-
PDF
- Split View
-
Views
-
Cite
Cite
Spyridoula Maraka, Naykky Singh Ospina, Rene Rodriguez-Gutierrez, Caroline J Davidge-Pitts, Todd B Nippoldt, Larry J Prokop, M Hassan Murad, Sex Steroids and Cardiovascular Outcomes in Transgender Individuals: A Systematic Review and Meta-Analysis, The Journal of Clinical Endocrinology & Metabolism, Volume 102, Issue 11, 1 November 2017, Pages 3914–3923, https://doi.org/10.1210/jc.2017-01643
Close - Share Icon Share
Abstract
Transgender individuals receive cross-sex hormonal therapy to induce desired secondary sexual characteristics despite limited data regarding its effects on cardiovascular health.
A comprehensive search of several databases up to 7 April 2015 was conducted for studies evaluating the effect of sex steroid use on lipids, myocardial infarction, stroke, venous thromboembolism (VTE), and mortality in transgender individuals. Pairs of reviewers selected and appraised the studies. A random-effects model was used to pool weighted mean differences and 95% confidence intervals (CIs).
We found 29 eligible studies with moderate risk of bias. In female-to-male (FTM) individuals, sex steroid therapy was associated with statistically significant increases in serum triglyceride (TG) levels at 3 to 6 months and at ≥24 months (21.4 mg/dL; 95% CI: 0.14 to 42.6) and in low-density lipoprotein cholesterol (LDL-C) levels at 12 months and ≥24 months (17.8 mg/dL; 95% CI: 3.5 to 32.1). High-density lipoprotein cholesterol (HDL-C) levels decreased significantly across all follow-up periods (highest at ≥24 months, −8.5 mg/dL; 95% CI: −13.0 to −3.9). In male-to-female (MTF) individuals, serum TG levels were significantly higher at ≥24 months (31.9 mg/dL; 95% CI: 3.9 to 59.9) without any changes in other parameters. Few myocardial infarction, stroke, VTE, and death events were reported (more frequently in MTF individuals).
Low-quality evidence suggests that sex steroid therapy may increase LDL-C and TG levels and decrease HDL-C level in FTM individuals, whereas oral estrogens may increase TG levels in MTF individuals. Data about important patient outcomes remain sparse.
The prevalence of transgender individuals as reported in the literature is increasing, with recent estimates of 6.8 per 100,000 males seeking transition to the female gender [male-to-female (MTF)] and 2.6 per 100,000 females seeking transition to the male gender [female-to-male (FTM)] (1). To decrease gender dysphoria, many transgender individuals receive cross-sex hormonal therapy, which suppresses natal secondary sexual characteristics and induces changes consistent with their desired gender. For MTF transition, the main components of cross-sex hormonal therapy are estrogens, antiandrogens (cyproterone acetate and spironolactone), or gonadotropin-releasing hormone (GnRH) agonists. For FTM transition, testosterone is the main treatment (2).
Adverse outcomes of sex steroid therapy in other settings, such as hormone replacement therapy in postmenopausal women, oral contraceptive pills in premenopausal women, or testosterone in males with hypogonadism, have been the subject of multiple studies (3–6). However, differences in patients’ clinical characteristics and hormonal regimens in these trials do not allow direct application of their results to transgender individuals receiving feminizing or masculinizing hormone therapy. To date, no randomized trial has evaluated the safety of any feminizing hormone regimen during MTF transition. For FTM transition, a small randomized trial with only a 3-month follow-up was conducted to assess the effects of estrogen deprivation on bone metabolism and vascular parameters (7). In 2010, a systematic review of 16 studies found very low-quality evidence suggesting that cross-sex hormonal therapy was associated with unfavorable changes in lipid profiles and inconclusive data about important patient cardiovascular outcomes (8). Since the publication of that review, more studies have become available, justifying a new quantitative synthesis of the evidence to inform discussions between clinicians and transgender individuals regarding treatment decisions. To this end, we conducted a systematic review and meta-analysis of available evidence on the effect of sex steroids on lipids and important cardiovascular outcomes in MTF and FTM transgender individuals.
Methods
We performed a systematic review and meta-analysis to estimate the effects of sex steroid treatment on (1) lipid profiles, (2) cardiovascular events, (3) venous thromboembolism (VTE), and (4) mortality in adolescent and adult transgender individuals. This report followed a rigorous systematic review protocol that was developed in collaboration with experts from the Endocrine Society and that adheres to the PRISMA statement (9).
Eligibility criteria
We included randomized trials, observational studies, and case series of adolescent and adult transgender individuals who used sex steroids, regardless of whether they had gender-confirming surgery or not. Eligible studies exposed MTF transgender individuals to cross-sex hormone therapy including estrogen, antiandrogens (cyproterone acetate and spironolactone), or GnRH agonists and FTM transgender individuals to testosterone. We included studies that provided a pre-post intervention comparison of participants followed up for at least 3 months and studies that compared transgender individuals (at least 3 months of therapy) with a control group. Outcomes of interest included changes in (1) lipid profile [total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) levels]; (2) cardiovascular events (myocardial infarction, transient ischemic attack, stroke); (3) VTE events; and (4) mortality.
To avoid selection bias and to ascertain a well-documented exposure for the pre-post intervention comparison, eligible studies excluded individuals who had received sex steroids—even when self-prescribed—before the initiation of the study. We also excluded studies for which information to determine eligibility was not available in the manuscript and whose authors did not respond to requests for that information. We included studies regardless of their publication status, language, or size. Review articles, commentaries, and letters that did not contain primary data were excluded.
Study identification
A comprehensive search of several databases from 1980 to 7 April 2015 was conducted. Databases included Ovid MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, and Scopus. An experienced librarian (L.J.P.) designed the search strategy with input from study investigators with expertise in conducting systematic reviews (S.M. and N.S.O.). Controlled vocabulary supplemented with key words was used to search for studies of outcomes of cross-sex hormone therapy in transgender individuals. The search strategy is available in the Supplemental Appendix. We reviewed the reference lists of narrative reviews and consulted with experts to identify additional references.
The search results were uploaded into a systematic review software program (DistillerSR; Ottawa, ON, Canada). Reviewers working independently and in duplicate reviewed all abstracts and titles for inclusion (S.M., N.S.O., and R.R.-G.). After abstracts were screened and potentially eligible studies were retrieved, the full-text publications were assessed for eligibility by both reviewers with excellent chance-adjusted inter-reviewer agreement (κ statistic = 0.82). Duplicate studies and studies with overlapping populations were excluded. Disagreements were resolved by consensus (the two reviewers discussed the discrepancy and reached a final decision).
Data collection and management
Working independently and in duplicate using a standardized form, reviewers collected the following information from each eligible study: (1) baseline clinical features: age, weight, body mass index, and whether MTF or FTM transition; (2) gender-confirming surgery proportion and definition; (3) proportion of patients with risk factors for cardiovascular events; (4) type of intervention (medication, dose, route, frequency) and duration of exposure at outcome assessment; and (5) outcomes (blood lipid fractions, number of cardiovascular events, VTE events, and deaths). We also extracted the definition of controls used in the applicable studies. Disagreements were resolved by discussion and consensus.
Risk of bias assessment
We used the Newcastle-Ottawa tool to evaluate the risk of bias in observational studies. This tool evaluates the selection of study cohorts, the comparability of the study cohorts, and the ascertainment of exposure and outcomes (10). For randomized trials, we used the Cochrane Collaboration tool for assessing risk of bias (11). Reviewers working independently assessed the risk of bias of included studies in duplicate. Any disagreements were resolved by consensus.
Author contact
To reduce reporting bias, we contacted by e-mail corresponding authors (or any other author if we were unable to reach the corresponding author) of each of the eligible studies in which clarification or more information was needed to determine eligibility or to complete the analysis. Three out of the ten contacted authors replied.
Meta-analysis
We conducted a random-effects meta-analysis using the DerSimonian-Laird random-effects method to pool mean differences for continuous outcomes and their associated 95% confidence intervals (CIs) (12). Longitudinal and cross-sectional studies were pooled separately. We subtracted the baseline measurement from the follow-up measurement of serum lipids when calculating the mean differences. Hence, negative values indicate a decrease from baseline and positive values indicate an increase. When comparing values against a control group, we subtracted the control value from the transgender group value; hence, a positive value indicates a higher value for the transgender group and a negative value indicates a lower value for the transgender group. We also planned to estimate the pooled cumulative incidence of cardiovascular events, thrombotic events, and deaths; however, varied follow-up durations across studies and the limited number of events in the included studies precluded pooling.
Inconsistency was assessed using the I2 statistic, with values <25% indicative of low inconsistency and values >75% indicative of high inconsistency not due to chance (13). Open Meta-Analyst was used for statistical analyses (14).
Subgroups and sensitivity analyses
A priori hypotheses to explore potential causes of heterogeneity included possible differences in population age (e.g., adolescents vs adults); different treatment regimens (e.g., oral vs transdermal estrogen, estrogens alone vs combination therapy); outcome characteristics (e.g., symptomatic vs all events); study design (e.g., controlled study vs single cohort); and study quality (e.g., blinded vs open outcome assessment, follow-up duration). Sensitivity analyses were conducted to explain possible inconsistencies across study results.
Results
Study identification
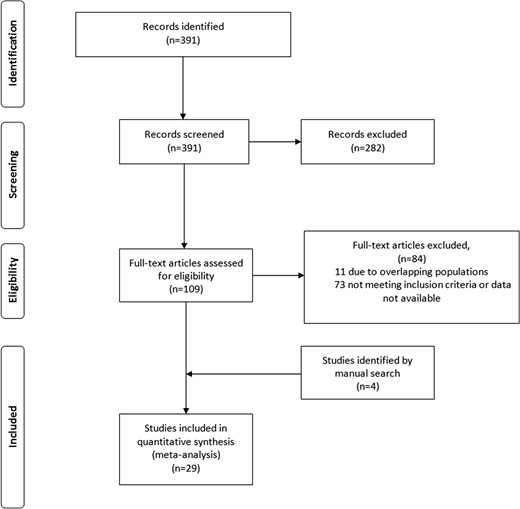
A total of 391 potentially eligible articles were identified through our systematic database search, of which 29 were ultimately eligible (7, 15–42) after exclusion of studies that represented overlapping populations (43–50). The study selection process is described in Fig. 1.
Supplemental Table 1 summarizes the characteristics of the included studies. Twenty-eight were cohort studies, and one was a randomized trial. Seventeen were before and after cross-sex hormone therapy studies, and four studies compared transgender participants with a control group. Twenty-one studies evaluated lipid fractions. Ten studies evaluated VTE (10 in MTF individuals and 8 in FTM individuals). Numbers of studies evaluating mortality, myocardial infarction, and stroke were 4, 3, and 2, respectively.
Across all studies, 4731 transgender patients were included. These included 3231 MTF participants (23 studies) and 1500 FTM participants (20 studies). No study provided information solely on adolescent populations. The mean age of the MTF group ranged from 19.3 to 43.7 years, and the mean age of the FTM group ranged from 21.7 to 37.5 years. MTF treatment regimens included various doses of oral, transdermal, or intramuscular (IM) estrogens, and some regimens included cyproterone acetate, GnRH agonists (goserelin, triptorelin), spironolactone, or anastrozole. Most FTM transgender individuals used various IM preparations of testosterone and some transdermal, subcutaneous, or oral testosterone. Exposure and follow-up ranged from 3 months to 41 years.
Risk of bias
We judged the observational studies to be at moderate risk of bias on the basis of representativeness of the exposed cohort [most were somewhat representative (clinic based)] and assessment of outcome (record linkage or self-report). The included randomized trial had an increased risk of bias because of unclear randomization and allocation concealment methods, lack of blinding, and high rate of loss to follow-up (see Supplemental Tables 2 and 3).
Meta-analysis
Serum lipids
In FTM transgender individuals, the meta-analysis (Table 1) showed a statistically significant increase in serum TG levels at 3 to 6 months of therapy (9 mg/dL; 95% CI: 2.5 to 15.5 mg/dL) and at ≥24 months (21.4 mg/dL; 95% CI: 0.1 to 42.6 mg/dL) compared with baseline. The serum LDL-C level showed a statistically significant increase when measured at 12 months (11.3 mg/dL; 95% CI: 5.5 to 17.1 mg/dL) and ≥24 months (17.8 mg/dL; 95% CI: 3.5 to 32.1 mg/dL). There was a statistically significant decrease in serum HDL-C level across all follow-up periods (highest at ≥24 months, −8.5 mg/dL; 95% CI: −13.0 to −3.9 mg/dL). Total serum cholesterol level changes were not statistically significant at any time period.
Change in Lipid Profile in FTM Transgender Individuals at Different Periods (Follow-Up to Baseline)
| . | Estimate . | 95% CI . | I2 . | Number of Studies . |
|---|---|---|---|---|
| . | 3–6 Months . | |||
| TG, mg/dL | 9 | (2.5 to 15.5) | 7.7 | 4 |
| LDL-C, mg/dL | 7.1 | (−3.2 to 17.3) | 0 | 3 |
| HDL-C, mg/dL | −6.5 | (−11.9 to −1.0) | 0 | 3 |
| TC, mg/dL | 10.6 | (−4.2 to 25.4) | 73.6 | 4 |
| . | Estimate . | 95% CI . | I2 . | Number of Studies . |
|---|---|---|---|---|
| . | 3–6 Months . | |||
| TG, mg/dL | 9 | (2.5 to 15.5) | 7.7 | 4 |
| LDL-C, mg/dL | 7.1 | (−3.2 to 17.3) | 0 | 3 |
| HDL-C, mg/dL | −6.5 | (−11.9 to −1.0) | 0 | 3 |
| TC, mg/dL | 10.6 | (−4.2 to 25.4) | 73.6 | 4 |
| . | 12 Months . | |||
|---|---|---|---|---|
| TG, mg/dL | 14.7 | (−2.7 to 32.1) | 75.4 | 9 |
| LDL-C, mg/dL | 11.3 | (5.5 to 17.1) | 0 | 8 |
| HDL-C, mg/dL | −8.1 | (−10.6 to −5.7) | 0 | 8 |
| TC, mg/dL | 12.0 | (−3.7 to 27.8) | 88.0 | 9 |
| . | 12 Months . | |||
|---|---|---|---|---|
| TG, mg/dL | 14.7 | (−2.7 to 32.1) | 75.4 | 9 |
| LDL-C, mg/dL | 11.3 | (5.5 to 17.1) | 0 | 8 |
| HDL-C, mg/dL | −8.1 | (−10.6 to −5.7) | 0 | 8 |
| TC, mg/dL | 12.0 | (−3.7 to 27.8) | 88.0 | 9 |
| . | ≥24 Months . | |||
|---|---|---|---|---|
| TG, mg/dL | 21.4 | (0.1 to 42.6) | 80.0 | 3 |
| LDL-C, mg/dL | 17.8 | (3.5 to 32.1) | 84.1 | 3 |
| HDL-C, mg/dL | −8.5 | (−13.0 to −3.9) | 70.7 | 3 |
| TC, mg/dL | 14.6 | (−5.1 to 34.3) | 90.2 | 3 |
| . | ≥24 Months . | |||
|---|---|---|---|---|
| TG, mg/dL | 21.4 | (0.1 to 42.6) | 80.0 | 3 |
| LDL-C, mg/dL | 17.8 | (3.5 to 32.1) | 84.1 | 3 |
| HDL-C, mg/dL | −8.5 | (−13.0 to −3.9) | 70.7 | 3 |
| TC, mg/dL | 14.6 | (−5.1 to 34.3) | 90.2 | 3 |
Abbreviation: TC, total cholesterol.
Change in Lipid Profile in FTM Transgender Individuals at Different Periods (Follow-Up to Baseline)
| . | Estimate . | 95% CI . | I2 . | Number of Studies . |
|---|---|---|---|---|
| . | 3–6 Months . | |||
| TG, mg/dL | 9 | (2.5 to 15.5) | 7.7 | 4 |
| LDL-C, mg/dL | 7.1 | (−3.2 to 17.3) | 0 | 3 |
| HDL-C, mg/dL | −6.5 | (−11.9 to −1.0) | 0 | 3 |
| TC, mg/dL | 10.6 | (−4.2 to 25.4) | 73.6 | 4 |
| . | Estimate . | 95% CI . | I2 . | Number of Studies . |
|---|---|---|---|---|
| . | 3–6 Months . | |||
| TG, mg/dL | 9 | (2.5 to 15.5) | 7.7 | 4 |
| LDL-C, mg/dL | 7.1 | (−3.2 to 17.3) | 0 | 3 |
| HDL-C, mg/dL | −6.5 | (−11.9 to −1.0) | 0 | 3 |
| TC, mg/dL | 10.6 | (−4.2 to 25.4) | 73.6 | 4 |
| . | 12 Months . | |||
|---|---|---|---|---|
| TG, mg/dL | 14.7 | (−2.7 to 32.1) | 75.4 | 9 |
| LDL-C, mg/dL | 11.3 | (5.5 to 17.1) | 0 | 8 |
| HDL-C, mg/dL | −8.1 | (−10.6 to −5.7) | 0 | 8 |
| TC, mg/dL | 12.0 | (−3.7 to 27.8) | 88.0 | 9 |
| . | 12 Months . | |||
|---|---|---|---|---|
| TG, mg/dL | 14.7 | (−2.7 to 32.1) | 75.4 | 9 |
| LDL-C, mg/dL | 11.3 | (5.5 to 17.1) | 0 | 8 |
| HDL-C, mg/dL | −8.1 | (−10.6 to −5.7) | 0 | 8 |
| TC, mg/dL | 12.0 | (−3.7 to 27.8) | 88.0 | 9 |
| . | ≥24 Months . | |||
|---|---|---|---|---|
| TG, mg/dL | 21.4 | (0.1 to 42.6) | 80.0 | 3 |
| LDL-C, mg/dL | 17.8 | (3.5 to 32.1) | 84.1 | 3 |
| HDL-C, mg/dL | −8.5 | (−13.0 to −3.9) | 70.7 | 3 |
| TC, mg/dL | 14.6 | (−5.1 to 34.3) | 90.2 | 3 |
| . | ≥24 Months . | |||
|---|---|---|---|---|
| TG, mg/dL | 21.4 | (0.1 to 42.6) | 80.0 | 3 |
| LDL-C, mg/dL | 17.8 | (3.5 to 32.1) | 84.1 | 3 |
| HDL-C, mg/dL | −8.5 | (−13.0 to −3.9) | 70.7 | 3 |
| TC, mg/dL | 14.6 | (−5.1 to 34.3) | 90.2 | 3 |
Abbreviation: TC, total cholesterol.
In MTF transgender individuals, the meta-analysis (Table 2) showed no statistically significant difference in serum LDL-C, HDL-C, and total cholesterol levels between baseline and any time periods. The serum TG level was significantly higher only at ≥24 months (31.9 mg/dL; 95% CI: 3.9 to 59.9 mg/dL). Using the cross-sectional studies that compared lipid values in the transgender population with those of control groups (natal males), the LDL-C level was statistically significantly lower in the transgender group (−20.3 mg/dL; 95% CI: −30.7 to −10.0 mg/dL; I2 = 0%). There was no difference in serum TG (23 mg/dL; 95% CI: −12.8 to 58.7 mg/dL; I2 = 63.1%), HDL-C (6.9 mg/dL; 95% CI: −5.1 to 18.8 mg/dL; I2 = 83.3%), or total cholesterol (−10 mg/dL; 95% CI: −32.5 to 12.5 mg/dL; I2 = 74.5%) level.
Change in Lipid Profile in MTF Transgender Individuals at Different Periods (Follow-Up to Baseline)
| . | Estimate . | 95% CI . | I2 . | Number of Studies . |
|---|---|---|---|---|
| . | 3–6 Months . | |||
| TG, mg/dL | 3.8 | (−11.6 to 19.3) | 63.5 | 6 |
| LDL-C, mg/dL | −3.1 | (−11.6 to 5.4) | 0 | 3 |
| HDL-C, mg/dL | 1.2 | (−5.7 to 8.1) | 70.0 | 5 |
| TC, mg/dL | −1.1 | (−9.4 to 7.2) | 0 | 4 |
| . | Estimate . | 95% CI . | I2 . | Number of Studies . |
|---|---|---|---|---|
| . | 3–6 Months . | |||
| TG, mg/dL | 3.8 | (−11.6 to 19.3) | 63.5 | 6 |
| LDL-C, mg/dL | −3.1 | (−11.6 to 5.4) | 0 | 3 |
| HDL-C, mg/dL | 1.2 | (−5.7 to 8.1) | 70.0 | 5 |
| TC, mg/dL | −1.1 | (−9.4 to 7.2) | 0 | 4 |
| . | 12 Months . | |||
|---|---|---|---|---|
| TG, mg/dL | 13.6 | (−6.4 to 33.6) | 89.5 | 10 |
| LDL-C, mg/dL | −5.7 | (−19.5 to 8.1) | 85.1 | 7 |
| HDL-C, mg/dL | 0.3 | (−4.3 to 5.0) | 77.4 | 8 |
| TC, mg/dL | −7.9 | (−19.9 to 4.1) | 82.5 | 10 |
| . | 12 Months . | |||
|---|---|---|---|---|
| TG, mg/dL | 13.6 | (−6.4 to 33.6) | 89.5 | 10 |
| LDL-C, mg/dL | −5.7 | (−19.5 to 8.1) | 85.1 | 7 |
| HDL-C, mg/dL | 0.3 | (−4.3 to 5.0) | 77.4 | 8 |
| TC, mg/dL | −7.9 | (−19.9 to 4.1) | 82.5 | 10 |
| . | ≥24 Months . | |||
|---|---|---|---|---|
| TG, mg/dL | 31.9 | (3.9 to 59.9) | 94.4 | 6 |
| LDL-C, mg/dL | 6.6 | (−9.7 to 22.9) | 83.9 | 5 |
| HDL-C, mg/dL | 0.4 | (−7.7 to 8.5) | 93.5 | 5 |
| TC, mg/dL | 9.5 | (−5.8 to 24.9) | 87.4 | 6 |
| . | ≥24 Months . | |||
|---|---|---|---|---|
| TG, mg/dL | 31.9 | (3.9 to 59.9) | 94.4 | 6 |
| LDL-C, mg/dL | 6.6 | (−9.7 to 22.9) | 83.9 | 5 |
| HDL-C, mg/dL | 0.4 | (−7.7 to 8.5) | 93.5 | 5 |
| TC, mg/dL | 9.5 | (−5.8 to 24.9) | 87.4 | 6 |
Abbreviation: TC, total cholesterol.
Change in Lipid Profile in MTF Transgender Individuals at Different Periods (Follow-Up to Baseline)
| . | Estimate . | 95% CI . | I2 . | Number of Studies . |
|---|---|---|---|---|
| . | 3–6 Months . | |||
| TG, mg/dL | 3.8 | (−11.6 to 19.3) | 63.5 | 6 |
| LDL-C, mg/dL | −3.1 | (−11.6 to 5.4) | 0 | 3 |
| HDL-C, mg/dL | 1.2 | (−5.7 to 8.1) | 70.0 | 5 |
| TC, mg/dL | −1.1 | (−9.4 to 7.2) | 0 | 4 |
| . | Estimate . | 95% CI . | I2 . | Number of Studies . |
|---|---|---|---|---|
| . | 3–6 Months . | |||
| TG, mg/dL | 3.8 | (−11.6 to 19.3) | 63.5 | 6 |
| LDL-C, mg/dL | −3.1 | (−11.6 to 5.4) | 0 | 3 |
| HDL-C, mg/dL | 1.2 | (−5.7 to 8.1) | 70.0 | 5 |
| TC, mg/dL | −1.1 | (−9.4 to 7.2) | 0 | 4 |
| . | 12 Months . | |||
|---|---|---|---|---|
| TG, mg/dL | 13.6 | (−6.4 to 33.6) | 89.5 | 10 |
| LDL-C, mg/dL | −5.7 | (−19.5 to 8.1) | 85.1 | 7 |
| HDL-C, mg/dL | 0.3 | (−4.3 to 5.0) | 77.4 | 8 |
| TC, mg/dL | −7.9 | (−19.9 to 4.1) | 82.5 | 10 |
| . | 12 Months . | |||
|---|---|---|---|---|
| TG, mg/dL | 13.6 | (−6.4 to 33.6) | 89.5 | 10 |
| LDL-C, mg/dL | −5.7 | (−19.5 to 8.1) | 85.1 | 7 |
| HDL-C, mg/dL | 0.3 | (−4.3 to 5.0) | 77.4 | 8 |
| TC, mg/dL | −7.9 | (−19.9 to 4.1) | 82.5 | 10 |
| . | ≥24 Months . | |||
|---|---|---|---|---|
| TG, mg/dL | 31.9 | (3.9 to 59.9) | 94.4 | 6 |
| LDL-C, mg/dL | 6.6 | (−9.7 to 22.9) | 83.9 | 5 |
| HDL-C, mg/dL | 0.4 | (−7.7 to 8.5) | 93.5 | 5 |
| TC, mg/dL | 9.5 | (−5.8 to 24.9) | 87.4 | 6 |
| . | ≥24 Months . | |||
|---|---|---|---|---|
| TG, mg/dL | 31.9 | (3.9 to 59.9) | 94.4 | 6 |
| LDL-C, mg/dL | 6.6 | (−9.7 to 22.9) | 83.9 | 5 |
| HDL-C, mg/dL | 0.4 | (−7.7 to 8.5) | 93.5 | 5 |
| TC, mg/dL | 9.5 | (−5.8 to 24.9) | 87.4 | 6 |
Abbreviation: TC, total cholesterol.
Important patient outcomes
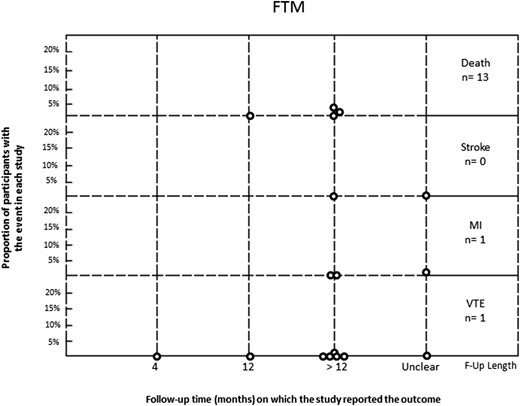
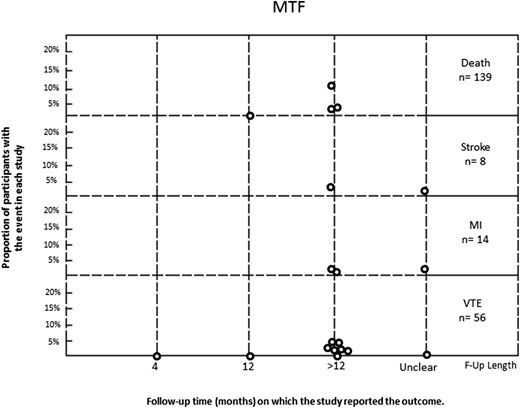
In the MTF group, 56 VTE events occurred in 1767 patients. Rates in the included studies varied from 0% to 5%. In the FTM group, VTE was reported in only one of 771 individuals. Individual study rates ranged from 0% to 0.34%. Stroke was reported in eight of 859 MTF participants and was not reported in any of the 340 FTM participants. Myocardial infarction was reported in 14 of 1073 MTF transgender individuals and one of 478 FTM transgender individuals. Mortality was reported in 139 of 1486 MTF participants and in 13 of 651 FTM participants, with a range in individual studies of 0% to 13% and 0% to 3%, respectively (Figs. 2 and 3). In the studies that reported cause of death, there were 23 cardiovascular-related deaths and 26 suicides in MTF transgender individuals, whereas there was one cardiovascular-related death and one suicide in FTM transgender individuals.
Rate of events (death, stroke, MI, VTE) in FTM transgender individuals treated with sex steroids. F-Up, follow up; MI, myocardial infarction.
Rate of events (death, stroke, MI, VTE) in MTF transgender individuals treated with sex steroids. F-Up, follow up; MI, myocardial infarction.
Subgroup and sensitivity analyses
We performed a sensitivity analysis that included FTM transgender individuals who were treated with only IM testosterone (six studies). The change in serum total cholesterol level became statistically significant with an increase of 10.2 mg/dL (95% CI: 1.7 to 18.6 mg/dL; I2 = 31.2%) at 12 months. Moreover, there was a statistically significant increase in serum LDL-C levels (11.9 mg/dL; 95% CI: 5.9 to 17.9 mg/dL; I2 = 0%) and a decrease in HDL-C level (−8.1 mg/dL; 95% CI: −10.9 to −5.3 mg/dL; I2 = 14.6%). There was no significant change in serum TG levels (8.9 mg/dL; 95% CI: −6.3 to 24.1 mg/dL; I2 = 53.7%).
We performed subgroup analyses comparing MTF transgender individuals treated with oral vs transdermal estrogens (in the setting of cross-sex hormonal therapy) with regard to lipid fraction changes across different time periods. The only significant interaction was observed in serum TG levels at 3 to 6 months, with the oral group experiencing an increase of 28.2 mg/dL (95% CI: 0.5 to 55.9 mg/dL; I2 = 0%) vs a decrease of 4.8 mg/dL (95% CI: −21.2 to 11.6 mg/dL; I2 = 0%) in the transdermal group (P = 0.04). Because of inconsistent reporting, we were unable to conduct the other preplanned subgroup analyses.
Discussion
We performed a systematic review and meta-analysis to summarize the effect of sex steroid therapy on lipid levels and important cardiovascular outcomes in transgender individuals. In FTM transgender individuals, sex steroid therapy was associated with an increase in LDL-C and TG levels and a decrease in HDL-C level. In MTF transgender individuals, sex steroid therapy was associated with an increase in TG level, which was driven by oral estrogen treatment. Data about important patient outcomes such as myocardial infarction, stroke, VTE, and mortality were insufficient to allow any meaningful assessment, although a higher incidence of these events was found among MTF transgender individuals. These results were driven by data from one center where a large number of MTF transgender individuals received a fairly high dose of oral estrogens (36). Therefore, the only identifiable effect of cross-sex hormone therapy appears to be on lipid fractions, which are surrogate outcomes of limited patient importance (51–53). The quality of evidence is low because of the uncontrolled and observational nature of the included studies, small number of events leading to imprecision of estimates, short and varied duration of follow-up, heterogeneity of treatment regimens, and inconsistency of results across studies that was unexplained by subgroup analyses (54).
Limitations and strengths
Incomplete searching and arbitrary study selection represent potential limitations of systematic reviews. However, the rigorous and comprehensive nature of our overlapping search strategies, without language restrictions and with a medical librarian’s input, should have minimized the possibility that we missed studies that could have substantially changed the inferences we drew (55). The risk of reporting bias is high, particularly when the body of evidence is based on small observational studies. We attempted to decrease the chances of reporting bias by contacting the authors; however, the response rate was low (56). Although it would have been clinically meaningful to evaluate the effects of some important patient characteristics on cardiovascular risk (e.g., smoking), we were unable to do so because of insufficient data. These limitations could not be overcome methodologically; however, our review exhibited important strengths because we sought to summarize the totality of the available evidence following a predesigned protocol, with reproducible judgments about study selection and quality and focused analyses, including an assessment of the effects of oral estrogen and IM testosterone therapy.
Implications for practice
Clinicians and transgender individuals need reliable evidence regarding the potential harm of sex steroid therapy to guide their management decisions. This review highlights the low quality of the available evidence and the overall uncertainty regarding the safety of cross-sex hormonal therapy.
Our findings suggest that in FTM transgender individuals, masculinizing hormone therapy (testosterone based) is associated with significant increases in LDL-C and TG levels and a decrease in HDL-C level. On the other hand, in MTF transgender individuals, feminizing hormone therapy (estrogen based) was associated with a statistically significant increase in TG levels; further analysis showed that the TG increase was seen with oral estrogen therapy in contrast to transdermal estrogen therapy, which led to a decrease in TG levels. Similar findings were reported in a previous systematic review (8). The magnitude of the observed decline in HDL-C level may appreciably increase the risk of cardiovascular events (57, 58), whereas the clinical effect of the observed changes in LDL-C and TG levels may be less (58–60). Nonetheless, because the lipid profile is a surrogate marker for overall cardiovascular health, the effect of these findings on important patient outcomes such as myocardial infarction and stroke during long-term therapy remains uncertain.
The effect of sex steroid use has been studied extensively in different patient populations, providing indirect evidence for clinical care of transgender individuals. Testosterone use in postmenopausal women has been associated with a reduction in total cholesterol, HDL-C, and TG levels and an increase in LDL-C level. However, in addition to the fact that higher doses are used in FTM transition, long-term safety data are sparse, and the quality of the evidence is low (61). A recent meta-analysis of 35 randomized trials that included older men found a significant cardiovascular risk in participants using oral testosterone, whereas neither IM nor transdermal testosterone significantly changed cardiovascular risk (62). Uncertainty remains regarding the applicability of these studies to the care of FTM transgender individuals.
A systematic review of studies including healthy men did not find an association of endogenous estrogen level with incident cardiovascular disease, including myocardial infarction, stroke, or death from coronary heart disease (63). In the Coronary Drug Program, 5 mg of conjugated estrogen therapy in men 30 to 64 years of age with a history of myocardial infarction was associated with increased cardiovascular mortality, whereas 2.5 mg was associated with a trend toward increased risk of VTE (4). A recent Cochrane Database systematic review found that estrogen hormone therapy in postmenopausal women conferred no protective effect for all-cause mortality or myocardial infarction but increased the risk of stroke and VTE events (64). Observational evidence warranting low confidence in the estimates suggests that compared with transdermal estrogen, oral estrogen may be associated with increased risk of VTE but not myocardial infarction (65). Although it is unclear how applicable these results are to the care of MTF transgender individuals, the increased prevalence of cardiovascular disease in the MTF transgender population raises concern about the extent to which estrogen preparations can cause harmful events; if so, modifications of cross-sex hormone therapy may be necessary.
Clinicians prescribing cross-sex hormonal therapy need to share with transgender individuals the current uncertainty regarding potential side effects of masculinizing/feminizing hormone therapy and make treatment decisions based on patients’ values, preferences, and context (54, 66).
Implications for research
We have identified important knowledge gaps regarding the effects of cross-sex hormone therapy on cardiovascular outcomes. First, there is a paucity of data regarding myocardial infarction, stroke, or VTE events and mortality in this population, and the studies evaluating lipid changes have had short follow-up times. Second, literature addressing this clinical question in the pediatric/adolescent population is completely lacking. Research is needed to ascertain the safety of hormonal therapies in transgender individuals. Conducting research with low risk of bias can be challenging because of multiple barriers to health care in this population (e.g., underreporting of side effects, high rate of loss to follow-up). However, it is possible to conduct randomized trials nested within study center cohorts to test the relative safety of different cross-sex hormone regimens. Moreover, medical centers that provide care to transgender individuals should make it a priority to conduct long-term follow-up studies evaluating important patient outcomes (67). In this context, observational studies in which baseline cardiovascular risk is assessed and balanced between study groups, with proper ascertainment of exposure and outcome measures, are feasible and urgently needed.
Conclusion
Low-quality evidence due to methodological limitations of included studies, imprecision, and heterogeneity suggests that sex steroid therapy may increase LDL-C and TG levels and decrease HDL-C levels in FTM transgender individuals, whereas oral estrogen may increase TG level in MTF transgender individuals. Data about important patient outcomes, such as myocardial infarction, stroke, VTE, and mortality, remain sparse.
Abbreviations
- CI
confidence interval
- FTM
female-to-male
- GnRH
gonadotropin-releasing hormone
- HDL-C
high-density lipoprotein cholesterol
- IM
intramuscular
- LDL-C
low-density lipoprotein cholesterol
- MTF
male-to-female
- TG
triglyceride
- VTE
venous thromboembolism.
Acknowledgments
Financial Support: This systematic review was funded by a contract from the Endocrine Society.
Disclosure Summary: The authors have nothing to disclose.
References
Author notes
These authors contributed equally to this study.