-
PDF
- Split View
-
Views
-
Cite
Cite
K. Miyanishi, Y. Kamo, H. Ihara, T. Naka, M. Hirakawa, Y. Sugioka, Risk factors for dysbaric osteonecrosis, Rheumatology, Volume 45, Issue 7, July 2006, Pages 855–858, https://doi.org/10.1093/rheumatology/kel013
Close - Share Icon Share
Abstract
Objectives. Dysbaric osteonecrosis (DON) is a complication of ineffective decompression following exposure to high-pressure environments. This study was designed to determine risk factors for the occurrence of DON in divers.
Methods. Fifty-six male divers received skeletal examinations by radiography to assess the occurrence of DON. A questionnaire was used to obtain clinical and diving information, including diving experience and maximum diving depth. Blood samples were collected to analyse the levels of plasminogen activator inhibitor (PAI)-1, cholesterol, triglyceride, low-density lipoprotein, very low-density lipoprotein, high-density lipoprotein, apolipoprotein A1 and apolipoprotein B.
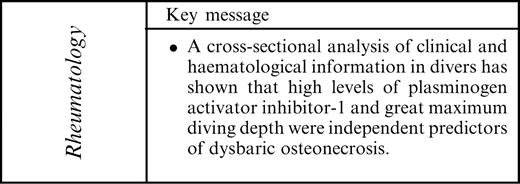
Results. Lesions of DON were detected in 31 of the 56 (55%) divers. Multivariate logistic regression analysis showed that high levels of PAI-1, a coagulation marker (odds ratio 4.281; P=0.0296) and great maximum diving depth (odds ratio 5.627; P=0.0231) were independent predictors of DON.
Conclusions. This study has shown the presence of coagulation abnormality in divers with DON. This result suggests that a pharmacological approach incorporating the use of an anticoagulant may represent a potential strategy for the prevention of DON.
Dysbaric osteonecrosis (DON) is a type of avascular bone necrosis seen in divers and compressed air workers [1–3]. The DON lesions characteristically occur in long bones that contain fatty marrow, such as the humerus, femur and tibia [4]. Ohta and Matsunaga demonstrated the radiographic features of DON [5]. When a lesion is juxta-articular, the articular surface may undergo collapse, resulting in osteoarthritic changes.
Although there is a clear aetiological association between hyperbaric exposure and DON, the underlying mechanisms of DON remain unclear [6]. Decompression-induced bubble formation in response to a reduction in ambient pressure is generally accepted as a causative mechanism for DON and decompression sickness [3, 6]. However, it has been reported that only 10% of compressed air workers who have experienced decompression sickness have DON and that 25% of those with DON have never had decompression sickness [7]. These data suggest that bubble formation is not sufficient to cause DON and that there may be some other predisposing factors for DON.
In this study we have conducted a cross-sectional analysis of clinical and haematological information in divers to determine risk factors for the occurrence of DON.
Materials and methods
Fifty-six male divers, who were engaged in shellfish collection or structural work in Ohura, Saga Prefecture, Japan, received radiographic skeletal examinations for DON after giving informed consent. The study was approved by the local ethics committee. The mean age of the study participants was 40.9±9.8 yr (mean±s.d.; range 21–64 yr).
Radiographic examination
Anteroposterior radiographs were obtained for six regions that included both sides of the shoulder, hip and knee joints. Radiographic findings were classified based on a system reported by Ohta and Matsunaga which includes type A (juxta-articular) and type B (head, neck and shaft) lesions [5].
Clinical information
A questionnaire was used to obtain personal clinical information that included age, history of bends and paraplegia, smoking and alcohol consumption. Smoking was defined as smoking of more than one cigarette per day. Alcohol consumption was defined as intake of more than 20 g of pure alcohol; this was based on a previous report [8]. The percentage of body fat was measured using a body fat analyser (TBF-215, TANITA Corporation, Tokyo, Japan). To generate diving profiles, the following information was collected: diving experience, maximum diving depth, average diving depth, average number of dives per day and average diving time per day.
Fasting blood samples were obtained to examine the blood levels of plasminogen activator inhibitor (PAI)-1, cholesterol, triglyceride, low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), high-density lipoprotein (HDL), apolipoprotein A1 (apoA1) and apolipoprotein B (apoB). Total PAI-1 was measured by latex photometric immunoassay [9]. Cholesterol and triglyceride levels were measured by enzymatic methods [10, 11]. Levels of LDL, VLDL and HDL were measured by polyacrylamide gel disc electrophoresis [12]. Serum levels of apoA1 and apoB were measured by turbidimetric immunoassay [13]. The ratio of apoB to apoA1 was calculated.
Statistical analysis
The incidence of type A and type B DON lesions was compared among the proximal humerus, proximal femur and knee joints using the χ2 test with Bonferroni methods for multiple comparisons.
Receiver operating characteristic curves were used to find the optimal cut-off level for clinical and haematological information, i.e. the level that can discriminate between divers with DON and those without DON. Univariate analyses were performed using logistic regression to identify significant variables predicting the presence of DON. Variables that were significant in univariate analyses (P<0.05) were taken as potential predictors of DON and were used as covariates in multivariate logistic regression analysis to identify independent predictors of DON. In this model, highly intercorrelated independent variables (r>0.7) were avoided. Odds ratios, adjusted odds ratios and 95% confidence intervals were computed for the variables. All analyses were performed using StatView software, version 5.0.1 (SAS Institute Inc., Cary, NC, USA).
Results
Radiographic examination
Radiography detected bone lesions in 31 of the 56 (55%) divers. DON lesions were seen in 39 of 112 (35%) proximal humeri, in 23 of 112 (21%) proximal femora and in 24 of 112 (21%) knee joints. The prevalence of type A lesions in the proximal humerus (32 out of 39 lesions, 82%) and in the proximal femur (15 out of 23 lesions, 65%) was significantly greater than in the knee joints (0 out of 24 lesions, 0%) (P<0.05). The number of affected regions per person was 1.5±1.8 (mean±s.d.) and ranged from 0 to 6.
Univariate and multivariate logistic regression analysis
The results of univariate logistic regression analysis are detailed in Table 1. Significant variables were age (P = 0.0035), history of bends (P = 0.0475), diving experience (P = 0.0041), maximum diving depth (P = 0.0013) and PAI-1 levels (P = 0.0053). Since age and diving experience were highly intercorrelated (r = 0.9309), age was not included as a covariate in the multivariate logistic regression analysis. The multivariate logistic regression analysis showed that PAI-1 levels greater than 38.0 ng/ml (odds ratio 4.281; P = 0.0296) and maximum diving depth greater than 35.0 m (odds ratio 5.627; P = 0.0231) were independent predictors of DON (Table 2).
Results of univariate logistic regression analysis
| . | DON+ (n = 31) . | DON− (n = 25) . | Cut-off level . | Regression coefficient . | Odds ratio . | 95% confidence interval . | P value . |
|---|---|---|---|---|---|---|---|
| Age (yr) | 44.5±8.9 | 36.4±9.0 | 43.0 | 1.751 | 5.758 | 1.776–18.671 | 0.0035 |
| Body fat (%) | 24.0±6.1 | 22.8±4.8 | 21.8 | 0.788 | 2.198 | 0.744–6.500 | 0.1544 |
| Bends | 23/31 (74%) | 12/25 (48%) | – | 1.136 | 3.115 | 1.012–9.583 | 0.0475 |
| Paraplegia | 9/31 (29%) | 4/25 (16%) | – | 0.764 | 2.148 | 0.573–8.048 | 0.2567 |
| Smoking | 22/31 (71%) | 20/25 (80%) | – | −0.492 | 0.611 | 0.175–2.133 | 0.4399 |
| Alcohol | 23/31 (74%) | 15/25 (60%) | – | 0.651 | 1.917 | 0.616–5.962 | 0.2611 |
| Diving experience (yr) | 24.9±10.5 | 17.1±11.1 | 25.0 | 1.686 | 5.400 | 1.705–17.106 | 0.0041 |
| Maximum diving depth (m) | 39.5±15.9 | 25.5±12.5 | 35.0 | 2.118 | 8.312 | 2.287–30.219 | 0.0013 |
| Average diving depth (m) | 14.5±7.6 | 11.0±3.9 | 15.0 | 1.088 | 2.969 | 0.933–9.443 | 0.0653 |
| Average number of dives per day (no) | 3.4±2.3 | 3.0±1.1 | 3.0 | 0.218 | 1.244 | 0.426–3.629 | 0.6893 |
| Average diving time per day (h) | 5.9±1.9 | 6.2±2.3 | 7.0 | 0.080 | 1.083 | 0.371–3.165 | 0.8836 |
| PAI-1 (ng/ml) | 59.1±34.6 | 37.5±17.7 | 38.0 | 1.631 | 5.111 | 1.624–16.085 | 0.0053 |
| Cholesterol (mg/dl) | 200.5±41.5 | 196.6±44.6 | 197.0 | 0.600 | 1.821 | 0.626–5.300 | 0.2711 |
| Triglyceride (mg/dl) | 151.8±129.9 | 151.8±122.8 | 109.0 | 0.114 | 1.121 | 0.390–3.224 | 0.8323 |
| Low-density lipoprotein (%) | 46.7±10.6 | 45.6±10.6 | 47.0 | 0.379 | 1.462 | 0.503–4.247 | 0.4856 |
| Very low-density lipoprotein (%) | 11.9±4.1 | 13.3±5.0 | 11.0 | −0.211 | 0.810 | 0.278–2.356 | 0.6982 |
| High-density lipoprotein (%) | 31.4±7.8 | 31.5±8.0 | 29.0 | 0.306 | 1.358 | 0.471–3.912 | 0.5713 |
| Apolipoprotein A1 (mg/dl) | 156.7±26.4 | 152.1±27.1 | 151.0 | 0.016 | 1.016 | 0.354–2.915 | 0.9770 |
| Apolipoprotein B (mg/dl) | 93.8±28.5 | 92.9±31.5 | 83.0 | 0.678 | 1.970 | 0.672–5.775 | 0.2167 |
| ApoB/ApoA1 ratio | 0.62±0.22 | 0.63±0.25 | 0.56 | 0.518 | 1.678 | 0.572–4.921 | 0.3454 |
| . | DON+ (n = 31) . | DON− (n = 25) . | Cut-off level . | Regression coefficient . | Odds ratio . | 95% confidence interval . | P value . |
|---|---|---|---|---|---|---|---|
| Age (yr) | 44.5±8.9 | 36.4±9.0 | 43.0 | 1.751 | 5.758 | 1.776–18.671 | 0.0035 |
| Body fat (%) | 24.0±6.1 | 22.8±4.8 | 21.8 | 0.788 | 2.198 | 0.744–6.500 | 0.1544 |
| Bends | 23/31 (74%) | 12/25 (48%) | – | 1.136 | 3.115 | 1.012–9.583 | 0.0475 |
| Paraplegia | 9/31 (29%) | 4/25 (16%) | – | 0.764 | 2.148 | 0.573–8.048 | 0.2567 |
| Smoking | 22/31 (71%) | 20/25 (80%) | – | −0.492 | 0.611 | 0.175–2.133 | 0.4399 |
| Alcohol | 23/31 (74%) | 15/25 (60%) | – | 0.651 | 1.917 | 0.616–5.962 | 0.2611 |
| Diving experience (yr) | 24.9±10.5 | 17.1±11.1 | 25.0 | 1.686 | 5.400 | 1.705–17.106 | 0.0041 |
| Maximum diving depth (m) | 39.5±15.9 | 25.5±12.5 | 35.0 | 2.118 | 8.312 | 2.287–30.219 | 0.0013 |
| Average diving depth (m) | 14.5±7.6 | 11.0±3.9 | 15.0 | 1.088 | 2.969 | 0.933–9.443 | 0.0653 |
| Average number of dives per day (no) | 3.4±2.3 | 3.0±1.1 | 3.0 | 0.218 | 1.244 | 0.426–3.629 | 0.6893 |
| Average diving time per day (h) | 5.9±1.9 | 6.2±2.3 | 7.0 | 0.080 | 1.083 | 0.371–3.165 | 0.8836 |
| PAI-1 (ng/ml) | 59.1±34.6 | 37.5±17.7 | 38.0 | 1.631 | 5.111 | 1.624–16.085 | 0.0053 |
| Cholesterol (mg/dl) | 200.5±41.5 | 196.6±44.6 | 197.0 | 0.600 | 1.821 | 0.626–5.300 | 0.2711 |
| Triglyceride (mg/dl) | 151.8±129.9 | 151.8±122.8 | 109.0 | 0.114 | 1.121 | 0.390–3.224 | 0.8323 |
| Low-density lipoprotein (%) | 46.7±10.6 | 45.6±10.6 | 47.0 | 0.379 | 1.462 | 0.503–4.247 | 0.4856 |
| Very low-density lipoprotein (%) | 11.9±4.1 | 13.3±5.0 | 11.0 | −0.211 | 0.810 | 0.278–2.356 | 0.6982 |
| High-density lipoprotein (%) | 31.4±7.8 | 31.5±8.0 | 29.0 | 0.306 | 1.358 | 0.471–3.912 | 0.5713 |
| Apolipoprotein A1 (mg/dl) | 156.7±26.4 | 152.1±27.1 | 151.0 | 0.016 | 1.016 | 0.354–2.915 | 0.9770 |
| Apolipoprotein B (mg/dl) | 93.8±28.5 | 92.9±31.5 | 83.0 | 0.678 | 1.970 | 0.672–5.775 | 0.2167 |
| ApoB/ApoA1 ratio | 0.62±0.22 | 0.63±0.25 | 0.56 | 0.518 | 1.678 | 0.572–4.921 | 0.3454 |
Results of univariate logistic regression analysis
| . | DON+ (n = 31) . | DON− (n = 25) . | Cut-off level . | Regression coefficient . | Odds ratio . | 95% confidence interval . | P value . |
|---|---|---|---|---|---|---|---|
| Age (yr) | 44.5±8.9 | 36.4±9.0 | 43.0 | 1.751 | 5.758 | 1.776–18.671 | 0.0035 |
| Body fat (%) | 24.0±6.1 | 22.8±4.8 | 21.8 | 0.788 | 2.198 | 0.744–6.500 | 0.1544 |
| Bends | 23/31 (74%) | 12/25 (48%) | – | 1.136 | 3.115 | 1.012–9.583 | 0.0475 |
| Paraplegia | 9/31 (29%) | 4/25 (16%) | – | 0.764 | 2.148 | 0.573–8.048 | 0.2567 |
| Smoking | 22/31 (71%) | 20/25 (80%) | – | −0.492 | 0.611 | 0.175–2.133 | 0.4399 |
| Alcohol | 23/31 (74%) | 15/25 (60%) | – | 0.651 | 1.917 | 0.616–5.962 | 0.2611 |
| Diving experience (yr) | 24.9±10.5 | 17.1±11.1 | 25.0 | 1.686 | 5.400 | 1.705–17.106 | 0.0041 |
| Maximum diving depth (m) | 39.5±15.9 | 25.5±12.5 | 35.0 | 2.118 | 8.312 | 2.287–30.219 | 0.0013 |
| Average diving depth (m) | 14.5±7.6 | 11.0±3.9 | 15.0 | 1.088 | 2.969 | 0.933–9.443 | 0.0653 |
| Average number of dives per day (no) | 3.4±2.3 | 3.0±1.1 | 3.0 | 0.218 | 1.244 | 0.426–3.629 | 0.6893 |
| Average diving time per day (h) | 5.9±1.9 | 6.2±2.3 | 7.0 | 0.080 | 1.083 | 0.371–3.165 | 0.8836 |
| PAI-1 (ng/ml) | 59.1±34.6 | 37.5±17.7 | 38.0 | 1.631 | 5.111 | 1.624–16.085 | 0.0053 |
| Cholesterol (mg/dl) | 200.5±41.5 | 196.6±44.6 | 197.0 | 0.600 | 1.821 | 0.626–5.300 | 0.2711 |
| Triglyceride (mg/dl) | 151.8±129.9 | 151.8±122.8 | 109.0 | 0.114 | 1.121 | 0.390–3.224 | 0.8323 |
| Low-density lipoprotein (%) | 46.7±10.6 | 45.6±10.6 | 47.0 | 0.379 | 1.462 | 0.503–4.247 | 0.4856 |
| Very low-density lipoprotein (%) | 11.9±4.1 | 13.3±5.0 | 11.0 | −0.211 | 0.810 | 0.278–2.356 | 0.6982 |
| High-density lipoprotein (%) | 31.4±7.8 | 31.5±8.0 | 29.0 | 0.306 | 1.358 | 0.471–3.912 | 0.5713 |
| Apolipoprotein A1 (mg/dl) | 156.7±26.4 | 152.1±27.1 | 151.0 | 0.016 | 1.016 | 0.354–2.915 | 0.9770 |
| Apolipoprotein B (mg/dl) | 93.8±28.5 | 92.9±31.5 | 83.0 | 0.678 | 1.970 | 0.672–5.775 | 0.2167 |
| ApoB/ApoA1 ratio | 0.62±0.22 | 0.63±0.25 | 0.56 | 0.518 | 1.678 | 0.572–4.921 | 0.3454 |
| . | DON+ (n = 31) . | DON− (n = 25) . | Cut-off level . | Regression coefficient . | Odds ratio . | 95% confidence interval . | P value . |
|---|---|---|---|---|---|---|---|
| Age (yr) | 44.5±8.9 | 36.4±9.0 | 43.0 | 1.751 | 5.758 | 1.776–18.671 | 0.0035 |
| Body fat (%) | 24.0±6.1 | 22.8±4.8 | 21.8 | 0.788 | 2.198 | 0.744–6.500 | 0.1544 |
| Bends | 23/31 (74%) | 12/25 (48%) | – | 1.136 | 3.115 | 1.012–9.583 | 0.0475 |
| Paraplegia | 9/31 (29%) | 4/25 (16%) | – | 0.764 | 2.148 | 0.573–8.048 | 0.2567 |
| Smoking | 22/31 (71%) | 20/25 (80%) | – | −0.492 | 0.611 | 0.175–2.133 | 0.4399 |
| Alcohol | 23/31 (74%) | 15/25 (60%) | – | 0.651 | 1.917 | 0.616–5.962 | 0.2611 |
| Diving experience (yr) | 24.9±10.5 | 17.1±11.1 | 25.0 | 1.686 | 5.400 | 1.705–17.106 | 0.0041 |
| Maximum diving depth (m) | 39.5±15.9 | 25.5±12.5 | 35.0 | 2.118 | 8.312 | 2.287–30.219 | 0.0013 |
| Average diving depth (m) | 14.5±7.6 | 11.0±3.9 | 15.0 | 1.088 | 2.969 | 0.933–9.443 | 0.0653 |
| Average number of dives per day (no) | 3.4±2.3 | 3.0±1.1 | 3.0 | 0.218 | 1.244 | 0.426–3.629 | 0.6893 |
| Average diving time per day (h) | 5.9±1.9 | 6.2±2.3 | 7.0 | 0.080 | 1.083 | 0.371–3.165 | 0.8836 |
| PAI-1 (ng/ml) | 59.1±34.6 | 37.5±17.7 | 38.0 | 1.631 | 5.111 | 1.624–16.085 | 0.0053 |
| Cholesterol (mg/dl) | 200.5±41.5 | 196.6±44.6 | 197.0 | 0.600 | 1.821 | 0.626–5.300 | 0.2711 |
| Triglyceride (mg/dl) | 151.8±129.9 | 151.8±122.8 | 109.0 | 0.114 | 1.121 | 0.390–3.224 | 0.8323 |
| Low-density lipoprotein (%) | 46.7±10.6 | 45.6±10.6 | 47.0 | 0.379 | 1.462 | 0.503–4.247 | 0.4856 |
| Very low-density lipoprotein (%) | 11.9±4.1 | 13.3±5.0 | 11.0 | −0.211 | 0.810 | 0.278–2.356 | 0.6982 |
| High-density lipoprotein (%) | 31.4±7.8 | 31.5±8.0 | 29.0 | 0.306 | 1.358 | 0.471–3.912 | 0.5713 |
| Apolipoprotein A1 (mg/dl) | 156.7±26.4 | 152.1±27.1 | 151.0 | 0.016 | 1.016 | 0.354–2.915 | 0.9770 |
| Apolipoprotein B (mg/dl) | 93.8±28.5 | 92.9±31.5 | 83.0 | 0.678 | 1.970 | 0.672–5.775 | 0.2167 |
| ApoB/ApoA1 ratio | 0.62±0.22 | 0.63±0.25 | 0.56 | 0.518 | 1.678 | 0.572–4.921 | 0.3454 |
Results of multivariate logistic regression analysis
| . | Cut-off level . | Regression coefficient . | Odds ratio . | 95% confidence interval . | P value . |
|---|---|---|---|---|---|
| Bends | – | −0.086 | 0.917 | 0.197–4.261 | 0.9124 |
| Diving experience (yr) | 25.0 | 1.121 | 3.068 | 0.716–13.142 | 0.1310 |
| Maximum diving depth (m) | 35.0 | 1.728 | 5.627 | 1.268–24.977 | 0.0231 |
| PAI-1 (ng/ml) | 38.0 | 1.454 | 4.281 | 1.155–15.864 | 0.0296 |
| . | Cut-off level . | Regression coefficient . | Odds ratio . | 95% confidence interval . | P value . |
|---|---|---|---|---|---|
| Bends | – | −0.086 | 0.917 | 0.197–4.261 | 0.9124 |
| Diving experience (yr) | 25.0 | 1.121 | 3.068 | 0.716–13.142 | 0.1310 |
| Maximum diving depth (m) | 35.0 | 1.728 | 5.627 | 1.268–24.977 | 0.0231 |
| PAI-1 (ng/ml) | 38.0 | 1.454 | 4.281 | 1.155–15.864 | 0.0296 |
Results of multivariate logistic regression analysis
| . | Cut-off level . | Regression coefficient . | Odds ratio . | 95% confidence interval . | P value . |
|---|---|---|---|---|---|
| Bends | – | −0.086 | 0.917 | 0.197–4.261 | 0.9124 |
| Diving experience (yr) | 25.0 | 1.121 | 3.068 | 0.716–13.142 | 0.1310 |
| Maximum diving depth (m) | 35.0 | 1.728 | 5.627 | 1.268–24.977 | 0.0231 |
| PAI-1 (ng/ml) | 38.0 | 1.454 | 4.281 | 1.155–15.864 | 0.0296 |
| . | Cut-off level . | Regression coefficient . | Odds ratio . | 95% confidence interval . | P value . |
|---|---|---|---|---|---|
| Bends | – | −0.086 | 0.917 | 0.197–4.261 | 0.9124 |
| Diving experience (yr) | 25.0 | 1.121 | 3.068 | 0.716–13.142 | 0.1310 |
| Maximum diving depth (m) | 35.0 | 1.728 | 5.627 | 1.268–24.977 | 0.0231 |
| PAI-1 (ng/ml) | 38.0 | 1.454 | 4.281 | 1.155–15.864 | 0.0296 |
Discussion
Several possible pathogenic mechanisms of steroid-induced osteonecrosis have been suggested by experimental studies, including thrombophilic and hypofibrinolytic coagulation abnormalities and hyperlipidaemia [14–17]. Vascular occlusion may be caused by mechanical interruption due to thrombi, lipid emboli or extravascular compression associated with enlargement of fat cells [14–17]. The combined use of an anticoagulant and a lipid-lowering agent has been shown to prevent steroid-induced osteonecrosis in rabbits [18]. Such prophylactic medication may be an ideal strategy for the prevention of DON.
On the basis of this knowledge of steroid-induced osteonecrosis, we hypothesized that coagulation abnormalities or hyperlipidaemia might be present in divers with DON. The haematological factors examined in this study have been reported to be risk factors for steroid-induced osteonecrosis [19–23]. The multivariate logistic regression analysis showed that a PAI-1 level greater than 38.0 ng/ml (cut-off level) is an independent predictor for the presence of DON in divers with an odds ratio of 4.281. Increased levels of PAI-1 may result in thrombus formation and thus contribute to the development of ischaemia. It is of note that high levels of PAI-1 were found in both divers with DON and patients with steroid-induced osteonecrosis [19–21], suggesting that these two disorders in part share coagulation abnormality as a common pathogenic pathway.
Since this is a cross-sectional study, the time of occurrence of high PAI-1 levels is unknown. High PAI-1 levels may potentially have been present in divers before their repeated decompressions. Other explanations include the hypothesis of nitrogen bubble-induced coagulation [24, 25]. Jones et al. [24, 25] hypothesized that the marrow adipose tissue can be injured by rapidly expanding nitrogen gas, which triggers local and systemic intravascular coagulation. Damage to marrow adipocytes may lead to further intravascular coagulation (presumably high levels of PAI-1), intramedullary stasis and ischaemia. Further study will be needed to determine the significance of coagulation in DON. It will be necessary to analyse other coagulation factors, including protein S, protein C and lipoprotein (a). Expression analysis of coagulation-related genes and proteins in local bone tissues may also be needed.
An important limitation of this study is that only divers were examined and that healthy controls were not. Average levels of haematological factors examined here in the normal population were not clarified. Our preliminary data showed that the PAI-1 level of five healthy volunteers (four men and a woman, age 30.2±9.5 yr) is 43.6±9.1 ng/ml.
The magnitude of pressure has been shown to be associated with the incidence of DON. McCallum and Walder [26] reported that osteonecrosis in caisson workers was directly related to the degree of pressure and the number of exposures. Ohta and Matsunaga [5] found that there was a high incidence of DON lesions in divers who usually dived to depths of greater than 30 m compared with divers who dived to shallower depths. Our observation of a significant association between maximum diving depth and development of DON corroborates this finding.
We found 13 divers suffering from spinal paraplegia. The maximum diving depth of divers with paraplegia (43±14 m) was significantly greater than that of those without paraplegia (30±15 m) (P = 0.011 by Student's t-test). Based on this observation, the paraplegia was most likely due to type II decompression sickness as a result of diving. However, the possibility of other spinal disorders such as lumbar disc herniation or lumbar spinal canal stenosis cannot be completely excluded.
In conclusion, this study has shown that a high level of PAI-1 is a risk factor for DON in divers. These results suggest that, in addition to strict decompression tables, a pharmacological approach using an anticoagulant such as warfarin may be a potential method for the prevention of DON.
We thank Mr Sou Fukawa (ANOVA, Asaka, Japan) for his useful advice on the statistical analysis. This research was supported by research funds to promote the hospital functions of Japan Labour Health and Welfare Organization.
The authors declare no conflicts of interest.
References
Medical Research Council Decompression Sickness Central Registry and Radiological Panel. Aseptic bone necrosis in commercial divers.
Kawashima M, Torisu T, Hayashi K, Kitano M. Pathological review of osteonecrosis in divers.
Catto M. Pathology of aseptic bone necrosis. In: Davidson JK, ed. Aseptic necrosis of bone. Amsterdam:
Chryssanthou CP. Dysbaric osteonecrosis. Etiological and pathogenetic concepts.
Walder DN. Bone necrosis. In: Jardine FM, McCallum RI, eds. Engineering and health in compressed air work.
Tsugane S, Fahey MT, Sasaki S, Baba S. Alcohol consumption and all-cause and cancer mortality among middle-aged Japanese men: seven-year follow-up of the JPHC study Cohort I. Japan Public Health Center.
Ono T, Sogabe M, Ogura M, Furusaki F. Automated latex photometric immunoassay for total plasminogen activator inhibitor-1 in plasma.
The recognition and management of hyperlipidaemia in adults: a policy statement of the European Atherosclerosis Society.
Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol.
Narayan KA, Narayan S, Kummerow FA. Disk electrophoresis of human serum lipoproteins.
Noma A, Hata Y, Goto Y. Quantitation of serum apolipoprotein A-I, A-II, B, C-II, C-III and E in healthy Japanese by turbidimetric immunoassay: reference values, and age- and sex-related differences.
Jones JP, Jr. Fat embolism, intravascular coagulation, and osteonecrosis.
Glueck CJ, Freiberg RA, Fontaine RN, Tracy T, Wang P. Hypofibrinolysis, thrombophilia, osteonecrosis.
Miyanishi K, Yamamoto T, Irisa T et al. Bone marrow fat cell enlargement and a rise in intraosseous pressure in steroid-treated rabbits with osteonecrosis.
Yamamoto T, Irisa T, Sugioka Y, Sueishi K. Effects of pulse methylprednisolone on bone and marrow tissues: corticosteroid-induced osteonecrosis in rabbits.
Motomura G, Yamamoto T, Miyanishi K, Jingushi S, Iwamoto Y. Combined effects of an anticoagulant and a lipid-lowering agent on the prevention of steroid-induced osteonecrosis in rabbits.
Glueck CJ, Glueck HI, Mieczkowski L, Tracy T, Speirs J, Stroop D. Familial high plasminogen activator inhibitor with hypofibrinolysis, a new pathophysiologic cause of osteonecrosis?
Glueck CJ, Fontaine RN, Gruppo R et al. The plasminogen activator inhibitor-1 gene, hypofibrinolysis, and osteonecrosis.
Miyanishi K, Yamamoto T, Irisa T et al. A high low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio as a potential risk factor for corticosteroid-induced osteonecrosis in rabbits.
Miyanishi K, Yamamoto T, Irisa T, Noguchi Y, Sugioka Y, Iwamoto Y. Increased level of apolipoprotein B/apolipoprotein A1 ratio as a potential risk for osteonecrosis.
Jones JP Jr, Ramirez S, Doty SB. The pathophysiologic role of fat in dysbaric osteonecrosis.
- anticoagulants
- low-density lipoproteins
- triglycerides
- diagnostic radiologic examination
- plasminogen activator inhibitor
- cholesterol
- high density lipoproteins
- apolipoproteins b
- blood coagulation
- diving
- plasminogen activator inhibitor 1
- apolipoprotein a-i
- osteonecrosis
- pharmacology
- coagulation process
- prevention




Comments