Abstract
Background
The role for surgery in patients with “unresectable” gastrointestinal stromal tumors (GIST) treated with imatinib is still not defined. The objective of this retrospective study was to evaluate the feasibility and benefit of this secondary surgery.
Methods
Progression-free survival (PFS) in a group of patients who underwent secondary surgery was compared to that of patients treated exclusively with imatinib.
Results
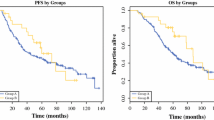
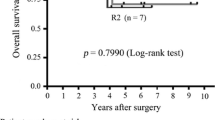
Of 180 patients with unresectable GIST treated with Imatinib, 22 (12%) underwent secondary surgery, following which one patient achieved a complete radiological response, 19 achieved a partial response (PR), in one patient the disease was stable, and in one patient there was reactivation of local occlusive disease after an initial PR. No patient with overall progression was to undergo surgery. At the beginning of imatinib therapy, five patients with metastases underwent emergency surgery [hemorrhage (n = 3) due to rupture of large necrotic masses], which ultimately resulted in three of the five patients dying postoperatively. A macroscopically complete resection was achieved in all primary tumors (5/5) and in ten of the 17 metastases. Pathological analysis revealed two complete response (CR) and 17 PR, and no treatment effect was evidenced in three patients. Two-year overall survival after surgery was 62%. The median PFS calculated from the initiation of imatinib therapy was 18.7 months for all operated patients and 23.4 months after planned surgery.
Conclusion
Primary tumors that become amenable to surgery with prior imatinib therapy, evolving necrosis and localized progression (to avoid life-threatening complications) could benefit from this secondary surgery. For the majority of other residual lesions, the potential benefit of secondary surgery should be evaluated in randomized studies in the future since PFS is similar to that reported among non-operated patients.





Similar content being viewed by others
References
Duffaud F, Blay JY. Gastrointestinal stromal tumors: biology and treatment. Oncology 2003;65:187–97
Miettinen M, Lasota J. Gastrointestinal stromal tumors-definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 2001; 438:1–12–26
Joensuu H, et al. Brief Report : Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal Tumor. N Engl J Med 2001;344:14
Verweij J, van Oosterom A, Blay JY, et al. Imatinibmesylate (STI-571 Glivec, Imatinib) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer 2003;39:2006–11
Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinibmesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472–80
Eisenberg BL, Judson I. Surgery and Imatinibin the management of GIST: emerging approaches to adjuvant and neoadjuvant therapy. Ann Surg Oncol 2004;11:465–75
Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 2004;364:1127–34
Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459–65
Lassau N, Lamuraglia M, Leclère J, Bonvalot S, Vanel D, Robert C, Tursz T. Doppler-Ultrasonography with perfusion software and contrast medium injection as an early evaluation tool of gastro intestinal stromal tumor (GIST) treated by imatinib: results of a prospective study. Proceedings ASCO 2004, vol 23, abstract 9048
Vanel D, Albiter M, Shapeero L, et al. Role of computed tomography in the follow up of hepatic and peritoneal metastases of GIST under imatinib mesylate treatment. A prospective study of 54 patients. Eur J Radiol 2005;54:118–23
Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol 1999;43(Suppl):S15–25
Kaplan EL, Meir P. Non-parametric estimation from incomplete observations. J Am Statist Assoc 1958;53:457–81
Demetri GD. Targeting c-kit mutations in solid tumors: scientific rationale and novel therapeutic options. Semin Oncol 2001; 28(Suppl 17):19–26
De Matteo RP, Lewis JJ, Leug D, Mudan S, Woodruff JM, Brennan M. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51–8
Scaife CL, Hunt KK, Patel SR, Benjamin RS, Burgess MA, Chen LL, Trent J, et al. Is there a role for surgery in patients with “unresectable” cKIT+ gastrointestinal stromal tumors treated with Imatinibmesylate? Am J Surg 2003;186:665–9
Bauer S, Hartman JT, Lang H, et al. Imatinib may enable complete resection in previously unresectable or metastatic GISTS. Proc ASCO 2004, 23:abstract 9023
Katz D, Segal A, Alberton Y, Jurim O, Reissman P, Catane R, Cherny NI. Neoadjuvant Imatinib for unresectable gastrointestinal stromal tumor. Anticancer Drugs 2004;15:599–602
Aparicio T, Boige V, Sabourin JC, Crenn P, Ducreux M, Le Cesne A, Bonvalot S. Prognostic factors after complete resection of primary gastro intestinal stromal tumors. Eur J Surg Oncol 2004;30:1098–103
Blay JY, Bonvalot S, Casali P, et al. GIST consensus meeting panelists. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20–21 March 2004, under the auspices of ESMO. Ann Oncol 2005;16(4):566–78
Benjamin RS, Blanke CD, Blay JY, Bonvalot S, Eisenberg B. Management of gastrointestinal stromal tumors in the imatinib era: selected case studies. Oncologist 2006;11:9–20
Bechtold RE, Chen MY, Stanton CA, Savage PD, Levine EA. Cystic changes in hepatic and peritoneal metastases from gastrointestinal stromal tumors treated with Imatinib. Abdom Imaging 2003;28:808–14
Van Oosterom AT, Judson IR, Verweij J, et al. European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Update of phase I study of Imatinib(STI 571) in advanced soft tissue sarcomas and gastrointestinal stromal tumors : a report of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 2002; 38(Suppl 5):S83–7
Van Coevorden F, Peterse H, Rodenhuis S. Is there a role for post Imatinib (salvage) surgery in gastro intestinal tumors? Connective Tissue Oncology Society. In: 9th Annual Scientific Meeting 2003, abstract 147
Desai J, Shankar S, Heinrich C, et al. Clonal evolution of resistance to Imatinibin patients with gastro intestinal tumors: molecular and radiologic evaluation of new lesions. Proc ASCO 2004;23: abstract 3010
Dileo P, Randhawa R, Vanonnenberg E, et al. Safety and efficacy of percutaneous radiofrequency ablation (RFA) in patients with GIST with clonal evolution of lesions refractory to imatinib. Proc ASCO 2004;23: abstract 9024
Wu PC, Langerman A, Ryan CW, et al. Surgical treatment of gastrointestinal stromal tumors in the Imatinib (STI-571) era. Surgery 2003;134:656–65
Blay JY, Berthaud P, Perol D, et al. Continuous versus intermittent Imatinib treatment in advanced GIST after one year: a prospective phase III randomised trial of the French sarcoma group. Proc ASCO 2004;23: abstract 9006
Acknowledgments
The authors thank Mrs. Lorna Saint-Ange for editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonvalot, S., Eldweny, H., Péchoux, C.L. et al. Impact of Surgery on Advanced Gastrointestinal Stromal Tumors (GIST) in the Imatinib Era. Ann Surg Oncol 13, 1596–1603 (2006). https://doi.org/10.1245/s10434-006-9047-3
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-006-9047-3