoraria, speaker’s bureau, advisory board). Suzanne George—Novartis (advisory board); Pfizer (advisory board). George D. Demetri—Novartis (honoraria, speaker’s bureau, advisory board); Pfizer (honoraria, advisory board).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raut, C.P., Wang, Q., Manola, J. et al. Cytoreductive Surgery in Patients with Metastatic Gastrointestinal Stromal Tumor Treated with Sunitinib Malate. Ann Surg Oncol 17, 407–415 (2010). https://doi.org/10.1245/s10434-009-0784-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-009-0784-y