-
PDF
- Split View
-
Views
-
Cite
Cite
Andreas L. Serra, Albin A. Schwarz, Franziska H. Wick, Hans-Peter Marti, Rudolf P. Wüthrich, Successful treatment of hypercalcemia with cinacalcet in renal transplant recipients with persistent hyperparathyroidism, Nephrology Dialysis Transplantation, Volume 20, Issue 7, July 2005, Pages 1315–1319, https://doi.org/10.1093/ndt/gfh925
Close - Share Icon Share
Abstract
Background. Cinacalcet lowers plasma parathyroid hormone (PTH) levels in primary and secondary hyperparathyroidism. The efficacy and safety of cinacalcet have not been examined in renal transplant patients with persistent hyperparathyroidism. The aim of this study was to evaluate the effect of cinacalcet as a novel therapy for the management of such patients.
Methods. Eleven renal allograft recipients with persistent hyperparathyroidism were treated with cinacalcet. The total study time was 10 weeks. Individual cinacalcet doses were adjusted to obtain a serum calcium in the predefined normal target range of 2.10–2.60 mmol/l.
Results. Serum calcium decreased significantly from 2.73±0.05 mmol/l to 2.44±0.05 and 2.42± 0.04 mmol/l after 2 and 10 weeks of treatment, respectively. All patients reached the target range rapidly and remained normocalcaemic throughout the study. Serum PTH significantly decreased 16.1 and 21.8% at study weeks 2 and 10, respectively, compared with week 0. Serum phosphate increased. Renal function remained stable and no allograft rejection was observed. From weeks 2 to 10, daily cinacalcet doses administered were 30 mg (n = 8), 15 mg (n = 1) and 60 mg (n = 1), respectively.
Conclusion. Cinacalcet was effective in correcting the hypercalcaemia associated with persistent hyperparathyroidism after renal transplantation. It appears to be safe. Thus, cinacalcet represents a promising alternative for parathyroidectomy in these patients.
Introduction
Tertiary hyperparathyroidism is common after renal transplantation [1,2]. The disorder is characterized by persistently elevated serum calcium and increased or inappropriately high-normal parathyroid hormone (PTH) levels. Parathyroidectomy represents the definitive treatment for persistent hyperparathyroidism and is recommended for symptomatic hypercalcaemia, or if the plasma calcium concentration remains abnormal for >1 year [3,4]. Many patients remain untreated because they do not meet the accepted guidelines for parathyroidectomy or do not wish to have surgery. Nevertheless, they may be at increased risk for bone disease and vascular calcification.
In primary [5,6] and secondary [7] hyperparathyroidism, the calcimimetic drug cinacalcet effectively lowers PTH levels. Since the efficacy and safety of cinacalcet in patients with a functioning kidney graft have not been studied, we designed a prospective study to examine the effect of cinacalcet on serum calcium and PTH levels in renal allograft recipients with persistent hyperparathyroidism.
Methods
Patients and study design
A prospective single centre, open label study was performed in 11 renal transplant recipients with persistent hyperparathyroidism and normal allograft function. The study was approved by the local ethics committee. All patients gave written informed consent. Patients were included if they met all of the following criteria: total serum calcium ≥2.60 mmol/l (measured at least twice within 6 months, normal range, 2.10–2.60 mmol/l), plasma intact PTH concentration ≥65 ng/l (normal range, 15–65 ng/l), duration of renal transplantation ≥6 months, ≥18 years of age, measured creatinine clearance (CrCl) ≥40 ml/min/1.73 m2, normal serum 1,25-dihydroxyvitamin D (normal range, 19.0–67.0 ng/l) and 25-hydroxyxvitamin D (normal range, 10–42 µg/l) levels, and stable maintenance immunosuppressive therapy over the last 6 months. Dietary calcium intake was not restricted but kept stable during the course of the study. Diuretics were not permitted during the study period. Exclusion criteria included pregnancy, elevated liver enzymes (serum aspartate aminotransferase and alanine aminotransferase), concurrent or preceding (within 3 months of inclusion) therapy with flecainide, thioridazine, bi- or tricyclic antidepressants, vitamin D sterols, calcium supplementation, bisphophonates or fluoride, and clinically suspected or biopsy-proven renal allograft rejection within 6 months before study start.
After inclusion, patients received 30 mg of cinacalcet once daily in the evening. The cinacalcet dosage was adapted every 2 weeks to keep the serum calcium in the predefined target range of 2.10–2.60 mmol/l independently of intact PTH level. Oral phosphate supplementation was stopped if the serum phosphate level was >0.87 mmol/l (normal range, 0.87–1.45 mmol/l). Blood pressure was measured at every study visit by an automatic blood pressure monitor (Boso-Medicus, Jungingen, Germany). The total study time was 10 weeks.
Laboratory analyses
Blood samples were collected for measurements of serum total and ionized calcium, serum phosphate, plasma intact PTH, plasma creatinine, serum urea, serum albumin, whole blood trough level of cyclosporin or tacrolimus and liver enzymes every 2 weeks after an overnight fast and before the administration of the immunosuppressive drugs. The glomerular filtration rate was calculated every 2 weeks according to the Modification of Diet in Renal Disease (MDRD) study group using the following values: plasma creatinine, serum urea, serum albumin, gender, race and age [8]. Urine was collected over 24 h at baseline and at week 10 for the measurement of creatinine clearance and fractional calcium and phosphate excretion rates. Calcium, phosphate and liver enzymes were measured by standard methods, creatinine by the modified Jaffé method and plasma intact PTH using a double antibody chemiluminescence immunoassay (Roche Diagnostics Switzerland). Serum 1,25-dihydroxyvitamin D and 25-hydroxyxvitamin D were measured by radioreceptor assay, and the whole blood concentration of cyclosporin and tacrolimus by immunoassay. All biochemical analyses were performed at the Department of Clinical Chemistry of our institution.
Statistical analysis
Means of continuous data were compared by the Student's t-test. P-values were two sided for the comparison with the baseline value, and those <0.05 were considered statistically significant. All laboratory results have been expressed as means±SE. Values that were not normally distributed were expressed as medians (minimum–maximum). All analyses were performed using SPSS software (version 12.0, SPSS Inc., Chicago, IL).
Results
Relevant demographic, clinical and laboratory features of our patients are presented in Table 1. All patients were Caucasians. Patient no. 3 had undergone parathyroidectomy 4 years ago. Three patients were on oral phosphate supplementation at study inclusion. One patient (no. 9) discontinued the study after 2 weeks because of visual problems (see below).
Patient demographics and characteristics at baseline (week 0)
| No. . | Age (years) . | Sex . | Dialysis (months) . | Time from RTx to study week 0 (months) . | Creatinine clearance (ml/min/1.73 m2) . | Immunosuppression . |
|---|---|---|---|---|---|---|
| 1 | 51 | M | 36 | 6 | 69.92 | CyA + MMF + PDN |
| 2 | 64 | M | 30 | 17 | 45.56 | CyA + MMF |
| 3 | 49 | F | 45 | 29 | 49.37 | Tac + MMF |
| 4 | 70 | F | 84 | 10 | 55.32 | CyA + MMF + PDN |
| 5 | 50 | F | 12 | 364 | 72.46 | Aza + PDN |
| 6 | 59 | F | 41 | 14 | 43.37 | CyA + Aza |
| 7 | 61 | M | 0 | 28 | 68.35 | CyA + MMF |
| 8 | 55 | F | 0 | 37 | 43.20 | CyA + MMF |
| 9 | 62 | F | 35 | 28 | 49.14 | CyA + MMF |
| 10 | 70 | M | 29 | 178 | 61.92 | CyA + Aza |
| 11 | 59 | M | 33 | 55 | 44.71 | CyA + MMF + PDN |
| 59.1±2.2 | 5 M, 6 F | 33 (0–84) | 28 (6–364) | 54.84±3.42 |
| No. . | Age (years) . | Sex . | Dialysis (months) . | Time from RTx to study week 0 (months) . | Creatinine clearance (ml/min/1.73 m2) . | Immunosuppression . |
|---|---|---|---|---|---|---|
| 1 | 51 | M | 36 | 6 | 69.92 | CyA + MMF + PDN |
| 2 | 64 | M | 30 | 17 | 45.56 | CyA + MMF |
| 3 | 49 | F | 45 | 29 | 49.37 | Tac + MMF |
| 4 | 70 | F | 84 | 10 | 55.32 | CyA + MMF + PDN |
| 5 | 50 | F | 12 | 364 | 72.46 | Aza + PDN |
| 6 | 59 | F | 41 | 14 | 43.37 | CyA + Aza |
| 7 | 61 | M | 0 | 28 | 68.35 | CyA + MMF |
| 8 | 55 | F | 0 | 37 | 43.20 | CyA + MMF |
| 9 | 62 | F | 35 | 28 | 49.14 | CyA + MMF |
| 10 | 70 | M | 29 | 178 | 61.92 | CyA + Aza |
| 11 | 59 | M | 33 | 55 | 44.71 | CyA + MMF + PDN |
| 59.1±2.2 | 5 M, 6 F | 33 (0–84) | 28 (6–364) | 54.84±3.42 |
RTx = renal transplantation; CyA = cyclosporin A; Tac = tacrolimus; MMF = mycophenolate mofetil; Aza = azathioprine; PDN = prednisone.
Patient demographics and characteristics at baseline (week 0)
| No. . | Age (years) . | Sex . | Dialysis (months) . | Time from RTx to study week 0 (months) . | Creatinine clearance (ml/min/1.73 m2) . | Immunosuppression . |
|---|---|---|---|---|---|---|
| 1 | 51 | M | 36 | 6 | 69.92 | CyA + MMF + PDN |
| 2 | 64 | M | 30 | 17 | 45.56 | CyA + MMF |
| 3 | 49 | F | 45 | 29 | 49.37 | Tac + MMF |
| 4 | 70 | F | 84 | 10 | 55.32 | CyA + MMF + PDN |
| 5 | 50 | F | 12 | 364 | 72.46 | Aza + PDN |
| 6 | 59 | F | 41 | 14 | 43.37 | CyA + Aza |
| 7 | 61 | M | 0 | 28 | 68.35 | CyA + MMF |
| 8 | 55 | F | 0 | 37 | 43.20 | CyA + MMF |
| 9 | 62 | F | 35 | 28 | 49.14 | CyA + MMF |
| 10 | 70 | M | 29 | 178 | 61.92 | CyA + Aza |
| 11 | 59 | M | 33 | 55 | 44.71 | CyA + MMF + PDN |
| 59.1±2.2 | 5 M, 6 F | 33 (0–84) | 28 (6–364) | 54.84±3.42 |
| No. . | Age (years) . | Sex . | Dialysis (months) . | Time from RTx to study week 0 (months) . | Creatinine clearance (ml/min/1.73 m2) . | Immunosuppression . |
|---|---|---|---|---|---|---|
| 1 | 51 | M | 36 | 6 | 69.92 | CyA + MMF + PDN |
| 2 | 64 | M | 30 | 17 | 45.56 | CyA + MMF |
| 3 | 49 | F | 45 | 29 | 49.37 | Tac + MMF |
| 4 | 70 | F | 84 | 10 | 55.32 | CyA + MMF + PDN |
| 5 | 50 | F | 12 | 364 | 72.46 | Aza + PDN |
| 6 | 59 | F | 41 | 14 | 43.37 | CyA + Aza |
| 7 | 61 | M | 0 | 28 | 68.35 | CyA + MMF |
| 8 | 55 | F | 0 | 37 | 43.20 | CyA + MMF |
| 9 | 62 | F | 35 | 28 | 49.14 | CyA + MMF |
| 10 | 70 | M | 29 | 178 | 61.92 | CyA + Aza |
| 11 | 59 | M | 33 | 55 | 44.71 | CyA + MMF + PDN |
| 59.1±2.2 | 5 M, 6 F | 33 (0–84) | 28 (6–364) | 54.84±3.42 |
RTx = renal transplantation; CyA = cyclosporin A; Tac = tacrolimus; MMF = mycophenolate mofetil; Aza = azathioprine; PDN = prednisone.
Serum calcium and phosphate concentration and plasma intact PTH level
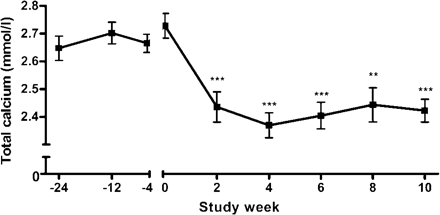
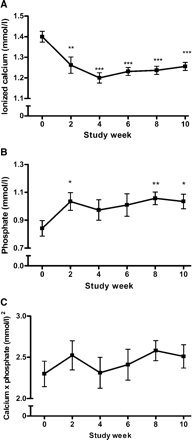
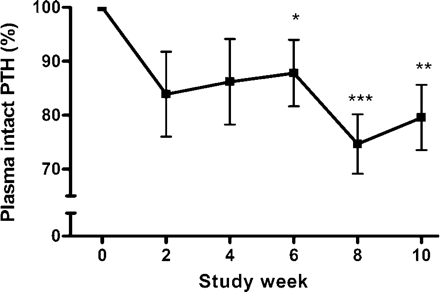
Prior to inclusion, all patients had persistent hypercalcaemia (Figure 1). After the individual cinacalcet doses were adjusted at week 2, total serum calcium remained within normal limits and ionized calcium <1.35 mmol/l in all patients until week 10 (Figures 1 and 2A). Importantly, no further cinacalcet dose adjustment had to be performed. The applied daily dosages of cinacalcet during the 8 weeks following the initial dose-finding period were as follows: 30 mg (n = 8), 15 mg (n = 1) and 60 mg (n = 1). Cinacalcet increased serum phosphate concentrations significantly at study weeks 2, 8 and 10, although oral phosphate supplements were stopped at week 6 (Figure 2B). The serum calcium × phosphate product remained unchanged throughout the study (Figure 2C). Intact PTH levels were reduced by 16.1% during the dose-finding period and remained suppressed throughout the study (Figure 3). The intact PTH levels from week 0 to 10 are indicated in Table 2.
Serum levels of total calcium over time. Prior to inclusion, patients had persistent hypercalcaemia (weeks −24 to 0). Cinacalcet reduced serum calcium concentration significantly, achieving normocalcaemia in all patients (<2.60 mmol/l) from weeks 2 to 10. ***P<0.001, **P<0.01, compared with week 0 (mean±SE).
Serum levels of ionized calcium, phosphate and total calcium × phosphate product. (A) Cinacalcet reduced ionized serum calcium concentration significantly throughout the study. Ionized serum calcium remained below 1.35 mmol/l in all patients from weeks 2 to 10. (B) Cinacalcet increased the mean serum phosphate concentration. The increase reached statistical significance at weeks 2, 8 and 10 despite discontinuation of prior oral phosphate supplements in two patients at week 6. (C) The mean serum total calcium × phosphate product remained unchanged throughout the study period. ***P<0.001, **P<0.01, *P<0.05, compared with week 0 (mean±SE).
Parathyroid hormone (PTH) levels over time. Cinacalcet reduced intact PTH plasma levels, expressed as mean percentage of initial PTH (100% = value of week 0) by 12.2% (week 6), 25.3% (week 8) and 21.8% (week 10), respectively. ***P<0.001, **P<0.01, *P<0.05, compared with week 0 (mean±SE).
Patient biochemistry between study weeks 0 and 10
| Parameter . | Study week . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 0 . | 2 . | 4 . | 6 . | 8 . | 10 . | |||||
| Plasma intact PTH (ng/l) | 176.0±24.0 | 147.6±25.6 | 147.8±28.6 | 153.9±23.9 | 133.0±31.2 | 135.6±17.6 | |||||
| Serum creatinine (µmol/l) | 118.8±10.0 | 123.5±10.0 | 122.5±10.0 | 126.8±13.1 | 121.7±15.8 | 125.7±12.9 | |||||
| Serum urea (mmol/l) | 8.18±0.77 | 9.62±1.06 | 9.44±0.94 | 8.80±1.12 | 8.21±1.18 | 10.01±1.42 | |||||
| MDRD GFR (ml/min/1.73 m2) | 56.65±5.75 | 52.87±5.41 | 52.96±4.93 | 52.05±4.75 | 54.88±6.53 | 51.96±5.25 | |||||
| CrCl (ml/min/1.73 m2) | 54.49±2.55 | 48.61±4.22 | |||||||||
| 24 h urine Ca to creatinine ratio | 0.42±0.12 | 0.39±0.12 | |||||||||
| Fractional Ca excretion (%) | 1.48±0.32 | 1.62±0.31 | |||||||||
| 24 h urine P to creatinine ratio | 2.89±0.52 | 2.41±0.15 | |||||||||
| Fractional phosphate excretion (%) | 37.35±3.28 | 30.87±2.92 | |||||||||
| Parameter . | Study week . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 0 . | 2 . | 4 . | 6 . | 8 . | 10 . | |||||
| Plasma intact PTH (ng/l) | 176.0±24.0 | 147.6±25.6 | 147.8±28.6 | 153.9±23.9 | 133.0±31.2 | 135.6±17.6 | |||||
| Serum creatinine (µmol/l) | 118.8±10.0 | 123.5±10.0 | 122.5±10.0 | 126.8±13.1 | 121.7±15.8 | 125.7±12.9 | |||||
| Serum urea (mmol/l) | 8.18±0.77 | 9.62±1.06 | 9.44±0.94 | 8.80±1.12 | 8.21±1.18 | 10.01±1.42 | |||||
| MDRD GFR (ml/min/1.73 m2) | 56.65±5.75 | 52.87±5.41 | 52.96±4.93 | 52.05±4.75 | 54.88±6.53 | 51.96±5.25 | |||||
| CrCl (ml/min/1.73 m2) | 54.49±2.55 | 48.61±4.22 | |||||||||
| 24 h urine Ca to creatinine ratio | 0.42±0.12 | 0.39±0.12 | |||||||||
| Fractional Ca excretion (%) | 1.48±0.32 | 1.62±0.31 | |||||||||
| 24 h urine P to creatinine ratio | 2.89±0.52 | 2.41±0.15 | |||||||||
| Fractional phosphate excretion (%) | 37.35±3.28 | 30.87±2.92 | |||||||||
MDRD GFR = calculated glomerular filtration rate according to the Modification of Diet in Renal Disease study group (extended version); CrCl = measured creatinine clearance; Ca = calcium; P = phosphate.
Patient biochemistry between study weeks 0 and 10
| Parameter . | Study week . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 0 . | 2 . | 4 . | 6 . | 8 . | 10 . | |||||
| Plasma intact PTH (ng/l) | 176.0±24.0 | 147.6±25.6 | 147.8±28.6 | 153.9±23.9 | 133.0±31.2 | 135.6±17.6 | |||||
| Serum creatinine (µmol/l) | 118.8±10.0 | 123.5±10.0 | 122.5±10.0 | 126.8±13.1 | 121.7±15.8 | 125.7±12.9 | |||||
| Serum urea (mmol/l) | 8.18±0.77 | 9.62±1.06 | 9.44±0.94 | 8.80±1.12 | 8.21±1.18 | 10.01±1.42 | |||||
| MDRD GFR (ml/min/1.73 m2) | 56.65±5.75 | 52.87±5.41 | 52.96±4.93 | 52.05±4.75 | 54.88±6.53 | 51.96±5.25 | |||||
| CrCl (ml/min/1.73 m2) | 54.49±2.55 | 48.61±4.22 | |||||||||
| 24 h urine Ca to creatinine ratio | 0.42±0.12 | 0.39±0.12 | |||||||||
| Fractional Ca excretion (%) | 1.48±0.32 | 1.62±0.31 | |||||||||
| 24 h urine P to creatinine ratio | 2.89±0.52 | 2.41±0.15 | |||||||||
| Fractional phosphate excretion (%) | 37.35±3.28 | 30.87±2.92 | |||||||||
| Parameter . | Study week . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 0 . | 2 . | 4 . | 6 . | 8 . | 10 . | |||||
| Plasma intact PTH (ng/l) | 176.0±24.0 | 147.6±25.6 | 147.8±28.6 | 153.9±23.9 | 133.0±31.2 | 135.6±17.6 | |||||
| Serum creatinine (µmol/l) | 118.8±10.0 | 123.5±10.0 | 122.5±10.0 | 126.8±13.1 | 121.7±15.8 | 125.7±12.9 | |||||
| Serum urea (mmol/l) | 8.18±0.77 | 9.62±1.06 | 9.44±0.94 | 8.80±1.12 | 8.21±1.18 | 10.01±1.42 | |||||
| MDRD GFR (ml/min/1.73 m2) | 56.65±5.75 | 52.87±5.41 | 52.96±4.93 | 52.05±4.75 | 54.88±6.53 | 51.96±5.25 | |||||
| CrCl (ml/min/1.73 m2) | 54.49±2.55 | 48.61±4.22 | |||||||||
| 24 h urine Ca to creatinine ratio | 0.42±0.12 | 0.39±0.12 | |||||||||
| Fractional Ca excretion (%) | 1.48±0.32 | 1.62±0.31 | |||||||||
| 24 h urine P to creatinine ratio | 2.89±0.52 | 2.41±0.15 | |||||||||
| Fractional phosphate excretion (%) | 37.35±3.28 | 30.87±2.92 | |||||||||
MDRD GFR = calculated glomerular filtration rate according to the Modification of Diet in Renal Disease study group (extended version); CrCl = measured creatinine clearance; Ca = calcium; P = phosphate.
Renal function and urinary excretion of phosphate and calcium
Renal function was assessed by serum creatinine, serum urea and calculated glomerular filtration rate according to the MDRD study group (extended version) every 2 weeks, and creatinine clearance was measured at baseline and week 10. Based on these determinations, renal function remained stable throughout (Table 2). No allograft rejection was observed. Blood pressure remained unchanged. Fractional phosphate excretion after 10 weeks of treatment with cinacalcet did not change significantly (P = 0.151). Similary, the fractional excretion of calcium, 24 h urine calcium to creatinine ratio and 24 h urine calcium excretion remained unchanged compared with baseline values (Table 2).
Immunosuppressive drug dosage and calcineurin inhibitor trough levels
Nine of the 11 patients were on a cyclosporin-based immunosuppression regimen (Table 1). Cyclosporin doses and trough levels remained unchanged throughout (Table 3). The doses of tacrolimus, mycophenolate mofetil, azathioprine and prednisone also remained constant.
Cyclosporin (n = 9) dose and whole blood through levels of study weeks 0–10
| Parameter . | Study week . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 0 . | 2 . | 4 . | 6 . | 8 . | 10 . | |||||
| Cyclosporin dose (mg/day) | 188.9±13.9 | 188.9±13.9 | 193.8±14.0 | 200.0±17.1 | 206.3±15.7 | 200±18.3 | |||||
| Cyclosporin dose (mg/kg/day) | 2.98±0.18 | 2.98±0.18 | 2.93±0.16 | 2.65±0.45 | 2.92±0.29 | 3.08±0.15 | |||||
| Cyclosporin trough level (µg/l) | 110.0±13.0 | 114.9±15.2 | 111.6±22.3 | 115.83±9.4 | 121.7±6.2 | 119.8±10.4 | |||||
| Parameter . | Study week . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 0 . | 2 . | 4 . | 6 . | 8 . | 10 . | |||||
| Cyclosporin dose (mg/day) | 188.9±13.9 | 188.9±13.9 | 193.8±14.0 | 200.0±17.1 | 206.3±15.7 | 200±18.3 | |||||
| Cyclosporin dose (mg/kg/day) | 2.98±0.18 | 2.98±0.18 | 2.93±0.16 | 2.65±0.45 | 2.92±0.29 | 3.08±0.15 | |||||
| Cyclosporin trough level (µg/l) | 110.0±13.0 | 114.9±15.2 | 111.6±22.3 | 115.83±9.4 | 121.7±6.2 | 119.8±10.4 | |||||
Cyclosporin (n = 9) dose and whole blood through levels of study weeks 0–10
| Parameter . | Study week . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 0 . | 2 . | 4 . | 6 . | 8 . | 10 . | |||||
| Cyclosporin dose (mg/day) | 188.9±13.9 | 188.9±13.9 | 193.8±14.0 | 200.0±17.1 | 206.3±15.7 | 200±18.3 | |||||
| Cyclosporin dose (mg/kg/day) | 2.98±0.18 | 2.98±0.18 | 2.93±0.16 | 2.65±0.45 | 2.92±0.29 | 3.08±0.15 | |||||
| Cyclosporin trough level (µg/l) | 110.0±13.0 | 114.9±15.2 | 111.6±22.3 | 115.83±9.4 | 121.7±6.2 | 119.8±10.4 | |||||
| Parameter . | Study week . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 0 . | 2 . | 4 . | 6 . | 8 . | 10 . | |||||
| Cyclosporin dose (mg/day) | 188.9±13.9 | 188.9±13.9 | 193.8±14.0 | 200.0±17.1 | 206.3±15.7 | 200±18.3 | |||||
| Cyclosporin dose (mg/kg/day) | 2.98±0.18 | 2.98±0.18 | 2.93±0.16 | 2.65±0.45 | 2.92±0.29 | 3.08±0.15 | |||||
| Cyclosporin trough level (µg/l) | 110.0±13.0 | 114.9±15.2 | 111.6±22.3 | 115.83±9.4 | 121.7±6.2 | 119.8±10.4 | |||||
Adverse events
Cinacalcet was well tolerated. No specific side effects were reported and no serious adverse events occurred during the 10 week treatment phase. All patients continued to take the study medications without interruption, except one subject. This patient discontinued the study after 2 weeks because she feared a possible deterioration of a pre-existing retinitis pigmentosa. Ophthalmological examination showed no changes in vision compared with pre-study tests.
Discussion
Our study demonstrated that cinacalcet treatment was effective in normalizing serum calcium in renal transplant patients with persistent hyperparathyroidism. The effect was sustained over 10 weeks, and renal graft function remained stable.
In primary hyperparathyroidism, cinacalcet administered twice daily was effective in normalizing serum calcium levels in 73% of patients with a mean serum calcium of 2.67 mmol/l at baseline, and relatively constant and well-preserved kidney function. As in our study, serum calcium was normalized rapidly, within 2 weeks. After individual doses of cinacalcet had been established, 100% of our renal transplant patients reached the predefined normal target range of a total serum calcium concentration of 2.10–2.60 mmol/l.
Successful renal transplantation leading to normalization of urinary phosphate excretion and renal calcitriol production may reverse hyperparathyroidism due to involution of the parathyroid glands [9]. This process takes a few months to several years. Our patients had persistently elevated serum calcium concentrations in the 6 months before cinacalcet treatment was started, and 63.7% of them were transplanted since >2 years.
Cinacalcet effectively lowers plasma parathyroid hormone levels in primary [5,6] and secondary [7] hyperparathyroidism. In our study, a significant reduction of plasma intact PTH of ∼18% was observed. In patients with primary hyperparathyroidism, the reduction was only 8%, with a mean PTH at baseline of 120 ng/l, compared with a 43% reduction in patients with secondary hyperparathyroidism and a mean baseline plasma PTH of 643 ng/l [7]. The development of hypercalcaemia correlates with the hyperplasia and nodular transformation of the parathyroid gland [10]. The degree of PTH reduction with cinacalcet treatment may reflect the degree of parathyroid hyperplasia and nodular transformation, with less reduction in patients with adenoma-like formations of the parathyroid glands.
The reduction of intact PTH was accompanied by an increase in serum phosphate concentration towards normal values and a trend to a reduced fractional phosphate excretion. An elevated serum calcium × phosphate product is a risk factor for cardiovascular mortality, at least in haemodialysis patients [8]. In our study, the observed increase in serum phosphate was counterbalanced by the decrease in serum calcium and therefore the serum calcium × phosphate product remained unchanged.
The reduction of intact PTH by cinacalcet could reduce tubular calcium reabsorption, leading to hypercalciuria and, if prolonged, increase the risk for nephrolithiasis [11] and osteopenia [12]. However, the 24 h urine calcium to creatinine ratios and 24 h urine calcium excretion remained unchanged, as compared with baseline values. A similar observation was also made in patients treated with cinacalcet for primary hyperparathyroidism [6]. The authors suggested that the observed reduction in serum calcium led to a decrease of filtered calcium load that compensated for the decrease in tubular calcium reabsorption. This could explain why despite a reduction in plasma PTH, overall urinary calcium excretion did not change.
During the observation period of our study, renal function remained unchanged and no renal allograft rejection was observed. Immunosuppression was kept constant throughout. In vivo interaction data of cinacalcet with immunosuppressive drugs are lacking. Cinacalcet inhibits cytochrome P450 2D6 (CYP2D6) and is metabolized by CYP3A4, CYP2D6 and CYP1A2. Therefore, no interactions with the metabolic transformation of cyclosporin or tacrolimus were expected. The cyclosporin dose and whole blood trough levels showed no relevant fluctuations.
In conclusion, the results of this study demonstrate that cinacalcet is efficacious and safe in normalizing serum calcium by lowering intact PTH in renal allograft recipients with hypercalcaemia due to persistent hyperparathyroidism. These beneficial effects were rapidly achieved and were sustained over 2 months. Thus, the treatment of persistent hyperparathyroidism with cinacalcet may represent a valuable alternative for parathyroidectomy in these patients. The long-term effects and treatment duration remain to be examined.
Conflict of interest statement. None declared.
References
Chatterjee SN, Friedler RM, Berne TV, Oldham SB, Singer FR, Massry SG. Persistent hypercalcemia after successful renal transplantation.
David DS, Sakai S, Brennan BL et al. Hypercalcemia after renal transplantation. Long-term follow-up data.
Kinnaert P, Nagy N, Decoster-Gervy C, De Pauw L, Salmon I, Vereerstraeten P. Persistent hyperparathyroidism requiring surgical treatment after kidney transplantation.
D'Alessandro AM, Melzer JS, Pirsch JD et al. Tertiary hyperparathyroidism after renal transplantation: operative indications.
Peacock M, Bilezikian JP, Klassen PS, Guo MD, Turner SA, Shoback D. Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism.
Shoback DM, Bilezikian JP, Turner SA, McCary LC, Guo MD, Peacock M. The calcimimetic cinacalcet normalizes serum calcium in subjects with primary hyperparathyroidism.
Block GA, Martin KJ, de Francisco AL et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group.
Bonarek H, Merville P, Bonarek M et al. Reduced parathyroid functional mass after successful kidney transplantation.
McCarron DA, Muther RS, Lenfesty B, Bennett WM. Parathyroid function in persistent hyperparathyroidism: relationship to gland size.
Parks JH, Coe FL. A urinary calcium-citrate index for the evaluation of nephrolithiasis.





Comments